Table of Contents
What is Bismuth Sulphite Agar (BSA)?
- Bismuth Sulphite Agar (BSA) is a selective and differential medium used for the isolation and presumptive identification of Salmonella spp, with a specific focus on Salmonella Typhi. Salmonella bacteria are responsible for various diseases such as gastroenteritis, sepsis, and enteric fever. BSA is a modified version of the original Wilson and Blair Medium, specifically designed for the isolation of Salmonella bacteria.
- Salmonella infections in humans are primarily caused by the ingestion of contaminated food, water, or milk that has been exposed to human or animal excreta. Among the Enterobacteriaceae family, the Salmonellae group is taxonomically complex. Different clinical types of Salmonella infections include gastroenteritis, bacteremia or septicemia, enteric fever, and a carrier state.
- BSA is considered the most productive medium for the isolation and preliminary identification of Salmonella Typhi and other Salmonella bacteria. It is recommended by various associations for the isolation and identification of Salmonella Typhi and other Salmonella strains from pathological materials, sewage, water, food, and other products.
- On Bismuth Sulphite Agar, Salmonella Typhi, Salmonella Enteritidis, and Salmonella Typhimurium typically grow as black colonies with a surrounding metallic sheen. This appearance is due to the production of hydrogen sulphide and the reduction of sulphite to black ferric sulphide. Salmonella Paratyphi A, on the other hand, grows as light green colonies.
- It is important to note that BSA may inhibit some strains of Salmonella species, and therefore, it should not be used as the sole selective medium for these organisms. Additionally, BSA has unique inhibitory properties against gram-positive organisms and coliforms, making it necessary to use a larger inoculum compared to other selective media.
- The components of Bismuth Sulphite Agar include peptone and HM peptone B as sources of carbon, nitrogen, long-chain amino acids, vitamins, and growth factors. Dextrose serves as the carbon source, while disodium phosphate maintains osmotic equilibrium. The inclusion of bismuth sulphite indicator and brilliant green inhibits the growth of intestinal gram-positive and gram-negative bacteria. Ferrous sulphate aids in the detection of hydrogen sulphide production.
- Clinical samples can be directly inoculated onto Bismuth Sulphite Agar, while food samples may require pre-enrichment prior to inoculation.
- Overall, Bismuth Sulphite Agar is a valuable medium for the selective isolation and presumptive identification of Salmonella spp, particularly Salmonella Typhi. It allows for the differentiation of lactose-fermenting salmonellae and is widely used in clinical, food, and environmental settings to detect and identify these pathogenic bacteria.
Principle of Bismuth Sulphite Agar (BSA)
The principle of Bismuth Sulphite Agar (BSA) revolves around its selective and differential properties that allow for the growth and presumptive identification of Salmonella spp, particularly Salmonella Typhi.
The components of BSA contribute to its functionality:
- Peptone and HM Peptone B: These serve as sources of carbon, nitrogen, long-chain amino acids, vitamins, and essential growth factors, providing the necessary nutrients for bacterial growth.
- Dextrose: Dextrose acts as the carbon source, supporting the metabolic needs of the bacteria.
- Disodium phosphate: Disodium phosphate helps maintain osmotic equilibrium in the medium, ensuring optimal growth conditions.
- Bismuth sulfite indicator and brilliant green: These substances work together to selectively inhibit the growth of gram-positive and gram-negative bacteria commonly found in the intestinal flora, thereby facilitating the isolation of Salmonella spp.
- Ferrous sulfate: Ferrous sulfate is incorporated into the medium to aid in the detection of hydrogen sulfide (H2S) production by Salmonella. When Salmonella bacteria produce H2S, it reacts with ferrous sulfate to form black ferric sulfide, resulting in the characteristic black colonies observed on BSA.
Based on their specific characteristics, Salmonella Typhi, Salmonella Enteritidis, and Salmonella Typhimurium typically display black colonies with a surrounding metallic sheen on BSA. This appearance is attributed to their ability to produce hydrogen sulfide and reduce sulfite to black ferric sulfide. In contrast, Salmonella Paratyphi A grows as light green colonies, allowing for its differentiation from other Salmonella species.
Clinical samples can be directly inoculated onto Bismuth Sulphite Agar for isolation and presumptive identification of Salmonella. However, when dealing with food samples, pre-enrichment of the sample is often performed prior to inoculation onto BSA to increase the chances of detecting low levels of Salmonella contamination.
Overall, the principle of Bismuth Sulphite Agar lies in its ability to selectively inhibit gram-positive and gram-negative bacteria, promote the growth of Salmonella spp, and allow for their presumptive identification based on characteristic colony appearance.
Composition of Bismuth Sulphite Agar (BSA)
| Ingredients | Gms/liter |
| Peptone | 10.000 |
| HM Peptone B # | 5.000 |
| Dextrose (Glucose) | 5.000 |
| Disodium phosphate | 4.000 |
| Ferrous sulfate | 0.300 |
| Bismuth sulfite indicator | 8.000 |
| Brilliant green | 0.025 |
| Agar | 20.000 |
Final pH: 7.7±0.2
Preparation of Bismuth Sulphite Agar (BSA)
The preparation and method of use of Bismuth Sulphite Agar (BSA) are as follows:
- Suspend 52.33 grams of Bismuth Sulphite Agar in 1000 ml of distilled water.
- Heat the mixture to boiling in order to dissolve the medium completely.
- It is important not to sterilize BSA in an autoclave or by fractional sterilization, as overheating can destroy the selectivity of the medium.
- Mix the medium well to disperse the suspension and pour thick plates, with approximately 25 ml of medium per plate. Ensure that the dishes are left uncovered during the solidification process.
- Prior to use, the plates should be dried, but be careful not to over-dry them.
- Correctly prepared plates will have a smooth, cream-like opacity with a pale straw color. There should be no sedimentation of the indicator.
- Inoculate the Bismuth Sulphite Agar plates with the specimen of interest.
- Incubate the inoculated plates for 48 hours at a temperature of 35°C.
- After 24 hours of incubation, examine the plates for the presence of typical colonies. If the plates show little or no growth after 48 hours, continue the incubation for an additional 18-24 hours.
- Note that the sensitivity of the medium largely depends on the uniform dispersion of precipitated bismuth sulfite in the final gel. Therefore, ensure that the dispersion is thorough before pouring the medium into sterile Petri plates.
Following these steps will help ensure the proper preparation and utilization of Bismuth Sulphite Agar for the isolation and presumptive identification of Salmonella species and other relevant microorganisms.
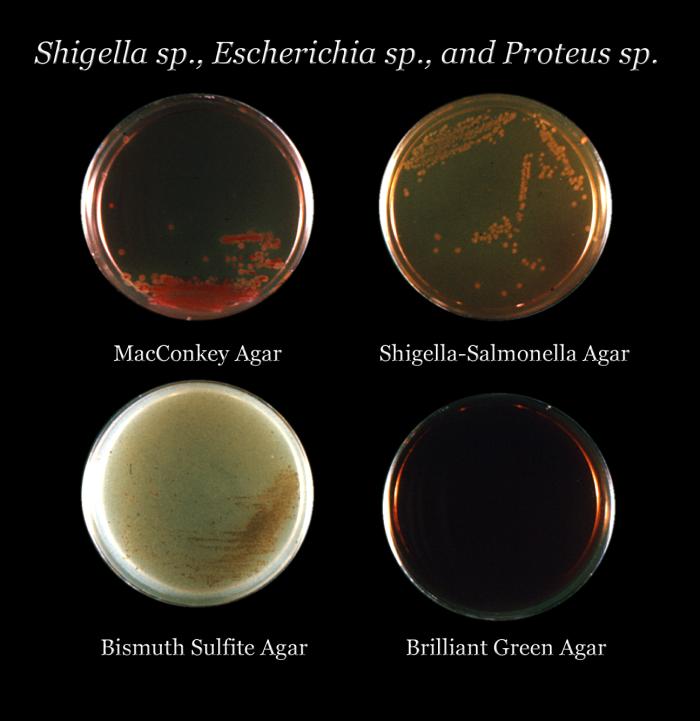
Result and Colony Characterisitcs of Different Organisms on Bismuth Sulphite Agar (BSA)
The interpretation of results on Bismuth Sulphite Agar (BSA) is as follows:
- Enterobacter aerogenes: This organism typically shows poor growth on BSA, and the colonies may appear brown-green. The color of the colonies can vary depending on the density of the inoculum.
- Enterococcus faecalis: BSA inhibits the growth of Enterococcus faecalis, so there will be no visible growth of this organism on the agar.
- Escherichia coli: Similar to Enterobacter aerogenes, Escherichia coli tends to display poor growth on BSA, and the colonies may appear brown-green. The color can vary based on the inoculum density.
- Salmonella Enteritidis: Salmonella Enteritidis shows good-luxuriant growth on BSA. The colonies will be black with a metallic sheen, resulting from the production of hydrogen sulfide (H2S) and reduction of sulfite to black ferric sulfide.
- Salmonella Typhi: Salmonella Typhi also exhibits good-luxuriant growth on BSA. The colonies will be black with a metallic sheen, similar to Salmonella Enteritidis.
- Salmonella Typhimurium: Salmonella Typhimurium, like the other Salmonella species, displays good-luxuriant growth on BSA. The colonies will be black with a metallic sheen.
- Salmonella Abony: Salmonella Abony, another member of the Salmonella genus, shows good-luxuriant growth on BSA. The colonies will be black with a metallic sheen.
The characteristic black colonies with a metallic sheen observed for Salmonella species on BSA are indicative of their ability to produce hydrogen sulfide and reduce sulfite, which are key reactions used for the presumptive identification of Salmonella.
Interpreting the growth and colony appearance on BSA helps in the presumptive identification of Salmonella spp, especially Salmonella Enteritidis, Salmonella Typhi, Salmonella Typhimurium, and Salmonella Abony. However, it is important to note that further confirmatory tests, such as biochemical or serological assays, are required for definitive identification of the isolated colonies.
| Organisms | Growth |
| Enterobacter aerogenes | Poor growth; Brown-green (depends on the inoculum density) |
| Enterococcus faecalis | Inhibited |
| Escherichia coli | Poor growth; Brown-green (depends on the inoculum density) |
| Salmonella Enteritidis | Good-luxuriant growth; Black with a metallic sheen |
| Salmonella Typhi | Good-luxuriant growth; Black with a metallic sheen |
| Salmonella Typhimurium | Good-luxuriant growth; Black with a metallic sheen |
| Salmonella Abony | Good-luxuriant growth; Black with a metallic sheen |
Quality Control of Bismuth Sulphite Agar (BSA)
The quality control of Bismuth Sulphite Agar (BSA) involves several parameters to ensure its effectiveness and reliability. Here are the key aspects of quality control for BSA:
- Appearance: BSA should have a light yellow to greenish yellow homogeneous free-flowing powder appearance.
- Gelling: The gelling property of BSA should be firm and comparable to a 2.0% agar gel.
- Colour and Clarity of prepared medium: After preparation, BSA should exhibit a greenish yellow color and appear opalescent with a flocculent precipitate formation in Petri plates.
- Reaction: The reaction of a 5.23% w/v aqueous solution of BSA at 25°C should have a pH of 7.7±0.2.
- pH: The pH range for BSA is between 7.50 and 7.90.
- Cultural Response: The cultural characteristics of BSA should be observed after incubation at 35-37°C for 40-48 hours.
Cultural response is assessed using specific organisms:
- Klesiella aerogenes ATCC 13048: Growth should be none to poor, with a colony color of brown-green, depending on the inoculum density.
- Enterococcus faecalis ATCC 29212: BSA should inhibit the growth of this organism, resulting in 0% recovery.
- Escherichia coli ATCC: Growth should be none to poor, with a colony color of brown-green, depending on the inoculum density.
- Salmonella Enteritidis ATCC 13076: There should be good-luxuriant growth of black colonies with a metallic sheen.
- Salmonella Typhi ATCC 6539: There should also be good-luxuriant growth of black colonies with a metallic sheen.
These cultural responses of specific organisms serve as indicators for the quality control of BSA, ensuring that it performs as expected and facilitates the selective isolation and identification of Salmonella species.
Uses of Bismuth Sulphite Agar (BSA)
Bismuth Sulphite Agar (BSA) has several important uses in microbiology, primarily for the selective isolation and preliminary identification of Salmonella Typhi and other Salmonellae. Here are the key uses of Bismuth Sulphite Agar:
- Isolation of Salmonella Typhi and other Salmonellae: BSA is particularly recommended for the selective isolation of Salmonella Typhi, the causative agent of typhoid fever, as well as other Salmonella species. It provides a suitable environment for the growth of Salmonella while inhibiting the growth of other bacteria, facilitating their isolation from various sources.
- Pathological materials: BSA is used to culture and isolate Salmonella from pathological specimens, such as clinical samples from patients suspected of having Salmonella infections. It helps in identifying the presence of Salmonella Typhi or other Salmonella strains in these materials.
- Sewage and water supplies: BSA is utilized in the analysis of sewage and water supplies for the presence of Salmonella bacteria. It aids in detecting the contamination of water sources by Salmonella, which is crucial for ensuring public health and preventing waterborne infections.
- Food testing: BSA is valuable in the examination of food samples for the presence of Salmonella contamination. It is employed to isolate and identify Salmonella Typhi and other Salmonella strains from various food products, including raw and processed foods, helping to monitor food safety and prevent foodborne outbreaks.
- Environmental testing: BSA is used in environmental microbiology for the detection of Salmonella in diverse environmental samples. This includes samples taken from animal and bird habitats, farms, and other settings where Salmonella contamination may occur. The selective and differential properties of BSA aid in the isolation and preliminary identification of Salmonella.
Overall, Bismuth Sulphite Agar is a valuable tool in the laboratory for the selective isolation and preliminary identification of Salmonella Typhi and other Salmonellae from different sources, including pathological materials, sewage, water supplies, and food. Its use helps in detecting and monitoring the presence of these pathogenic bacteria, enabling appropriate control measures and ensuring public health safety.
Limitations of Bismuth Sulphite Agar (BSA)
Bismuth Sulphite Agar (BSA) has several limitations that should be considered when using this medium. Here are the key limitations of BSA:
- Inhibitory to some Salmonella strains: BSA may inhibit the growth of certain strains of Salmonella species. Therefore, it should not be relied upon as the sole selective medium for the isolation of these organisms. Supplementing with other selective media may be necessary to ensure comprehensive detection.
- Requires larger inoculum: BSA tends to require a larger inoculum compared to other selective media. This is because the medium has a unique inhibitory action against gram-positive organisms and coliforms. A higher bacterial load may be needed for successful growth and detection of Salmonella on BSA.
- Variation in colony appearance: While typical Salmonella colonies on BSA display a black color with a metallic sheen, this appearance may vary with different Salmonella species. Near heavy inoculation, black colonies with a metallic sheen may be observed. However, isolated colonies of certain Salmonella species can show green colonies instead.
- Inhibition of Shigella species: BSA predominantly inhibits the growth of Shigella species, with the exceptions being Shigella flexneri and Shigella sonnei. Therefore, BSA may not be suitable for the selective isolation of all Shigella species.
- Inhibition of certain Salmonella species: BSA can also inhibit the growth of specific Salmonella species, such as Salmonella Sendai, Salmonella Berta, Salmonella Gallinarum, and Salmonella Abortus-equi. This limitation should be considered when using BSA for the detection and identification of Salmonella.
It is important to be aware of these limitations when working with Bismuth Sulphite Agar. Using alternative or complementary selective media, as well as performing additional confirmatory tests, can help overcome these limitations and ensure accurate detection and identification of Salmonella and other relevant pathogens.
FAQ
What is Bismuth Sulphite Agar (BSA)?
Bismuth Sulphite Agar (BSA) is a selective and differential medium used for the isolation and preliminary identification of Salmonella Typhi and other Salmonella species from various sources.
What is the composition of Bismuth Sulphite Agar?
BSA contains peptone, HM peptone B, dextrose, disodium phosphate, bismuth sulfite indicator, brilliant green, and ferrous sulfate.
What is the principle of Bismuth Sulphite Agar?
The principle of BSA involves the inhibition of gram-positive organisms and coliforms while allowing the selective growth of Salmonella species. It also facilitates the detection of hydrogen sulfide production, which results in the formation of black colonies with a metallic sheen.
What are the uses of Bismuth Sulphite Agar?
BSA is used for the selective isolation and preliminary identification of Salmonella Typhi and other Salmonella species from pathological materials, sewage, water supplies, and food samples.
Can Bismuth Sulphite Agar inhibit the growth of some Salmonella strains?
Yes, BSA may inhibit the growth of certain Salmonella strains. Therefore, it is not recommended as the sole selective medium for these organisms.
How does Bismuth Sulphite Agar differentiate between Salmonella species?
BSA allows for the differentiation of Salmonella species based on colony appearance. Salmonella Enteritidis, Salmonella Typhi, Salmonella Typhimurium, and Salmonella Abony typically exhibit black colonies with a metallic sheen, while Salmonella Paratyphi A grows as light green colonies.
Does Bismuth Sulphite Agar inhibit the growth of Shigella species?
Yes, BSA generally inhibits the growth of most Shigella species, except for Shigella flexneri and Shigella sonnei.
Are there any limitations of Bismuth Sulphite Agar?
Yes, BSA may inhibit certain Salmonella and Shigella species. It also requires a larger inoculum compared to other selective media and may show variation in colony appearance depending on the species and inoculum density.
How should Bismuth Sulphite Agar be prepared?
To prepare BSA, the medium is suspended in distilled water, heated to dissolve completely, and poured into plates. Autoclaving or fractional sterilization should be avoided.
How long should Bismuth Sulphite Agar be incubated for optimal results?
Inoculated BSA plates should be incubated for 48 hours at a temperature of 35°C. If little or no growth is observed after 48 hours, an additional 18-24 hours of incubation may be required before considering the plates as negative.
References
- https://exodocientifica.com.br/_technical-data/M027.pdf
- https://microbeonline.com/bismuth-sulfite-agar-composition-preparation-uses-and-colony-morphology/
- https://en.wikipedia.org/wiki/Bismuth_sulfite_agar
- https://www.neogen.com/categories/microbiology/bismuth-sulfite-agar/
- https://www.thermofisher.com/order/catalog/product/CM0201B
- https://www.dalynn.com/dyn/ck_assets/files/tech/PB68.pdf
- https://us.vwr.com/assetsvc/asset/en_US/id/8041218/contents
- https://legacy.bd.com/europe/regulatory/Assets/IFU/Difco_BBL/273300.pdf
- http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0201&c=UK&lang=EN
