Table of Contents
What is Bile Solubility Test?
- The Bile Solubility Test is a specialized biochemical assay employed primarily for the differentiation of Streptococcus pneumoniae from other alpha-hemolytic Streptococci. This test has garnered significance in the realm of microbiology due to the inherent challenges in distinguishing between S. pneumoniae and Streptococcus pseudopneumoniae.
- The foundational principle of the bile solubility test lies in the lysis or dissolution of bacterial cells when exposed to specific bile salts, such as sodium desoxycholate, under controlled conditions.
- Organisms that undergo lysis in the presence of these bile salts are deemed positive, while those that remain intact are categorized as negative. The underlying mechanism driving this lysis is believed to be the activation of autolytic enzymes, although the exact modus operandi remains a subject of scientific inquiry.
- Central to the bile solubility test is the role of autolytic enzymes, specifically amidases, present within the bacterial cells. These enzymes, when activated by bile salts, instigate autolysis by cleaving the bond between an alanine and muramic acid within the peptidoglycan structure.
- This enzymatic action results in the disintegration of the peptidoglycan backbone, leading to the dissolution of the bacterial cell. Notably, Streptococcus pneumoniae is uniquely sensitive to this process, rendering it “bile soluble,” whereas other alpha-hemolytic Streptococci remain resistant.
Definition of Bile Solubility Test
The bile solubility test is a biochemical assay used to differentiate Streptococcus pneumoniae from other alpha-hemolytic Streptococci based on the lysis of bacterial cells in the presence of specific bile salts.
Objectives of Bile Solubility Test
- Species Differentiation: One of the primary objectives of the bile solubility test is to discern Streptococcus pneumoniae from other alpha-hemolytic Streptococci. Given the close similarities among these bacterial species, this test provides a reliable method for accurate identification.
- Lysis Detection: The test is designed to ascertain the capability of a bacterial organism to undergo lysis when exposed to specific bile salts. This lysis, indicative of the organism’s sensitivity to bile, serves as a distinguishing feature for certain bacterial species, particularly S. pneumoniae.
In essence, the bile solubility test is a pivotal tool in microbiological diagnostics, facilitating precise differentiation and understanding of bacterial behavior in the presence of bile salts.
Principle of Bile Solubility Test
The bile solubility test is a diagnostic assay employed to distinguish Streptococcus pneumoniae from other alpha-hemolytic Streptococcus species. This differentiation is crucial given the clinical significance of S. pneumoniae and the close resemblance it shares with other streptococci.
The foundational principle of the bile solubility test revolves around the susceptibility of pneumococcal cells to lysis upon exposure to sodium desoxycholate, a type of bile salt. When introduced to the colony under specific conditions of time and temperature, S. pneumoniae undergoes lysis, while other streptococci remain intact. This lysis is attributed to the presence of an intracellular autolytic enzyme, specifically an amidase, within the pneumococcus. When cultivated on artificial media, this enzyme prompts the organism to undergo rapid autolysis.
Role of Bile Salts: Bile salts, such as sodium desoxycholate, play a pivotal role in the test. They are believed to modify the surface tension of the medium, leading to rearrangements in the cell membrane. While the exact mechanism remains a subject of scientific inquiry, prevailing hypotheses suggest that bile salts expedite the lysis of pneumococcal cells by activating the autolytic enzyme. This activation is a manifestation of the “Autolysis reaction,” which differentiates S. pneumoniae from other alpha-hemolytic streptococci, given its unique susceptibility to both bile solubility and Optochin.
Enzymatic Action: Central to the test’s mechanism is the Lyt-A enzyme, a cell-bound autolysin amidase. This enzyme becomes operative in the presence of surface-active reagents, such as bile salts. Upon exposure to sodium deoxycholate solution, either at 2% or 10% concentrations, there is a sequential degradation of the bacterial cell. Initially, the solution diminishes the surface tension between the medium and the cell membrane interface. Subsequently, the disruption of the Streptococcus pneumoniae cell ensues, leading to the activation of the intracellular autolytic enzyme. This enzyme cleaves the bond between an alanine and muramic acid within the peptidoglycan structure, culminating in the autolysis of the bacterial cell.
Microorganisms Tested
The bile solubility test is typically employed for:
- Alpha-hemolytic, catalase-negative, Gram-positive cocci in chains that exhibit a characteristic central depression or mucoid colony morphology, suggestive of S. pneumoniae.
- Gram-positive cocci in lancet-shaped pairs derived from positive blood cultures.
In essence, the bile solubility test offers a robust and reliable mechanism to differentiate S. pneumoniae from other closely related streptococci, leveraging the unique biochemical reactions of the organism in the presence of bile salts.
Requirements for Bile Solubility Test
To conduct the bile solubility test, a specific set of reagents and supplies are essential. Ensuring the availability and proper handling of these components is crucial for the accuracy and reliability of the test results.
Reagents:
- Bile Salts:
- Composition: The primary reagent required is a 10% bile salt solution, predominantly composed of sodium desoxycholate.
- Preparation: To prepare this solution, 10 grams of sodium desoxycholate should be dissolved in 100 ml of distilled water.
- Storage: Once prepared, the solution should be aliquoted in small volumes to reduce the risk of contamination. It is imperative to store the solution between 15 to 30°C. Storing the reagent at colder temperatures may lead to its thickening, which can compromise its efficacy.
- Shelf Life: Typically, the bile salt solution remains viable for approximately 270 days post-preparation.
- 0.85% NaCl: A sterilized solution of 0.85% sodium chloride is required.
- Broth Culture Medium: A suitable broth culture medium, such as Brain Heart Infusion (BHI) broth, is essential for cultivating the bacterial samples.
Supplies:
- Loops: Inoculation loops are necessary for transferring and streaking bacterial samples.
- Test Tubes or Slides: Depending on the method employed (direct or indirect), test tubes or slides are required to hold the bacterial samples and reagents during the test.
- Pipettes: These are essential for the accurate transfer and measurement of reagents and bacterial suspensions.
In summary, the bile solubility test necessitates a meticulous arrangement of specific reagents and supplies. Proper preparation, storage, and handling of these components are paramount to ensure the test’s precision and reproducibility.
Test Organism
- Any alpha-hemolytic, catalase-negative, Gram-positive cocci in chains with a flattened centre or a mucoid colony shape that looks like it could be S. pneumoniae.
- Any Gram-positive cocci in pairs that look like lancets from a successful blood culture. Grow the isolate(s) to be tested on a blood agar plate (BAP) for 18–24 hours at 35–37°C with less than 5% CO2 (or in a candle-jar).
Procedure of Bile Solubility Test
Do some preliminary tests, like a Gram stain and a catalase, to make sure that the test isolate is a Streptococcus.
1. Spot Test/Plate Method of Bile Solubility Test
The direct plate method is a straightforward technique employed in the bile solubility test, aiming to discern the susceptibility of bacterial colonies, notably Streptococcus pneumoniae, to bile salts directly on the agar plate.
Procedure:
- Reagent Application: Begin by placing a drop of 10% bile solubility reagent adjacent to a suspected colony that has been cultivated for 18 to 24 hours. Care should be taken to ensure the dropper tip does not come into contact with the agar surface.
- Colony Interaction: Gently roll the drop of bile reagent over several representative colonies by tilting the plate. This action should be executed with caution to prevent dislodging the colonies from the agar surface.
- Incubation: Once the bile reagent has been applied, the plate is positioned upright and incubated at a temperature range of 35°C to 37°C. The incubation period typically spans 15 to 30 minutes, or until the reagent drop has evaporated. To expedite the evaporation process, the plate lid can be left slightly ajar. Alternatively, a heat block can be employed in lieu of a conventional incubator.
- Observation: Post-incubation, the plate is scrutinized for any morphological changes in the colonies. The primary indicator of positive bile solubility is the disintegration or solubility of the colony. It is essential to differentiate between genuine colony disintegration and instances where the colony might merely float away due to the reagent.
The direct plate method offers a rapid and efficient means to assess the bile solubility of bacterial colonies directly on the culture medium. By observing the interaction of the colonies with the bile reagent, this method provides immediate insights into the bacterial species present. Proper execution, coupled with meticulous observation, is crucial to ensure the accuracy and reliability of the results derived from this method.
2. Test Tube Method for Bile Solubility Test
The test tube method is a widely employed technique in the bile solubility test, designed to ascertain the susceptibility of bacterial cells, specifically Streptococcus pneumoniae, to bile salts. This method is characterized by its indirect approach, utilizing test tubes for the incubation and observation of bacterial reactions to bile reagents.
Procedure:
- Preparation of Suspension: Begin by dispensing approximately 0.5 ml of sterile saline or an appropriate broth into a small test tube. Subsequently, a dense suspension of the organism is prepared in this saline, aiming for a consistency equivalent to the no.1 McFarland standard. This suspension is then agitated either manually or using a vortex mixer to ensure uniformity.
- Division of Suspension: The prepared suspension is apportioned equally into two test tubes. These tubes are distinctly labeled – one as “TEST” and the other as “CONTROL.”
- Addition of Reagents: Into both the “TEST” and “CONTROL” tubes, introduce two to five drops of the bile reagent. Following this, the contents of the tubes are gently mixed to ensure even distribution of the reagent.
- Incubation: The tubes are then incubated at a temperature of 35°C for a duration of three hours. During this period, the tubes are examined hourly for any signs of clearing. Alternatively, for a more detailed analysis, the contents of each tube can be periodically assessed using a Gram stain or methylene blue wet mount to detect cell lysis. This examination can be conducted at 15-minute intervals.
- Observation: Post incubation, the tubes are scrutinized for turbidity clearance. A clear solution in the “TEST” tube, as compared to the “CONTROL,” indicates positive bile solubility, suggesting the presence of Streptococcus pneumoniae.
The test tube method offers a systematic and reliable approach to differentiate Streptococcus pneumoniae from other alpha-hemolytic Streptococci. By observing the lysis or dissolution of bacterial cells in the presence of bile salts, this method provides valuable insights into the bacterial species under examination. Proper execution and interpretation of the test are paramount to ensure accurate results.
3. Direct slide blood culture test
The direct slide blood culture test is a method employed in the bile solubility test to directly assess the susceptibility of bacteria in blood culture broth to bile salts. This technique is particularly useful for rapid identification and differentiation of bacterial species.
Procedure:
- Sample Preparation: Begin by placing a drop of blood culture broth onto a clean, grease-free glass slide.
- Reagent Application: To the aforementioned drop, add one drop of bile reagent. Allow the mixture to air dry on the slide. This ensures that the bacterial cells from the blood culture interact with the bile reagent.
- Control Preparation: For comparison and to ensure the validity of the test, a control slide is prepared. On a separate glass slide, combine one drop of blood culture broth with one drop of water. Allow this mixture to dry. This control helps differentiate the specific effects of the bile reagent from any changes that might occur due to the drying process or other factors.
- Gram Staining: Once the slides (both test and control) are air-dried, they undergo the Gram staining procedure. This differential staining technique allows for the visualization of bacterial cells under a microscope.
- Microscopic Examination: Post-staining, the slides are examined under a microscope for the presence of cocci. The interaction of the cocci with the bile reagent, as evidenced by changes in their morphology or integrity, provides insights into their bile solubility.
The direct slide blood culture test offers a streamlined approach to assess the bile solubility of bacteria directly from blood culture broths. By juxtaposing the test slide with a control, this method ensures accurate differentiation and identification of bacterial species based on their reactions to bile salts. Proper execution and microscopic examination are paramount to derive reliable and conclusive results from this test.
Result interpretation of Bile Solubility Test

The bile solubility test is a diagnostic tool employed to differentiate Streptococcus pneumoniae from other alpha-hemolytic Streptococci. Proper interpretation of the results is crucial for accurate identification.
1. Test Tube Method:
- Positive Result: A clearing or loss of turbidity in the “TEST” tube, relative to the “CONTROL” tube, within a span of 3 hours indicates bile solubility. Microscopic observation may also reveal the lysis of cells, further confirming the positive result.
- Negative Result: Absence of clearing or persistent turbidity suggests bile insolubility.
2. Direct Slide Blood Culture Method:
- Positive Result: Complete lysis of all cocci in the smear, coupled with the presence of intact bacteria in the control smear, signifies bile solubility.
- Negative Result: If cocci remain intact, the organism is bile insoluble.
3. Direct Plate Method:
- Positive Result: Disintegration or flattening of the bacterial colony within 30 minutes, leaving behind an area of alpha-hemolysis where the colonies were previously situated, indicates bile solubility.
- Negative Result: The absence of any change in the colony’s integrity within 30 minutes suggests bile insolubility.
Reporting Results
- For alpha-hemolytic colonies derived from catalase-negative, lancet-shaped, Gram-positive cocci that demonstrate bile solubility (either through the spot or tube test), the organism can be definitively reported as Streptococcus pneumoniae.
- In cases where bile solubility is not demonstrated, the organism is likely a member of the viridans group streptococci. However, it’s noteworthy that a subset of bile-resistant organisms might still be S. pneumoniae. In such scenarios, further testing is recommended, especially from colonies that exhibit typical pneumococcal characteristics.
Interpreting the results of the bile solubility test with precision is paramount for accurate bacterial identification. Recognizing the distinct outcomes of the various methods ensures that Streptococcus pneumoniae is correctly differentiated from other closely related streptococci.
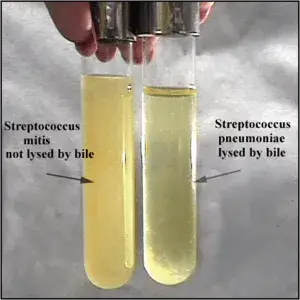
Quality Control
Quality control (QC) is an integral component of any diagnostic procedure, ensuring the accuracy, reliability, and consistency of test results. In the context of the bile solubility test, specific controls are employed to validate the efficacy of the test and the reagents used.
Control Organisms and Expected Results:
- Streptococcus pneumoniae:
- Incubation: Aerobic conditions for 24-48 hours at a temperature range of 33-37°C.
- Expected Outcome: Positive growth is observed. Upon the addition of the bile reagent, the colonies dissolve typically within approximately 30 minutes, indicating bile solubility.
- Streptococcus mutans:
- Incubation: Aerobic conditions for 24-48 hours at a temperature range of 33-37°C.
- Expected Outcome: Positive growth is observed. However, even after the addition of the bile reagent, the colonies remain intact for about 30 minutes, indicating bile insolubility.
- Enterococcus faecalis ATCC 29212: This organism serves as a negative control, demonstrating bile insolubility.
- Streptococcus mitis ATCC 49456: Another negative control, this organism also exhibits bile insolubility.
Reagent Quality Control:
- Before utilizing any new lot of sodium deoxycholate reagent, it is imperative to test it with known positive and negative controls. This ensures the reagent’s functionality and reliability.
- The bile reagent should be clear and exhibit a very light amber hue. If any deviations from this appearance are noted, the reagent should not be used.
Incorporating quality control measures in the bile solubility test is paramount to ensure the validity of the results. By using known control organisms and ensuring the quality of reagents, potential errors can be minimized, and the reliability of the test outcomes can be bolstered. Proper adherence to these QC protocols ensures that the test remains a robust tool in differentiating Streptococcus pneumoniae from other Streptococci.
| Streptococcus pneumoniae (TubeTest) | Positive |
| Streptococcus sanguinis (TubeTest) | Negative |
| Streptococcus pneumoniae (Spot Test) | Positive |
| Streptococcus sanguinis (Spot Test) | Negative |
Troubleshooting in Bile solubility test
In the realm of microbiological diagnostics, the bile solubility test is a pivotal tool for differentiating Streptococcus pneumoniae from other alpha-hemolytic streptococci. However, like any diagnostic procedure, it may present challenges that require troubleshooting. Here are some key considerations:
- Partial Solubility: One common challenge encountered in the bile solubility test is partial clearing or partial solubility. It’s crucial to understand that partial solubility should not be interpreted as a positive indication for the identification of pneumococci. Only complete solubility is indicative of S. pneumoniae.
- Optochin Sensitivity: Strains that exhibit partial solubility and have optochin inhibition zones measuring less than 14 mm should not be classified as pneumococci. Optochin sensitivity is a distinguishing feature of S. pneumoniae, and any deviation from the expected inhibition zone diameter can lead to misidentification.
- Mnemonic for Recall: To assist in remembering the key characteristics of Streptococcus pneumoniae, one can use the mnemonic: “Streptococcus pneumoniae is A BOSS.” This stands for:
- Alpha hemolytic
- Bile Soluble
- Optochin Sensitive
The bile solubility test, while a robust diagnostic tool, requires careful interpretation. Partial solubility and variations in optochin sensitivity can lead to misidentification if not properly addressed. Utilizing mnemonics and understanding the nuances of the test can aid in accurate identification and differentiation of Streptococcus pneumoniae from other streptococci. Proper troubleshooting ensures the reliability and accuracy of the test outcomes, reinforcing its value in microbiological diagnostics.
Precautions
The bile solubility test, while instrumental in differentiating Streptococcus pneumoniae from other alpha-hemolytic streptococci, necessitates meticulous execution to ensure accuracy. Here are the precautions to be observed:
- Reagent Concentration: While the 10% deoxycholate solution is commonly used, some protocols recommend a 2% concentration. If the 2% concentration is desired, the 10% solution should be appropriately diluted with sterile water to achieve this concentration.
- Plate Handling: After introducing the reagent to the plates, it’s essential to handle them with care. Avoid shaking or any undue movement. Some alpha-hemolytic colonies might not disintegrate during the plate technique but could detach and float, leading to potential misinterpretation.
- pH Sensitivity: Sodium deoxycholate can precipitate in acidic suspensions (with a pH of 6.5 or below), which can result in false-negative outcomes. Therefore, when conducting the tube test, it’s crucial to ensure that the pH remains above 6.8.
- Capsule Absence: Some studies, such as those by Downie, have indicated that the absence of a capsule in S. pneumoniae might influence its sensitivity to bile salt-induced lysis. This factor should be considered when interpreting results.
- Differentiation of Haemophilus Species: The bile solubility reagent can also be employed to differentiate between bile-soluble Haemophilus species (like H. influenzae and H. aegypticus) and bile-insoluble Haemophilus species. This underscores the versatility of the reagent but also necessitates caution to avoid cross-reactivity or misinterpretation.
The bile solubility test, while a robust tool in microbiological diagnostics, demands careful adherence to protocol and awareness of potential pitfalls. By observing the aforementioned precautions, one can ensure the reliability and accuracy of the test outcomes, facilitating precise bacterial identification and differentiation.
Limitations of Bile Solubility Test
The bile solubility test, while being a valuable diagnostic tool in microbiology, is not without its limitations. These constraints can influence the accuracy and reliability of the test results. The following are the notable limitations of the bile solubility test:
- Incomplete Lysis: Certain strains of S. pneumoniae may not undergo lysis in the presence of bile, potentially due to the loss of a virulence factor or capsule. Consequently, the absence of lysis does not necessarily rule out the presence of S. pneumoniae.
- Specificity: The bile solubility test is primarily designed to differentiate S. pneumoniae from other alpha-hemolytic streptococci. It is not universally applicable to all bacterial species.
- Reagent Concentration: A high concentration of bile salts can inhibit the normal autolysis of S. pneumoniae. Evaporation can lead to increased concentration of the reagent, potentially affecting test outcomes.
- Old Cultures: The test is unreliable with older cultures that have undergone autolysis.
- pH Sensitivity: When conducting the bile solubility tube test using saline or unbuffered broth, it’s crucial to ensure a neutral pH to prevent false-negative reactions.
- Plate Method Challenges: During the direct plate method, there’s a risk of dislodging the colony, potentially leading to false-positive results. Ambiguous results from the direct plate method may necessitate the use of alternative methods for clarity.
- Reagent Storage: Cold storage of the bile reagent can cause it to thicken, necessitating warming before use.
- Limited Scope: The bile solubility test primarily identifies the presence of Streptococcus pneumoniae among other alpha-hemolytic Streptococci. It does not provide a comprehensive bacterial profile.
- Optochin Susceptibility Test: In cases of incomplete or partial autolysis, the bile solubility test may suggest the presence of Streptococcus pneumoniae. However, confirmation often requires the “Optochin susceptibility test.”
- Spot Test Limitations: During the spot test, uneven plate positioning can cause the reagent to run, potentially washing away non-pneumococcal colonies and leading to false-negative results.
- Age of Colonies: Colonies older than 24 hours might have lost their active enzyme, which can result in false-negative outcomes.
- Incomplete Lysis: Only about 85% of pneumococcal strains will exhibit complete lysis in the bile solubility test. The remaining strains might not lyse due to various factors, necessitating further testing.
While the bile solubility test is an essential tool in microbiological diagnostics, understanding its limitations is crucial for accurate interpretation and subsequent clinical decision-making. Proper execution, coupled with awareness of these constraints, ensures that the test remains a reliable method for differentiating Streptococcus pneumoniae from other closely related streptococci.
Uses of Bile Solubility Test
The bile solubility test is a pivotal diagnostic tool in microbiology, designed to assess the susceptibility of bacterial organisms to bile salts. The primary uses of this test are as follows:
- Lysis Determination: The test is employed to ascertain the capability of a bacterial organism to undergo lysis when exposed to bile salts. Lysis, in this context, refers to the disintegration or dissolution of the bacterial cell membrane, leading to the release of its intracellular contents.
- Differentiation of S. pneumoniae: One of the primary applications of the bile solubility test is to differentiate and identify Streptococcus pneumoniae from other alpha-hemolytic Streptococci. Given the clinical significance of S. pneumoniae and its close resemblance to other Streptococci, this test provides a reliable method for its accurate identification.
- Qualitative Assessment: The bile solubility test serves as a qualitative assay, enabling the differentiation between bile-soluble and bile-insoluble organisms. This distinction is crucial in microbiological diagnostics, as it aids in the identification and understanding of specific bacterial species based on their biochemical reactions in the presence of bile salts.
The bile solubility test, with its specific applications, remains an indispensable tool in the realm of microbiological diagnostics. By evaluating the reactions of bacterial species to bile salts, this test offers valuable insights into their identity and characteristics, facilitating accurate diagnosis and subsequent clinical interventions.
Flashcard on Bile Solubility Test
Quiz Practice
What is the primary purpose of the bile solubility test?
a) To differentiate between Gram-positive and Gram-negative bacteria.
b) To determine antibiotic susceptibility.
c) To differentiate Streptococcus pneumoniae from other alpha-hemolytic streptococci.
d) To determine the metabolic activity of bacteria.
Which enzyme is activated in S. pneumoniae in the presence of bile salts leading to its lysis?
a) Catalase
b) Lysozyme
c) Amidase
d) Lipase
What happens to S. pneumoniae colonies in the presence of bile salts during the bile solubility test?
a) They turn pink.
b) They multiply rapidly.
c) They disintegrate or flatten.
d) They turn blue.
Which of the following is NOT a method for performing the bile solubility test?
a) Direct plate method
b) Test tube method
c) Direct slide blood culture method
d) Agar diffusion method
In which pH range can sodium deoxycholate form a precipitate, potentially leading to false-negative results in the bile solubility test?
a) Above 7.5
b) 6.5 and below
c) Between 6.8 and 7.2
d) Above 8.0
Which organism is used as a positive control in the bile solubility test?
a) Streptococcus mutans
b) Staphylococcus aureus
c) Streptococcus pyogenes
d) Streptococcus pneumoniae
What concentration of sodium deoxycholate solution is commonly used in the bile solubility test?
a) 1%
b) 2%
c) 5%
d) 10%
Which of the following is NOT a result interpretation in the bile solubility test?
a) Clearing or loss of turbidity
b) Disintegration or flattening of the colony
c) Color change to green
d) Lysis of cells when observed microscopically
The absence of which structure in S. pneumoniae might influence its sensitivity to bile salt-induced lysis?
a) Flagella
b) Pili
c) Capsule
d) Cell wall
Which mnemonic can be used to remember the key characteristics of Streptococcus pneumoniae?
a) Streptococcus pneumoniae is A KING
b) Streptococcus pneumoniae is A BOSS
c) Streptococcus pneumoniae is A STAR
d) Streptococcus pneumoniae is A HERO
FAQ
What is the primary purpose of the bile solubility test?
The bile solubility test is primarily used to differentiate Streptococcus pneumoniae from other alpha-hemolytic streptococci.
How does the bile solubility test work?
The test is based on the ability of S. pneumoniae to undergo lysis in the presence of bile salts, specifically sodium desoxycholate.
Is the bile solubility test specific for S. pneumoniae?
While the test is highly indicative of S. pneumoniae, some strains might not lyse in the presence of bile. Further confirmatory tests might be required in such cases.
How long does it take to get results from the bile solubility test?
The test typically takes a few hours, with results often observable within 15-30 minutes, depending on the method used.
What does a positive bile solubility test look like?
A positive test is indicated by the disintegration or flattening of the S. pneumoniae colony or a clearing/loss of turbidity in a test tube.
Can the bile solubility test be used for organisms other than streptococci?
While primarily designed for S. pneumoniae, the reagent can also differentiate between bile-soluble and bile-insoluble Haemophilus species.
Are there any limitations to the bile solubility test?
Yes, some strains of S. pneumoniae might not lyse in the presence of bile. Additionally, the test is not reliable with old cultures that have autolyzed, and the pH of the medium can affect results.
How is the bile solubility test different from the optochin susceptibility test?
While both tests are used to identify S. pneumoniae, the bile solubility test relies on the organism's lysis in the presence of bile salts, whereas the optochin susceptibility test determines the organism's sensitivity to the optochin antibiotic.
What precautions should be taken while performing the bile solubility test?
Care should be taken to ensure the correct concentration of the reagent, proper pH of the medium, and handling of the plates to avoid false results.
Can the bile solubility test be used for clinical diagnosis?
The bile solubility test is primarily a laboratory tool for bacterial identification. Clinical diagnosis should consider this test in conjunction with clinical symptoms, other diagnostic tests, and patient history.
