Table of Contents
Amoeba (less often spelled ameba, or amoeba, plural am(o)ebas or am(o)ebae) is often referred to as an amoeboid, is kind of unicellular organism that has the capability of changing its form, mostly by retracting and expanding pseudopods. Amoebae do not belong to a single taxonomic class, They are found in all major lineages of the eukaryotic species. Amoeboid cell types are not restricted to protozoa, as well as the fungi, algae, and animals.
Microbiologists frequently employ the words “amoeboid” as well as “amoeba” in conjunction with any organism that has amoeboid motion.
In earlier classification systems, amoebae were classified in the subphylum or class Sarcodina which is a classification of single-celled organisms with pseudopods or move via protoplasmic flow. However, studies on molecular phylogenetics have revealed the fact that Sarcodina does not constitute a single phylogenetic species that has members who share common descent. Thus, amoeboid species are not grouped together with another group.
The most well-known amoeboid protists are Chaos carolinense as well as Amoeba proteus Both are widely grown and researched in labs and classrooms. Other species that are well-known include “brain-eating amoeba” Naegleria fowleri as well as an intestinal parasite Entamoeba histolytica, which is responsible for amoebic dysentery. Then there is Multicellular “social amoeba” or slime mould Dictyostelium discoideum.
Amoeba Under Microscope
Amoebas are single-celled organisms. Therefore, they can only be observed with a microscope. There are techniques that can be employed to study these creatures.
The most basic method involves viewing amoebas using the microscope, without staining. This is an easy technique that allows students to watch them live while they move about. The other method involves staining and fixing to give a clearer view of the organelles and the structure of the living organism.
Simple or Direct Method
Amoebas are found flourishing and living in shallow ponds containing organic matter.
Requirement
To observe amoebas through a microscope, learners must have these items:
- The water sample is taken from a pond that contains organic matter
- Pondweed that has been gathered from a water pond
- Petri dish
- A compound light microscope
- Water
- A dropper
Procedure
To conduct this experiment students can examine a sample of water from a pond directly to determine the species or perform simple cultivation to increase the growth and increase the number of amoebae. Amoeba culture is an easy practice that involves the following steps:
- Place a few pondweeds inside a peri-dish, and then add water to cover the weed
- Place the culture in the dark for a few days, and wait until a brown scum appears on the surface
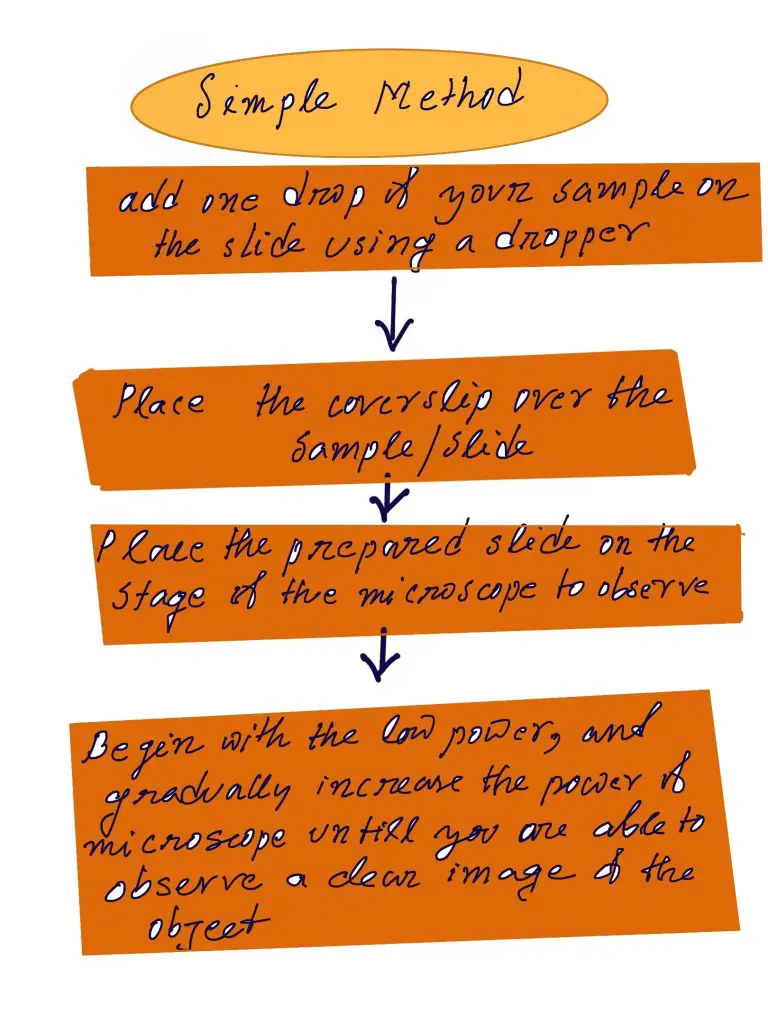
Procedure for Microscopy
- Utilizing a dropper, drop some droplets of your sample onto an optical microscope slide (a sample of pond water, or a tiny sample of the culture)
- Cover the sample gently with a coverslip. place it on the stage of the microscope to view
- Begin with low power, and gradually increase the power until you are able to observe the object
Observation
If viewed from a distance, amoebas appear as the colorless (transparent) jelly that moves through the field at a very slow pace when they change their shape. When it changes form, it can be visible protruding long finger-like projections (drawn and then withdrawn).
Fixing and Staining Technique
Requirements
- Amoeba culture
- A bench-top MSE centrifuge
- Neff’s saline, sea water
- Moist chamber
- Cover slip
- Sodium hydroxide (NaOH)
- Distilled water
- Nissenbaum’s fixative
- Lugol’s Iodine
- Formalin seawater
- Carnoy’s fixative
- Heidenhain’s iron Haematoxylin
Procedure
In this method, the sample (amoebae) is initially cultured with this method and the slopes of saline-agar. After the culture, a small portion of the sample is concentrated by centrifuge (at 3 Krpm for 10-minutes).
The process continues with the next few steps:
- Clean the pellet with 75 percent of seawater or Neff’s Saline or any other solution that is suitable.
- Let the specimen settle on the coverslip within an environment that is moist until the amoebae exhibit a normal morphology of locomotion (the coverslip could be treated using sodium hydroxide based on the kind of amoeba)
Fixing
- After settling the coverslip. Take a pipette of freshly prepared Nissenbaum’s fixative onto the sample and let sit for five minutes
- Clean the sample using acidified HgCl2 for around 7 minutes.
- Clean the sample using 50 percent or 35%, 15 percent ethanol for approximately 5 minutes
- The sample is then washed using distilled water for around 5 minutes
Note: A few other fixatives that are available include Carnoy’s Fixative Lugol’s Iodine, Seawater formalin.
Staining
The aim of staining amoebae is to enhance the visibility of mitotic structures. The stains that are able to stain amoeba cells include:
- Heidenhain’s iron Haematoxylin
- Kernechtrot (Nuclear Red)
- Modified Field’s Stain
- Klein’s Silver relief stain
Heidenhain’s iron hematoxylin staining is performed using the steps listed below:
- Inoculation of the sample within 2 percent ammonium ferric-sulfate for approximately 1 and a quarter hours
- Rinsing the sample with distillate water
- Inoculation of the sample with 0.5 percent haematoxylin and Ammonium Ferric Sulphate, 2% for approximately 1 and 1/2 hours
- Clean the sample using tap water
- After staining, put the slide onto the microscope to view.
Observation
If you look at the microscope, we will see tiny dark spots within the cytoplasm of the animal and the cytoplasm will be light stained.
The direct examination of a species (without staining) offers a significant benefit in that amoebae remain alive and mobile when observed under a microscope.
It allows students to observe the finger-like projections (pseudopods) lengthen and shrink as the organism travels around or takes in substrates. This technique, however, doesn’t let students see the organelles inside the cells.
Fixing and staining on contrary eliminate the amoeba. This means that students won’t have the opportunity to see the organism’s movements within the view of the microscope However, staining enhances the contrast, which allows students to have a clearer perspective of the organelles inside the cell.
