Table of Contents
What is Benedict’s Test?
Benedict’s Test is a widely recognized chemical assay employed for the identification of reducing sugars within a given solution. Reducing sugars, by definition, are carbohydrates that possess free aldehyde or ketone groups, enabling them to donate electrons and subsequently reduce other molecules. Notably, this category encompasses all monosaccharides and certain disaccharides, oligosaccharides, and polysaccharides.
The fundamental principle underlying Benedict’s Test is the reaction between the reducing sugar and Benedict’s reagent, resulting in a characteristic brick-red precipitate. This color change serves as a visual indicator of the presence of reducing sugars. While the intensity of the resultant color can provide a rough estimate of sugar concentration, it does not allow for precise quantification, rendering the test primarily qualitative with semi-quantitative potential.
Historically, Benedict’s Test has been instrumental in differentiating between reducing and non-reducing carbohydrates, offering a reliable alternative to the Fehling’s test. Its applications extend beyond mere differentiation; for instance, it serves as a preliminary test for the detection of glucose in urine, a potential indicator of diabetes mellitus.
The inception of this test can be attributed to the pioneering work of Stanley Rossiter Benedict, an American chemist and biochemist. His contributions have cemented the test’s position as a cornerstone in carbohydrate analysis within scientific and medical realms.
In summary, Benedict’s Test stands as a pivotal analytical method in the realm of carbohydrate research, offering a robust and reliable means to detect the presence of reducing sugars. Its enduring relevance is a testament to its efficacy and the foundational work of Stanley Rossiter Benedict.
Benedict’s Test Definition
Benedict’s Test is a qualitative chemical assay used to detect the presence of reducing sugars in a solution, characterized by the formation of a brick-red precipitate upon reaction with Benedict’s reagent.
Objectives of Benedict’s Test
Benedict’s Test, a cornerstone in carbohydrate analysis, serves several pivotal objectives within scientific and medical research:
- Detection of Reducing Sugars: One of the primary purposes of the test is to ascertain the presence of reducing sugars within a given sample solution. Reducing sugars, by their inherent nature, possess free aldehyde or ketone groups, enabling them to participate in redox reactions.
- Diagnosis of Diabetes Mellitus: The test holds clinical significance as it facilitates the detection of glucose in urine samples. The presence of glucose in urine can be indicative of diabetes mellitus, making Benedict’s Test a valuable preliminary diagnostic tool.
- Estimation of Sugar Concentration: While primarily qualitative, the test offers semi-quantitative insights. The intensity of the brick-red precipitate formed upon reaction with Benedict’s reagent can provide an approximate gauge of the concentration of reducing sugars in the sample.
- Carbohydrate Differentiation: Beyond mere detection, the test aids in the differentiation and identification of extracted carbohydrates. By discerning between reducing and non-reducing sugars, it offers a deeper understanding of the carbohydrate composition of the sample.
Principle of Benedict’s Test
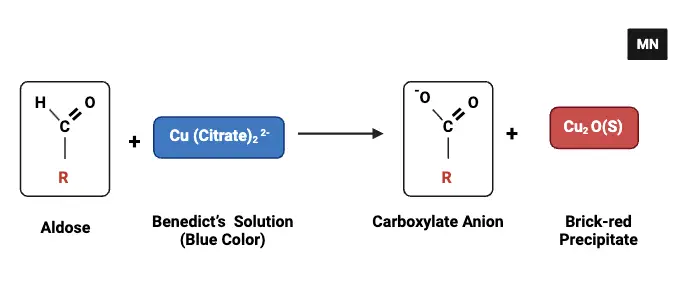
Benedict’s Test is a chemical assay grounded in the principle of redox reactions, specifically tailored to detect the presence of reducing sugars in a solution. The underlying mechanisms and chemical interactions that facilitate this detection can be delineated as follows:
- Alkaline Environment Creation: The Benedict reagent contains sodium carbonate (Na2CO3), which elevates the pH of the mixture, ensuring an alkaline milieu. This alkaline environment is pivotal for the subsequent chemical transformations of reducing sugars.
- Tautomerism of Reducing Sugars: Under the influence of the warm alkaline conditions, reducing sugars undergo tautomerism, transitioning into enediols. These enediols are characterized by their potent reducing capabilities.
- Interaction with Cupric Ions: The Benedict reagent is fortified with copper sulfate (CuSO4), a source of cupric ions (Cu2+). Enediols, in their capacity as strong reducing agents, facilitate the reduction of these cupric ions to cuprous ions (Cu+). The chemical representation of this interaction is: CuSO4→Cu2++SO42−
- Role of Sodium Citrate: To circumvent the potential precipitation of cupric hydroxide, sodium citrate is incorporated into the Benedict solution. It acts as a chelating agent, forming a loosely bound cupric sodium citrate complex. This complex ensures a consistent release of cupric ions, preventing their precipitation.
- Formation of Red Precipitate: The cuprous ions, once formed, react with hydroxide ions to produce cuprous hydroxide. Upon heating, this compound decomposes to yield cuprous oxide (Cu2O), which is discernible as a bright red precipitate. The progression of reactions can be represented as: Cu++OH−→CuOH
CuOH→Cu2O+H2O (Upon heating) - Color Intensity as an Indicator: The resultant color of the reaction mixture serves as an indirect measure of the reducing sugar concentration in the sample. The hue can range from greenish (indicative of lower concentrations) to brick-red (signifying higher concentrations).
- Reagent Preparation: The Benedict reagent is prepared by dissolving sodium citrate and sodium carbonate in hot water, followed by the addition of a cupric sulfate pentahydrate solution. Constant stirring ensures a uniform mixture.
In summation, the Benedict’s Test is a meticulously designed chemical assay that leverages the redox properties of reducing sugars to detect their presence. The formation of a red precipitate serves as the definitive visual confirmation of this process, with its intensity offering insights into the sugar concentration.
Materials Required of Benedict’s Test
To conduct the Benedict’s Test, a systematic and precise procedure designed to detect the presence of reducing sugars, a specific set of materials and reagents are essential. The following list provides a comprehensive overview of the required materials:
- Sample Solution: This is the primary substance under investigation. It could be an unknown carbohydrate solution or, in clinical settings, a urine sample to detect glucose presence.
- Test Tubes: Essential glassware for holding the sample solution and reagents during the test. They should be clean and dry to ensure accuracy.
- Test-Tube Holders: These tools are used to securely hold the test tubes, especially when subjected to heating, ensuring safety during the procedure.
- Pipettes: Precision instruments used for transferring specific volumes of the sample solution and reagents to the test tubes.
- Bunsen Burner: A source of flame, essential for heating the test tubes during the procedure to facilitate the chemical reactions.
- Benedict’s Reagent: The primary reagent used in the test, it contains compounds like sodium carbonate, sodium citrate, and copper sulfate, which interact with reducing sugars to produce a color change.
- Water Bath: An equipment used to maintain the test tubes at a constant temperature, ensuring uniform heating and facilitating the desired chemical reactions.
- Positive Control (5% Glucose): A known reducing sugar solution, typically 5% glucose, is used as a positive control to validate the test’s efficacy and to compare the results of the unknown sample.
- Negative Control (Distilled Water): Distilled water serves as the negative control, ensuring that any observed reactions are solely due to the presence of reducing sugars and not any extraneous factors.
Benedict’s Reagent Preparation
Benedict’s Reagent, a fundamental component in the Benedict’s Test, is meticulously prepared to ensure its efficacy in detecting reducing sugars. The following steps provide a detailed procedure for the preparation of this reagent:
- Quantitative Measurement:
- Begin by accurately measuring the following chemicals:
- Copper sulfate (CuSO4): 17.3 grams
- Sodium citrate (Na3C6H5O7): 173 grams
- Anhydrous sodium carbonate (Na2CO3): 100 grams (Alternatively, one can use 270 grams of sodium carbonate decahydrate (Na2CO3.10H2O))
- Begin by accurately measuring the following chemicals:
- Volumetric Flask Utilization:
- Transfer the measured chemicals into a 1000 mL volumetric flask. This flask facilitates precise volume measurements, ensuring the correct concentration of the reagent.
- Addition of Distilled Water:
- Gradually add distilled water into the volumetric flask, ensuring the final volume reaches the 1000 mL mark. Distilled water is preferred due to its purity, eliminating potential contaminants that could interfere with the test.
- Dissolution of Components:
- Once all components are in the flask, gently shake the mixture to facilitate the dissolution of the chemicals. Ensure a homogenous solution is achieved, with all components fully dissolved.
Procedure of Benedict’s Test
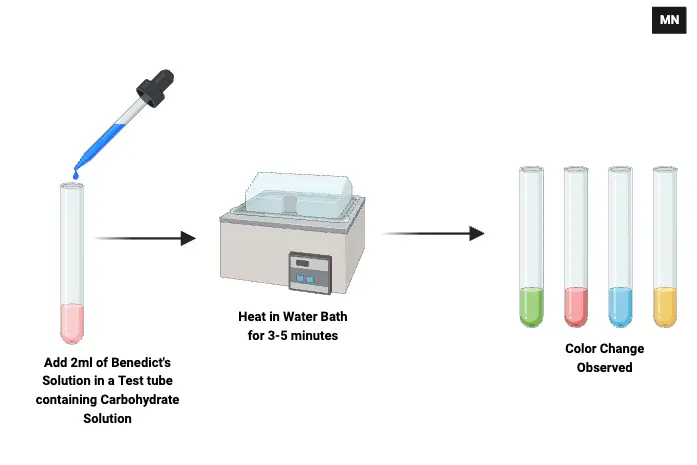
- Sample Preparation:
- Begin by transferring 1 mL of the sample solution, which could be a carbohydrate solution or urine, into a clean, dry test tube.
- As controls, take 1 mL of 5% glucose solution in one test tube and 1 mL of distilled water in another, both placed in separate dry test tubes.
- Reagent Addition:
- To each of the test tubes, including the controls, add 2 mL of Benedict’s reagent. Ensure uniform addition across all test tubes to maintain consistency.
- Heating:
- Position the test tubes in a water bath, ensuring they are securely placed.
- Heat the test tubes in the water bath for approximately 5 minutes. Alternatively, the test tubes can be directly heated over a flame for a duration of 3–5 minutes. Ensure consistent heating to facilitate the necessary chemical reactions.
- Observation:
- Post-heating, carefully observe any changes in the test tubes. The emergence of a brick-red colored precipitate is indicative of a positive result, signaling the presence of reducing sugars in the sample.
Observation and Results of Benedict’s Test
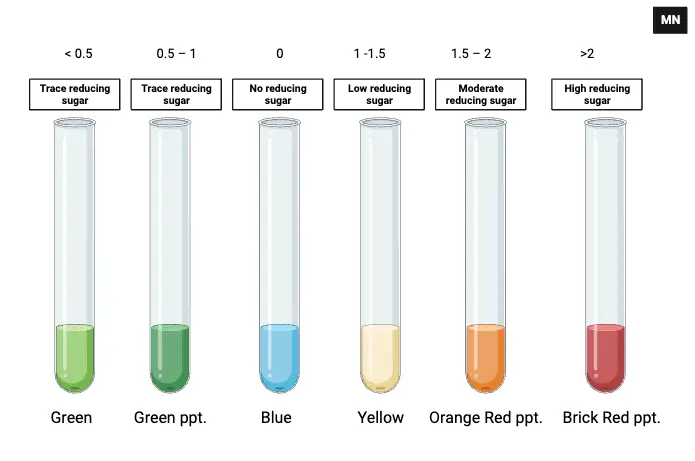
The Benedict’s Test, a diagnostic tool for the detection of reducing sugars, yields results that can be visually interpreted based on the color changes observed post-reaction. The following provides a comprehensive guide to interpreting these results:
- Color Changes:
- Blue: This color indicates a negative result, signifying the absence of reducing sugars in the sample.
- Green (Solution): A greenish hue in the solution suggests trace amounts of reducing sugar, with an approximate concentration of less than 0.5 g%.
- Green (Precipitate): The formation of a green precipitate indicates trace levels of reducing sugar, falling within the concentration range of 0.5 – 1 g%.
- Yellow Precipitate: A yellowish precipitate denotes a low concentration of reducing sugar, typically between 1 – 1.5 g%.
- Orange-Red Precipitate: An orange-red precipitate suggests a moderate concentration of reducing sugar, ranging from 1.5 – 2 g%.
- Brick-Red Precipitate: A pronounced brick-red precipitate is indicative of a high concentration of reducing sugar, exceeding 2 g%.
- Semiquantitative Evaluation:
- The observed color shades can be utilized for a semiquantitative assessment of the reducing sugar concentration in the sample. The intensity and shade of the color serve as proxies for the sugar concentration, allowing for a rough estimation.
- Reporting:
- Blue: Negative result, indicating no presence of reducing sugar.
- Green: Trace result, signifying a minimal amount of reducing sugar.
- Orange: Positive (+) result, indicating a moderate presence of reducing sugar.
- Brick Red: Strongly positive (++) result, denoting a substantial amount of reducing sugar.
In summation, the interpretation of the Benedict’s Test results hinges on the observed color changes. These changes, ranging from blue to brick-red, offer insights into the presence and approximate concentration of reducing sugars in the examined sample. Proper interpretation ensures accurate diagnostic outcomes and a deeper understanding of the sample’s carbohydrate composition.
| Shade of Color | Approx. Concentration of Reducing Sugar (in g%) | Indication |
|---|---|---|
| Blue | 0 | No reducing sugar |
| Green (Solution) | < 0.5 | Trace reducing sugar |
| Green (Precipitate) | 0.5 – 1 | Trace reducing sugar |
| Yellow Precipitate | 1 – 1.5 | Low reducing sugar |
| Orange-Red Precipitate | 1.5 – 2 | Moderate reducing sugar |
| Brick-Red Precipitate | >2 | High reducing sugar |
Advantages of Benedict’s Test
- Simplicity:
- The Benedict’s Test is characterized by its straightforward procedure. It requires minimal materials, making it easy to set up and execute. This simplicity ensures that it can be readily employed in various settings, from advanced laboratories to basic research environments.
- Time-Efficiency:
- One of the test’s salient features is its rapid turnaround time. The procedure, from sample preparation to result interpretation, can be completed in a relatively short duration, making it especially valuable in time-sensitive scenarios.
- Safety:
- The reagents used in the Benedict’s Test are non-toxic, ensuring the safety of the individuals conducting the test. This non-toxicity also reduces the environmental impact, as disposal concerns are minimized.
- Cost-Effectiveness:
- The test is economically advantageous, given the inexpensive nature of the required reagents and materials. This cost-effectiveness ensures its accessibility and widespread adoption, especially in settings with budgetary constraints.
- Versatility:
- The Benedict’s Test is not limited to a binary outcome. It offers both qualitative results, indicating the presence or absence of reducing sugars, and semi-quantitative results, providing an approximate gauge of the sugar concentration. This dual capability ensures a comprehensive understanding of the sample’s carbohydrate composition.
Limitation of Benedict’s Test
- Semiquantitative Nature:
- While the Benedict’s Test can indicate the presence of reducing sugars, it does not provide an exact quantification. Instead, it offers a semiquantitative estimation based on the intensity of the color change, which may not precisely reflect the sugar concentration.
- Interference from Other Chemicals:
- Certain chemicals present in urine, such as creatinine, ascorbic acid, and urate, can impede the Benedict’s reaction, potentially skewing the results.
- These chemicals may slow down the reaction, leading to delayed or muted color changes, which can be misinterpreted.
- Potential for False Positives:
- The test can yield false-positive results in the presence of specific substances. Drugs like penicillin, isoniazid, streptomycin, salicylates, and p-aminosalicylic acid can react with the Benedict reagent, producing color changes even in the absence of reducing sugars.
- Such false positives necessitate caution in result interpretation, especially when analyzing samples from individuals on medication.
- Requirement for Subsequent Analysis:
- The Benedict’s Test, being a preliminary assay, often requires follow-up tests for the definitive identification of the carbohydrate in question. This adds an additional step to the analytical process.
Applications/Uses of Benedict’s Test
- Biochemical Analysis:
- Detection of Unknown Carbohydrates: The test serves as a reliable tool for the identification of unknown carbohydrates in biochemical analyses. By discerning between reducing and non-reducing sugars, it offers insights into the carbohydrate composition of the sample.
- Analysis of Carbohydrate Extracts: In biochemistry, the test is instrumental in the analysis and identification of carbohydrate extracts, aiding researchers in understanding their structural and functional attributes.
- Clinical Diagnostics:
- Diagnosis of Diabetes Mellitus: The Benedict’s Test holds clinical significance as a rapid presumptive diagnostic tool for diabetes mellitus. By detecting glucose in urine samples, it provides preliminary insights into potential glucose metabolism disorders.
- Quality Control: In clinical settings, the test is employed for quality control purposes, specifically for the detection and quantification of simple sugars. This ensures the purity and integrity of samples and solutions used in various medical procedures.
- Economic and Time Efficiency:
- Cost-Effective: Owing to the straightforward preparation of the Benedict reagent, the test is cost-effective, making it accessible for widespread use.
- Rapid Results: The test is characterized by its quick turnaround time, yielding results in a short duration, which is especially valuable in time-sensitive scenarios.
- Versatility: The Benedict’s Test can be both qualitative, providing a binary outcome of presence or absence, and semi-quantitative, offering an approximate gauge of the reducing sugar concentration.
Precautions of Benedict’s Test
- Accurate Measurement:
- Precision in measurement is paramount. Ensure that all reagents and samples are measured accurately using appropriate instruments. Any deviation from the specified quantities can lead to skewed results.
- Controlled Heating:
- Avoid rapid heating of the mixture. Instead, opt for a gradual increase in temperature by placing the test tubes in a water bath. This controlled heating minimizes the risk of sudden reactions or splattering of the solution.
- Safe Handling of Test Tubes:
- Always employ a test-tube holder when heating the solution. This not only ensures a stable grip but also protects the hands from potential burns or injuries.
- Position the test tube in such a manner that its opening is not directed towards oneself or others. This orientation minimizes the risk of any accidental spillage or splatter reaching individuals.
- Repeated Heating:
- Before concluding a negative result, it is advisable to heat the solution at least three times. This ensures that any potential reactions have had ample opportunity to occur, thereby reducing the likelihood of false negatives.
- Safety First:
- Always prioritize safety. Wear appropriate protective gear, such as gloves and safety goggles, when conducting the test. This minimizes the risk of chemical exposure and potential injuries.


