Table of Contents
What is Prions?
- Prion is an abnormal or misfolded protein that causes fatal disease in animals and humans by transmitting their misfolded shape onto normal variants of the same protein.
- Prion causes untreatable, fatal, and transmissible neurodegenerative diseases in both humans and animals. In this disease, a progressive decline is occurring in brain function.
- In 1982 an American neurologist and biochemist Stanley Benjamin Prusiner first coined the term Prion. The term prion derived from “proteinaceous infectious particle”.
- Their mode of infection is totally different from bacteria, viruses, and other infectious pathogens; they lack genetic material.
- Prions are made of a protein called prion protein (PrP).
- This disease is very rare, about 350 new cases reported each year in the US.
- In this disease, the PrP accumulates in brain cells and starts to form clumps and then it starts damaging and killing nerve cells.
- It damages the brain cells by forming tiny holes that appear sponge-like structure under a microscope.
- The incubation period of prion disease is about 5 to 20 years.
Characteristics of Prion Protein
- Prions are smaller than a virus and can be seen under an electron microscope.
- They only appear in electron microscopes when they have aggregated and formed a cluster.
- Prions lack nucleic acids. Hence they are resistant to those procedures which are used to destroy pathogens by breaking down nucleic acid.
- Prions do not trigger a host immune response because they are the abnormal version of a normal protein.
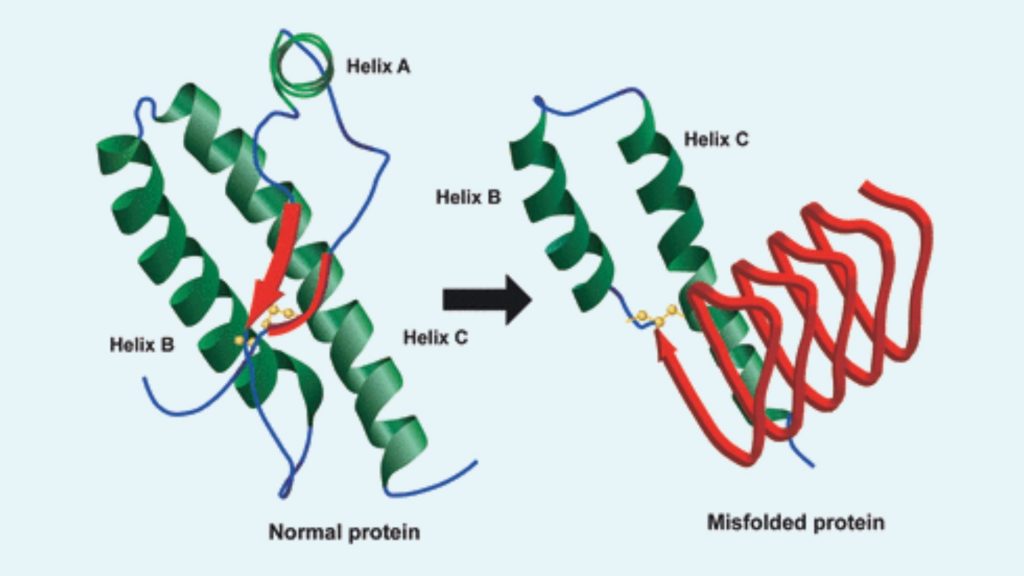
- The normal PrP is composed of a flexible coil known as alpha helices, whereas in abnormal PrP these helices are stretched out into densely packed structures called beta-sheets.
- PrP is resistant to proteases enzyme, heat, ionizing radiation, and formaldehyde treatments.
- In 2011 researchers found that prions can be transmitted through airborne particles such as aerosols.
- The prion can be decontaminated by protein hydrolysis or reduction or destruction of protein tertiary structure, which can be accomplished by the sodium hypochlorite, sodium hydroxide, and strongly acidic detergents such as LpH.
- Researchers currently study ozone sterilization as a potential method for prion denaturation and deactivation.
Structure of Prion Protein
The prion protein exists throughout the body of a healthy animal and people. There are present two forms of prion protein such as;
- PrPC, it is considered as the normal form of protein. Here C refers to ‘cellular’ PrP. this protein is structurally well defined.
- PrPSc, is the infectious form of the prion protein. Here Sc refers to ‘scrapie’, it is a prion disease that occurs in sheep. This protein is polydisperse and defined at a relatively poor level.
PrPC
- This is the normal protein which is found in membranes of cells.
- It contains 209 amino acids and one disulfide bond.
- The molecular weight is about 35–36 kDa.
- It mainly appears in alpha-helical structure.
- There are present different topological forms of this protein such as; 1 cell surface form anchored via glycolipid and 2 transmembrane forms.
- This protein is not sedimentable means Centrifugation techniques cannot separate this protein.
- These proteins are bound copper (II) ions with high affinity.
- Proteinase K enzyme can readily digest the PrPc Protein.
- The enzyme phosphoinositide phospholipase C (PI-PLC) liberates this protein from the cell surface by cleaving the glycophosphatidylinositol (GPI) glycolipid anchor.
- This protein helps in cell-cell adhesion, intracellular signaling in vivo and may be involved in cell-cell communication in the brain.
PrPsc
- It is the infectious isoform of PrP or known as the prion.
- This protein can convert the normal PrPc proteins into abnormal infectious form by altering their conformation, or shape.
- Although the 3D shape of this protein is not clear, it assumed that it has a β-sheet structure.
Replication of Prion Protein or Mode of Action
There are two hypotheses on prion protein replication such as;
- Heterodimer model
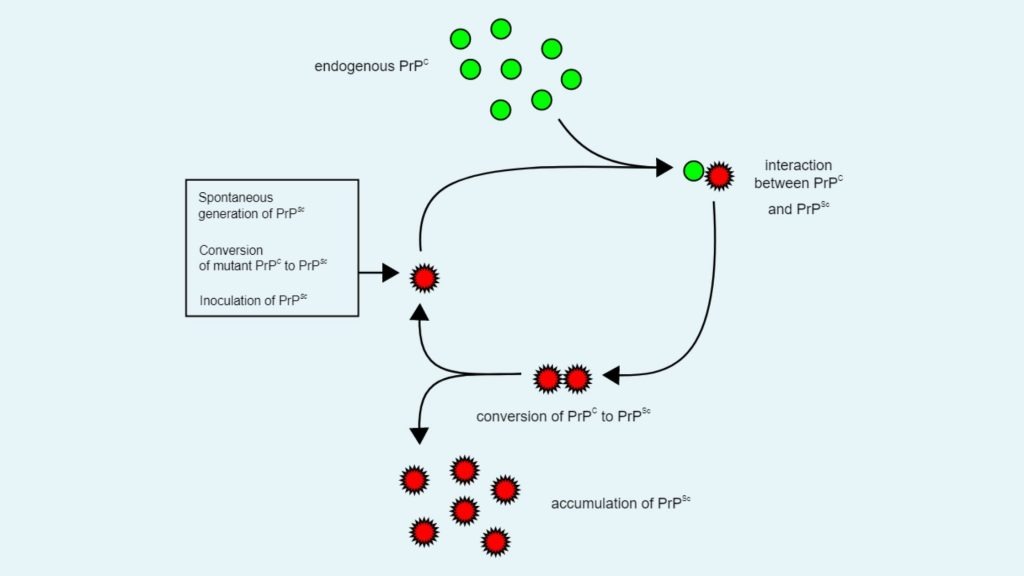
- This is the first model to explain how prion proteins are replicate. According to this replication model, a single PrPSc molecule attached with a single PrPC molecule and then it catalyzes the conversion of PrPC to PrPSc.
- After that two PrPSc molecules combined with each other and can go on to convert more PrPC.
- Fibril model
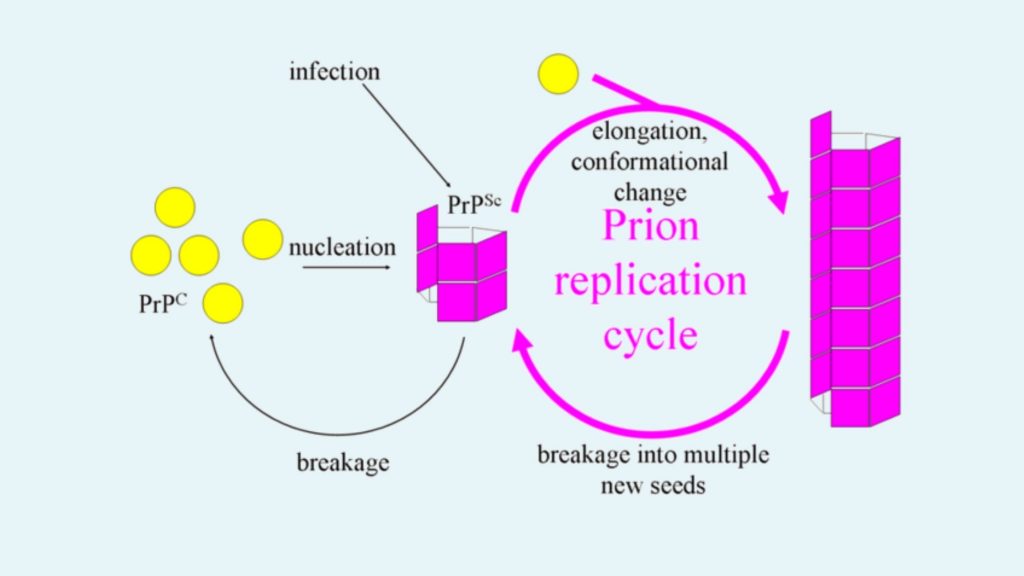
- According to this model PrPSc appears as fibrils.
- The end portion of this fibril is combined with PrPC and converted into PrPSc.
Normal function PrP
- Regulated cell death
There are several prion-like proteins in our body such as MAVS, RIP1, and RIP3. This protein polymerizes into filamentous amyloid fibers and initiates regulated cell death.
- long-term memory: It helps to maintain long-term memory.
- Stem cell renewal: Studies of Whitehead Institute for Biomedical Research found that PrP is necessary for an organism’s self-renewal of bone marrow.
- Innate immunity: It plays a role in innate immunity and contains antiviral properties against many viruses, including HIV.
Types of Prion Diseases
1. Prion diseases in Human
- Creutzfeldt-Jakob disease (CJD)
- It is also called Subacute spongiform encephalopathy or Neurocognitive Disorder due to Prion Disease.
- CJD is a fatal degenerative brain disorder and was first discovered in 1920.
- The symptoms of this disease include memory problems, behavioral changes, poor coordination, visual disturbances, dementia, involuntary movements, blindness, weakness, and coma.
- In most of the cases this disease occurs spontaneously and in few cases it is inherited from a person’s parents.
- CJD has no treatment.
- Most of the cases this disease affects those people above 60 years old.
- This disease is classified as the transmissible spongiform encephalopathy.
- CJD can be transmitted through the contaminated harvested human brain products, corneal grafts, dural grafts, or electrode implants and human growth hormone.
- There are three types of CJD such as; Sporadic (sCJD), this occurs due to the spontaneous misfolding of prion-protein.Familial (fCJD), occurs by inherited mutation in the prion-protein gene. Acquired CJD, this can be occurred by direct contact with the contaminated tissue of an infected person.
- Gerstmann–Sträussler–Scheinker syndrome
- Gerstmann–Sträussler–Scheinker syndrome was first reported in 1936.
- It is a rare, usually familial, fatal neurodegenerative disease.
- This disease only affects patients from 20 to 60 years in age.
- It is classified as transmissible spongiform encephalopathies (TSE).
- The symptoms include progressive ataxia, pyramidal signs, and even adult-onset dementia.
- Fatal insomnia
- This is a rare disorder that results in trouble sleeping and it worsens over time.
- Other symptoms of this disease are speech problems, coordination problems, dementia and death.
- It occurs by the mutation of gene encoding protein PrPC.
- There are two forms of this disease such as fatal familial insomnia (FFI), which is autosomal dominant and sporadic fatal insomnia (sFI) which is due to an inherited mutation.
- This can be diagnosed by sleep study and PET scan.
- Kuru
- It is a very rare, incurable and fatal neurodegenerative disorder.
- This disorder is found in Fore people of Papua New Guinea.
- It is a transmissible spongiform encephalopathy (TSE).
- The symptoms include progressive cerebellar ataxia, or loss of coordination and control over muscle movements.
- The term kuru is a Fore word kuria or guria (“to shake”). Kúru itself means “trembling”.
- This disease is also called “laughing sickness”.
- Variably protease-sensitive prionopathy
- This disease is also known as Protease Sensitive Prionopathy.
- This disease is occurring in just 2 or 3 out of every 100 million people.
- The symptoms are speech deficits (aphasia and/or dysarthria) and progressive cognitive and motor decline (dementia, ataxia, parkinsonism, psychosis, aphasia and mood disorder).
- This disease only affects those people who are above 70 years.
Other diseases are include Familial spongiform encephalopathy, Iatrogenic Creutzfeldt–Jakob disease (iCJD), Variant Creutzfeldt–Jakob disease (vCJD), Familial Creutzfeldt–Jakob disease (fCJD), Sporadic Creutzfeldt–Jakob disease (sCJD).
2. Prion diseases in Animal
- Scrapie
- It is a fatal, degenerative disease which affects the nervous systems of sheep and goats.
- It is a transmissible spongiform encephalopathies (TSEs).
- This disease is not transmissible to humans.
- The symptoms of this disease include lip smacking, altered gaits, convulsive collapse and compulsively scrape off their fleeces against rocks, trees or fences.
- The most effective way to prevent the transmission of scrapie disease is to quarantine and kill those affected.
- Bovine spongiform encephalopathy
- This disease is also known as mad cow disease.
- It is a neurodegenerative disease of cattle.
- The symptoms of this disease include abnormal behavior, trouble walking, and weight loss.
- The incubation time of this disease is four to five years.
- This disease can be transmitted to humans in the form of variant Creutzfeldt–Jakob disease (vCJD) by consuming contaminated food.
- Camel spongiform encephalopathy
- It is also known as mad camel disease and similar to mad cow disease.
- This disease affects camels.
- Chronic wasting disease
- It is also known as Zombie deer disease and is a transmissible spongiform encephalopathy (TSE).
- This disease affects mule deer, white-tailed deer, red deer, sika deer, elk, caribou, and moose.
- The symptoms of these diseases include chronic weight loss and clinical signs compatible with brain lesions, aggravated over time, always leading to death.
- This disease can be transmitted by consuming the brain, spinal cord, eyes, spleen, tonsils, lymph nodes of infected animals.
Other diseases include Spongiform encephalopathy (unknown if transmissible) affects Ostrich. Exotic ungulate encephalopathy (EUE) affects Nyala, Oryx, Greater Kudu. Feline spongiform encephalopathy (FSE) affects cats. Transmissible mink encephalopathy (TME) affects Mink.
Transmission of Prion Disease
Prion disease can be transmitted by this following methods;
- Acquired: When the individual has directly exposed to PrP contaminated food or medical equipment. If the person is exposed to urine, saliva, and other body fluids of infected animals.
- Inherited: The misfolded PrP can be produced by the presence of a mutation in a gene that codes for PrP.
- Sporadic: Misfolded PrP is formed without any causes.
Symptoms of Prion Disease
- Apathy, agitation, and depression.
- Thinking, memory, and judgment difficulty.
- confusion or disorientation.
- Myoclonus or involuntary muscle spasms.
- Ataxia or loss of coordination.
- Insomnia or trouble sleeping.
- difficult or slurred speech
- impaired vision or blindness
Diagnosis of Prion Disease
- Prion disease is difficult to diagnose because their symptoms are similar to other neurodegenerative disorders. Brain biopsy is the only way to confirm Prion Disease, but this process only can be performed after death.
- Currently, there are several tests which can be used to diagnose prion disease such as Magnetic resonance imaging (MRI), Cerebrospinal fluid (CSF) testing, Electroencephalography (EEG).
Treatment of Prion Disease
There is no effective treatment procedure for prion disease. Biologists are trying to find out an effective treatment for prion diseases.
Prevention of Prion Disease
- Don’t eat cattle from countries where BSE occurs.
- Don’t use the brain and spinal cord of a cow as a food.
- Don’t donate organs, blood if you are a prion patient, because blood is a good carrier of prion.
- Sterilize medical instruments that are recently coming in contact with the nervous tissue of someone with suspected prion disease.
- Currently, the Inherited or sporadic forms of prion disease can not be prevented.
Properties of the Agents
Scrapie was among the agents in this class that was adapted for rapid passage in mice and other rodents of small size that made it easier to study due to the smaller time incubation in these rodents. The majority of knowledge about the biological and physical properties of these agents was obtained from studies conducted on mice and Hamsters.
Up to now, studies using the isolated brains of kuru as well as CJD brains have revealed no fundamental difference from scrapie strains, apart from limitations to the host range. In recent times, rodent-adapted versions of the kuru as well as CJD are now accessible. The most reliable test method that can be used to test these drugs is a bioassay where the end-point titration process is performed. These agents are difficult to research due to the lengthy incubation times that can last for over 5 years in certain systems.
Physical Properties
- Filterable up to 25-50nm average pore size
- The temperature is stable at 90oC for 30 min, however, autoclaving effectively kills it. However, some cases of CJD were transmitted via an autoclaved instrument.
- Hydrophobic with a tendency to aggregation of the infectious unit with cell elements.
- The range of sizes varies from 40S to 500S on the rate-zonal sucrose gradients
- The size of the target determined by ionizing radiation can range between 64000 and 150,000 daltons
- Gel filtration in the aftermath of the zwitterionic treatment results in= 50,000 mol.
- Density vary from 1.08-1.30 g/ml
The above physical characteristics suggest that the monomeric variant of this virus may be tiny, however they should be considered with care. The well-known characteristic of the virus to self-aggregation could result in falsely low results for studies using irradiation to activate it.
Physical Inactivation Profile
- Resistant or only partially inactivated by aldehydes and related compounds (formaldehyde, glutaldehyde, B-propriolactone), Nucleases (RNAse, DNAse), Heat (80oC), UV and ionizing radiation, Mild organic solvents (ethanol, xylenes), Nonionic and nondenaturing anionic detergents
- Moderately inactivated by Ether, Acetone, Ethylene oxide
- Effectively inactivated by Autoclaving (134oC, 15 minutes), Proteases (pronase, trypsin, proteinase K), Denaturing detergents (SDS), Strong chaotropic ions (thiocyanate, trichloroacetate). Harsh organic solvents (phenol, chloroform, 2-chloroethanol), Strong oxidizing agents (periodate, hypochlorite, permanganate), Urea, Sodium hydroxide.
- While they are extremely resistant to the majority of disinfectants however, they are tolerant to a variety of processes that are readily suited to use in regular sterilization and disinfection.
Conventional Biological Properties
- It is filterable and easy to titrate.
- Replicate in high amounts (up to 108-1010 infectious units/gram of brain)
- Replicates first within lymph nodes the spleen and thymus. Then in the brain. Evidence of spread of the virus through neural pathways and also from the spleen into the thoracic nerve via autonomic nerves.
- Host range restriction and the ability to adapt to new hosts
- Variations in strain (as defined by the host’s specificity, incubation time as well as the topography of lesions in the brain and the ability to cause amyloid plaques, as well as the ability to select for different isolates of the “wildstock” virus)
- Control of host genes influences susceptibility and immunity to infections.
- Interference between slow-replicating as well as fast-replicating strains
- Tissue culture is not able to replicate in a limited way.
- Essential protein component for replication (as demonstrated by protease digestion; chemical modification by diethylpyrocarbonate, butanedione; inactivation by detergents such as SDS, ionic inactivation with guanidinium, thiocyanate, trichloroacetic acid; denaturation by urea and phenol)
Unconventional Biological Properties
- Long incubation period
- Insufficient immunogenicity or host immune
- Chronic progressive illness that is not any remission or Relapse
- Induction of spongiform changes as well as neuronal loss and the development of gliosis
- The absence of a specific viral particles by EMR
- Nucleic acids that are not specific and resistance to processes that inhibit nucleic acids like low pH, nucleases, UV radiation at 237nm, hydrolysis of zinc, activation of hydroxylamine and psoralens
- Immunosuppression, immunepotentiation or antiviral medicines. Splenectomy can affect the progression of an infection.
- There is no CPE in the tissue culture
- Unusual spectrum of resistance certain chemical and physical treatments
- Induction of amyloid/scrapie-associated fibril and the conversion of the normal host-coded protease-sensitive proteins (PrP) to a protease-resistant homologue.
The lengthy incubation time of these agents set them apart from other viral infections. They appear to reproduce at a constant rate after inoculation. They manifest clinically once they are able to enter the CNS and achieve a critical dose. The absence of any discernable immune response is a mystery however this could be explained through the possibility that the agent could be a modified protein in the host.
Evidence suggests that the Modified PrP is integral, but it is not the only component that makes up the drug. It is not clear if there is a nucleic acid component. Certain physical characteristics suggest that an NA is not an integral component that makes up the infection like resistance to proteases, UV radiation at 237nm., zinc hydrolysis as well as photochemical inhibition through psoralens as well as chemical modification through the hydroxylamine.
Certain conventional viruses have some or all of these traits, but. It is evident the NA genome that is present is extremely tiny (less than thirty bases) and could not contain the PrP protein.
Theories on the nature of Prions
There are two main theories to explain what the characteristics of the prion are
“Protein only” hypothesis
Prions are made up of protein materials that are able to fold in various, abstract ways. At the very least, at one point can be transmissible to other prion proteins which can cause disease in a manner similar to the spread of viral infections. The principal PrPC translation product will need to be transformed into a modifiedform that is infectious in the cell infected by PrPSc. This conversion can be catalyzed through PrPSc (Autocatalysis) Every scrapie strain is identified by a distinct variant of PrPSc, which alters an initial precursor, resulting in a substance that resembles its own. The conversion of a strain when it is passed through an alternative host could be explained by the different variants of the PrPSc gene.
In a more elaborate model, the conversion of PrPC into PrPSc could be triggered by a host-coded “converting enzyme” induced by PrPSc. The variety of species of the scrapie can be explained by an array of different enzymes that convert, each one activated by the corresponding PrPSc strain.
“Nucleoprotein or Virino” hypothesis
The prion comprises tiny nucleic acids and host-coded protein. Different strains are linked to variations in the nucleic acids similar to the case of viral DNA.
The overwhelming majority of evidence currently confirms this “protein only” hypothesis. In two families that appear to be unrelated one of which is from the US and the other one in the UK, Prusiner et al. discovered that the ataxic form of the Gerstmann-Straussler disorder is associated with a mutation in codon 102 on one of the PrP alleles, ranging from protoline and leucine. The mutation was not detected in healthy individuals or in patients with other types of sporadic or acquired prions. It is believed that this mutation could play a role in the causes for the illness. It is possible that in the sporadic instances in GSS and CJD A somatic mutation may give an altered PrP that has a higher ability to transform to PrPGSS or PrPCJD. There is a possibility that, in these patients the pathogenic PrPSc form naturally but it is not often. Another possibilities is that these significantly increase susceptibility to the “true” infecting agent. Further mutations of The PrP gene may have also been discovered in families that are prone to CJD or GSS.
Scrapie prions that were continuously passed around in hamsters , are then inoculated into mice, they have an incubation duration of at least 500 days that decreases to 140 days upon the next mouse passage and remains constant thereafter. Similar sequences of events can be observed in Hamsters. In hamsters, the “nucleoprotein theory” attributes this adaption to classical mutations and selection. However, the protein-only theory suggests that the transformation of host PrPC through exogenous, heterogeneous PrPSc is not a common event, but when it happens and the newly created PrPSc is now that of the host type is able to catalyze further conversions both effectively and quickly.
Prusiner et al. created transgenic mice that expressed the PrP gene from hamsters. They inoculated them with hamster-derived prions. The results were shocking as the time of incubation was decreased from over 500 days(in control mice) and up to 75 days in transgenic mice. In the same way the susceptibility to prions that are derived from mice was reduced. The prions produced by transgenic mice that were infected with the hamster-derived prions was infectious to hamsters but not mice. However, transgenic mice that were that were infected with mouse-derived PRions generated prions that were infectious for mouse but not so for Hamsters. These results suggest that hamster-derived prions transform the “transgenic” hamster PrPC but not the endogenous murine PrPC to PrPSc. The barrier between species could have to do with the lack of efficiency by which PrPC is transformed by the heterologus PrPSc.
A murine gene expressing the GSS proline-to-leucine switch was introduced into mice. The male who was the founder and 34 offspring transgenic with the mutant transgene showed neurological signs between 7 and 39 weeks of age . They passed away within one month. The brains displayed massive spongiform damage, however, in a surprising twist, despite the striking symptoms PrPSc was indiscernible. Mice that expressed hamster PrPC or a normal version of murine PrPC didn’t develop symptoms. Brain extracts derived from these mice carried CNS degeneration to a few people who received the vaccine. Based on inference, this study indicate that PrP mutations can cause GSS and the familial CJD.
Therefore, the evidence favors the protein-only hypothesis. The primary reason this hypothesis is still accepted with apprehension is the existence of prions in different strains. There are at most two distinct murine prions (distinguishable through their incubation time) Each of them are able to reproduce indefinitely by one inbred mouse strain, even though there is only one PrP gene. The mouse is homozygous to this gene. A variety of strains of scrapie have been identified. This means that PrPC will need to transform into as many different configurations as there are prions. Based on these new findings it is possible to conclusively conclude that the PrP protein has an important part in the pathogenesis of Spongiform Encephalopathies. It is also possible that a modified PrP protein forms at least a portion or the entire of the agent that causes infection.
A study conducted in 2015 found the fact that multisystem atrophy (MSA) is a very rare neurodegenerative condition in humans is caused by misfolding of the protein known as alpha-synuclein. Therefore, it is also classified as a prion disorder.
Read Also
Except animal and human prions are also found in fungi. These fungal prions help us to understand mammalian prions. Fungal Prions do not cause disease in their host body.
