Table of Contents
What is chromatography?
- Chromatography is a biophysical technique used for the separation, identification, and purification of the components within a mixture for qualitative and quantitative analysis. It is a powerful tool utilized across various scientific disciplines and often serves as the sole method for separating complex mixtures.
- The term “chromatography” was coined by the Russian botanist Mikhail Tswett in 1906. However, it wasn’t until 1952 that James and Martin described the first analytical use of chromatography, specifically gas chromatography for analyzing fatty acid mixtures.
- Different chromatographic procedures take advantage of various factors such as size differences, binding affinities, charges, and other properties to achieve material separation. In chemical analysis, chromatography involves dissolving the mixture in a fluid solvent, which can be either a gas or a liquid, referred to as the mobile phase. This mobile phase carries the mixture through a system such as a column, capillary tube, plate, or sheet, where a material called the stationary phase is fixed.
- The stationary phase possesses surface sites with which the constituents of the mixture interact differently, leading to variations in their affinities for the stationary phase. Consequently, the constituents travel at different apparent velocities within the mobile fluid, resulting in their separation. The separation is based on the differential partitioning between the mobile and stationary phases. Even subtle differences in a compound’s partition coefficient influence its retention on the stationary phase, thereby impacting the separation process.
- Chromatography can be categorized as preparative or analytical. Preparative chromatography aims to separate mixture components for subsequent use, serving as a purification method. However, due to the production involved, preparative chromatography is associated with higher costs. On the other hand, analytical chromatography is typically performed with smaller sample amounts and is utilized to determine the presence or measure the relative proportions of analytes within a mixture. It is important to note that these two types are not mutually exclusive, and both have their specific applications in scientific research and analysis.
Chromatography Definition
Chromatography is a laboratory technique used to separate, identify, and purify the components of a mixture based on their differential affinities for a stationary phase and a mobile phase.
Chromatography Overview
“Chromatography” is an analytical method that is used to separate the chemical mixture into its constituents in order that the distinct elements can then be fully examined. There are a variety of chromatography e.g. gas chromatography, liquid Ion Exchange Chromatography as well as affinity chromatography. However, all of them employ the same fundamental principles.
Chromatography is an separation method which every organic chemist as well as biochemist is aware of. Imyself, as an organic chemist, have regularly performed chromatographic separations of various mixtures of chemicals in the lab. In actuality I was looking through some of the research slide when I discovered a picture of a real separation chromatographic that I performed within the laboratory. It seems like that image could make a great start base for this post!
Let me first describe what I was trying accomplish here. I had two reactionants ‘A” and “B”. I allowed them to react with each with respect to certain conditions of reaction, to create a product called known as ‘C’. Once the reaction was complete I had the reaction mixture which contained unreacted A, unreacted C and my desired C. The next step was to isolate A C, B and A in order to identify and analyze pure C.
In the beginning, as illustrated in the left section, I used the thin layer analysis (TLC) plate. It is essentially an rectangular glass, which is which is coated in a fine layer silica. I placed a tiny amount of the reaction mix just above the surface of the dish (denoted by an elongated line) and then placed the plate into a jar that was filled with an organic solvent that was suitable (in this instance, a the 1:1 volume-by-volume mixture of hexane and ethyl acetate was utilized) and only enough volume to cover into the bottom of the plate. Then, by capillary force, the solution began ascending up the silica plate and as you can see, the reaction mixture was separated into three distinct spots. shades by the time it been able to reach the mark on the front of the solvent.
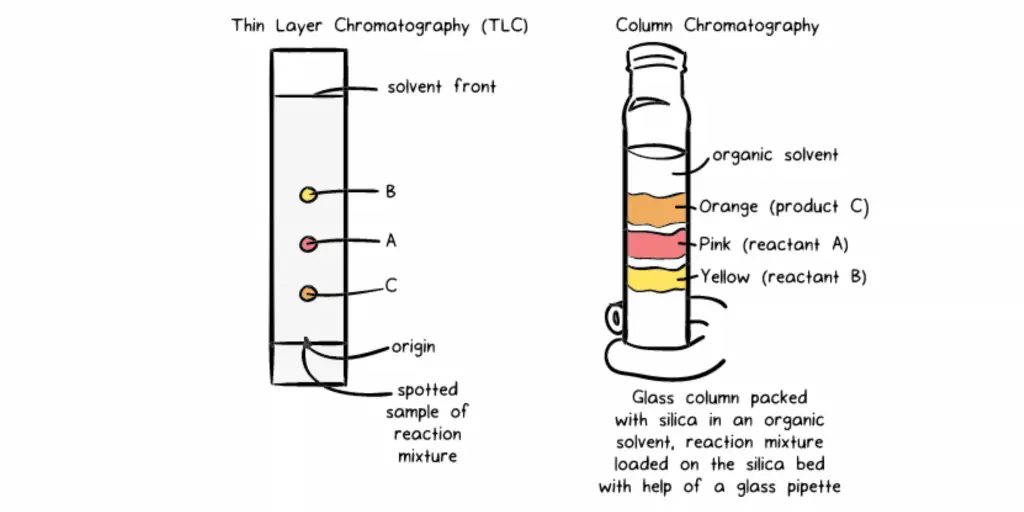
Then, to perform separation I put together the glass column (as as shown on the right side of the image). I made a glass-based column with an attached stopcock at the bottom, then inserted an elastomer plug at the base of the column and filled it with a mixture made of silica gel (prepared with the presence of an organic solvent). After the column was filled and the volume of solvent above the bed was reduced by less than five millimeters at a time, I carefully poured reaction mixture onto the silica-filled bed on the upper part of the column using the glass pipette. I shut the stopcock then let the liquid flow slow through the column. I continuously added solution from top to bottom of column. As you can see, the reaction mix began to split into 3 distinct band: orange, pink and yellow which correspond to unreacted B unreacted A, and C, which is the product I wanted in turn. I separated the individual bands into separate flasks and was capable of obtaining pure C!
Principle of Chromatography – How does chromatography work
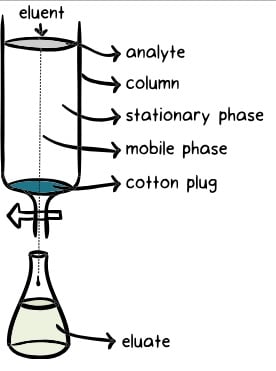
- The principle of chromatography involves the separation of components within a mixture based on their interactions with a stationary phase and a mobile phase. In chromatography, the analyte, or the mixture being analyzed, is combined with a liquid or gaseous mobile phase, which is pumped through a stationary phase.
- The stationary phase and mobile phase are typically designed to have contrasting properties, with one being hydrophilic (having an affinity for water) and the other being lipophilic (having an affinity for nonpolar substances). The components of the analyte interact differently with these phases based on their polarity. Components with higher polarity spend more time interacting with the stationary phase and are thus more retarded, while components with lower polarity interact less and are less retarded.
- As the mobile phase carries the analyte through the stationary phase, the different components of the mixture separate from each other. Each component elutes, or exits, the stationary phase at a specific time called the retention time. The separation is based on the varying interactions and affinities of the components with the stationary and mobile phases.
- During the chromatographic process, the eluted components pass through a detector where their signals are recorded. These signals are then plotted to create a chromatogram, which provides a visual representation of the separation and helps in identifying and quantifying the different components present in the sample.
- The separation in chromatography is influenced by factors such as adsorption (liquid-solid interaction), partition (liquid-liquid interaction), and differences in molecular weights. These factors determine the specific interactions between the stationary phase, mobile phase, and the substances in the mixture, leading to the separation of molecules from each other.
- In summary, chromatography works by exploiting the differential interactions and affinities of components with a stationary phase and a mobile phase. This separation technique relies on the principles of adsorption, partition, and molecular characteristics to separate and analyze the components of a mixture.
Types of Chromatography
Commonly used chromatography techniques are
- Column chromatography
- Ion-exchange chromatography
- Gel-permeation chromatography
- Affinity chromatography
- Paper chromatography
- Thin-layer chromatography
- Gas chromatography (GS)
- Dye-ligand chromatography
- Hydrophobic interaction chromatography
- Pseudoaffinity chromatography
- High-pressure liquid chromatography (HPLC)
Chromatography terms
Chromatography involves various terms and concepts that are important to understand in order to grasp the principles and techniques of this separation method. Here are some key chromatography terms:
| Term | Definition |
|---|---|
| Analyte | The substance to be separated during chromatography. |
| Analytical chromatography | The use of chromatography to determine the existence and concentration of analytes in a sample. |
| Bonded phase | A stationary phase covalently bonded to support particles or the inner wall of the column tubing. |
| Chromatogram | The visual output of a chromatograph, showing peaks or patterns corresponding to different components of the mixture. |
| Chromatograph | An instrument that enables chromatographic separation, such as gas chromatography or liquid chromatography. |
| Chromatography | A physical method of separation that distributes components between a stationary phase and a mobile phase. |
| Eluent | The solvent or solvent mixture used in elution chromatography, synonymous with the mobile phase. |
| Eluate | The mixture of solute and solvent exiting the column in elution chromatography. |
| Effluent | The stream flowing out of a chromatographic column, often used interchangeably with eluate. |
| Eluite | A more precise term for solute or analyte, referring to a sample component leaving the chromatographic column. |
| Eluotropic series | A list of solvents ranked by their eluting power in chromatography. |
| Immobilized phase | A stationary phase fixed on support particles or the inner wall of the column tubing. |
| Mobile phase | The phase that moves through the chromatography system, carrying the sample being separated. |
| Preparative chromatography | The use of chromatography to purify larger quantities of a substance for further use, rather than for analysis. |
| Retention time | The time taken for a specific analyte to pass through the system, from the column inlet to the detector, under set conditions. |
| Sample | The matter being analyzed in chromatography, which can be a single component or a mixture of components. |
| Solute | The sample components in partition chromatography. |
| Solvent | A substance capable of dissolving another substance; in chromatography, it refers to the liquid mobile phase. |
| Stationary phase | The substance fixed in place for the chromatography procedure, such as the silica layer in thin-layer chromatography. |
| Detector | The instrument used for qualitative and quantitative detection of analytes after separation. |
This table provides a concise overview of the key terms related to chromatography.
These terms and concepts provide a foundation for understanding and discussing the principles, techniques, and results of chromatography.
Column chromatography
- Column chromatography is a widely used technique for the purification of biomolecules, particularly proteins, based on their characteristic features such as size, shape, net charge, stationary phase used, and binding capacity. It falls under the category of adsorption chromatography, where the separation is achieved through the differential adsorption of components onto a stationary phase.
- In column chromatography, a column is packed with a stationary phase material, often a solid support such as silica or resin beads. The sample to be separated is applied to the top of the column, followed by the addition of a mobile phase or wash buffer. The mobile phase, which can be a liquid, flows through the column, carrying the components of the sample along with it.
- As the mobile phase moves through the column material, the different components of the sample interact differently with the stationary phase. Components that have stronger interactions with the stationary phase will bind more tightly and elute from the column at a slower rate, while components with weaker interactions will elute more quickly.
- The samples progress through the column material and accumulate at the bottom of the column in a time- and volume-dependent manner. This accumulation allows for the separation and purification of the desired biomolecules based on their specific characteristics.
- The choice of stationary phase material and mobile phase composition depends on the properties of the biomolecules being purified. The stationary phase material provides a surface for adsorption, while the mobile phase acts as a carrier for the sample components.
- Column chromatography is a versatile technique that can be applied to various types of biomolecules and is particularly valuable for large-scale purification in biotechnology and pharmaceutical industries. It allows for the isolation of specific biomolecules from complex mixtures and plays a crucial role in the production of pure and high-quality biomolecular samples.
- Overall, column chromatography, as a form of adsorption chromatography, is a powerful tool for the purification and separation of biomolecules based on their unique characteristics, contributing to advancements in various scientific and industrial fields.
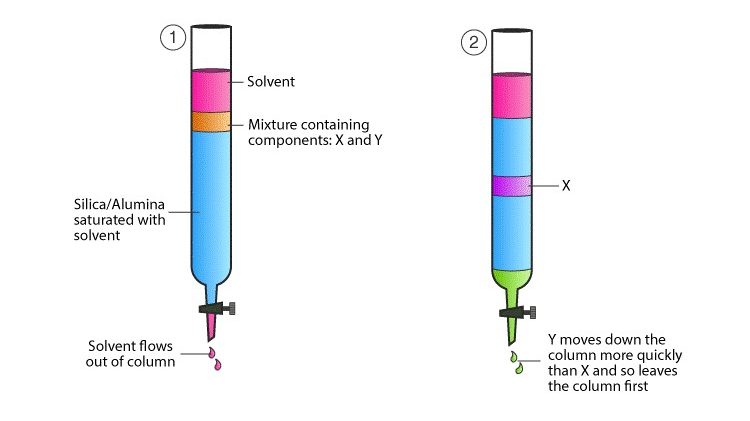
Partition chromatography
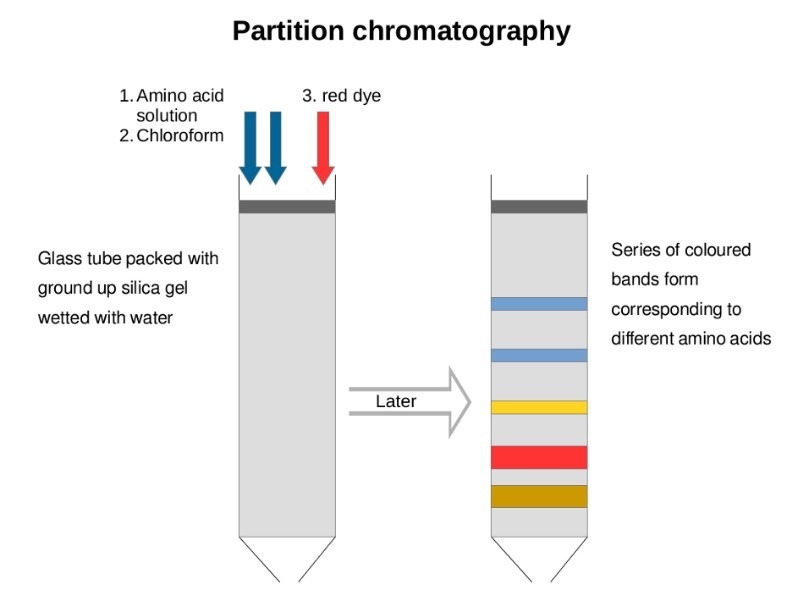
In this kind of chromatography, the constituents in the mix are separated in two phases because of variations in the distribution coefficient (Kd) that is the ratio of the concentration of solutes between two distinct phases. The distribution of solutes in both phases are dependent on the differences in solubility. In partition chromatography, there is a stationary while the mobile phase can be gas or liquid.

Gel- permeation (molecular sieve) chromatography
- Gel-permeation chromatography, also known as molecular sieve chromatography, is a method used to separate macromolecules based on their differences in molecular sizes. It is commonly employed to determine the molecular weights of proteins and to reduce salt concentrations in protein solutions.
- In gel-permeation chromatography, a column is packed with a stationary phase composed of inert molecules that have small pores. The solution containing molecules of varying sizes is continuously passed through the column at a constant flow rate. The larger molecules, which are too big to enter the pores, become trapped and retained between the particles within a restricted area. These molecules move more slowly through the column due to their size.
- On the other hand, smaller molecules can diffuse into the pores of the stationary phase material. As the size of the molecules decreases, they can penetrate deeper into the porous structure. Consequently, smaller molecules have shorter retention times and pass through the column more rapidly.
- The most commonly used column material for gel-permeation chromatography is Sephadex G, which is composed of dextran. Additionally, materials such as agarose and polyacrylamide can also be utilized as column materials.
- Gel-permeation chromatography provides a valuable technique for determining the molecular weights of proteins and separating macromolecules based on their size differences. It allows for the characterization and purification of biomolecules in various research fields, including biochemistry, biotechnology, and pharmaceutical sciences.
- In summary, gel-permeation chromatography employs a stationary phase with small pores to separate macromolecules based on their molecular sizes. Larger molecules are retained within the column, while smaller molecules can penetrate the pores and elute more rapidly. This technique serves as an important tool for the analysis and purification of proteins and other macromolecules.
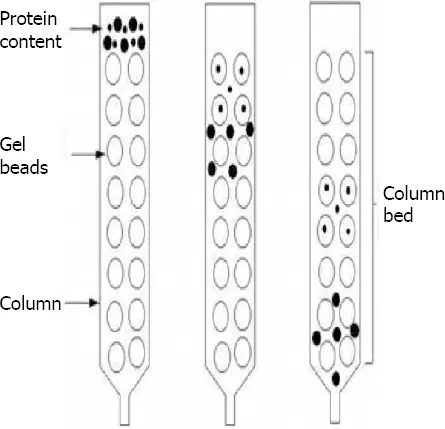
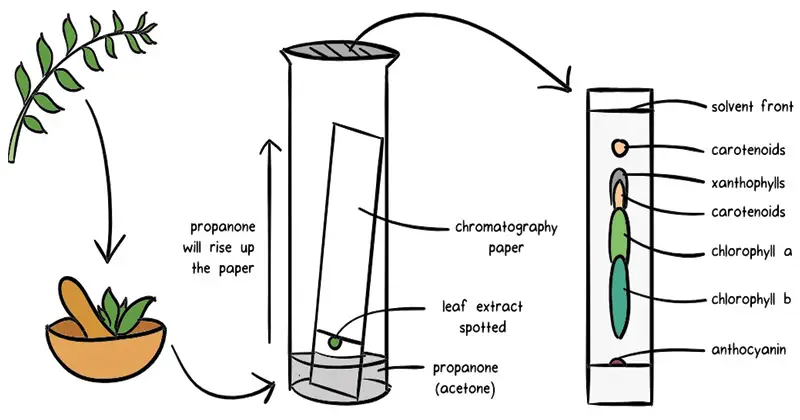
Paper chromatography
- Paper chromatography is a type of chromatographic technique that utilizes a layer of cellulose-saturated paper as the support material. The stationary phase in this method is the layer of water settled in the pores of the filter paper, while the mobile phase is a suitable fluid placed in a developing tank.
- The process of paper chromatography involves the separation of components in a mixture based on their differential affinities to the stationary and mobile phases. Since the cellulose paper is highly saturated with water, it acts as the stationary “liquid phase” where the sample components will interact.
- To perform paper chromatography, a small spot of the sample mixture is applied to the paper as a concentrated spot. The paper is then placed in a developing tank containing the mobile phase, which is allowed to migrate up the paper through capillary action. As the mobile phase moves, it carries the sample components along with it.
- During the migration process, the components in the sample mixture will interact differently with the cellulose paper and the mobile phase. Some components may have a stronger affinity for the stationary phase and will move more slowly, while others may have a weaker affinity and will move more rapidly.
- As the mobile phase continues to migrate up the paper, the different components of the sample will separate and form distinct bands or spots at different positions on the paper. Each component’s position on the paper is determined by its affinity for the stationary phase and the rate at which it is carried by the mobile phase.
- Once the migration is complete, the paper is removed from the developing tank and allowed to dry. The separated components can then be visualized by applying appropriate detection techniques such as UV light, staining, or spraying with chemical reagents. The resulting pattern of spots or bands on the paper, known as a chromatogram, provides information about the composition of the sample mixture.
- Paper chromatography is a simple and inexpensive method that is commonly used for qualitative analysis and separation of organic compounds, such as plant pigments, amino acids, and small molecules. It is widely employed in fields like biochemistry, forensic science, and environmental analysis, where quick and portable separation techniques are required.
- In summary, paper chromatography involves the separation of components in a mixture using cellulose-saturated paper as the stationary phase and a suitable fluid as the mobile phase. Through capillary action, the mobile phase migrates up the paper, carrying the sample components along and causing them to separate based on their affinity for the stationary and mobile phases. Paper chromatography is a widely used technique for qualitative analysis and is especially useful in situations where simplicity, cost-effectiveness, and portability are important considerations.
Thin-layer chromatography
- Thin-layer chromatography (TLC) is a type of chromatographic technique that falls under the category of “solid-liquid adsorption” chromatography. In this method, a solid adsorbent substance is coated onto glass plates, serving as the stationary phase. Various solid substances commonly used in column chromatography, such as alumina, silica gel, and cellulose, can be employed as the adsorbent material in TLC.
- The process of thin-layer chromatography involves the separation of components in a mixture based on their differential interactions with the stationary and mobile phases. The mobile phase, typically a liquid solvent, travels upward through the stationary phase on the thin plate by capillary action. As the solvent moves, it carries the mixture components with it, causing them to separate on the plate with different flow rates.
- The separation on the TLC plate depends on factors such as the polarity of the sample components, the nature of the solid phase (adsorbent material), and the polarity of the solvent used as the mobile phase. Components with higher affinity for the stationary phase will move more slowly, while those with greater affinity for the mobile phase will move more rapidly up the plate.
- To visualize the separated components, various techniques can be employed. If the molecules in the sample are colorless, fluorescent, radioactive, or lack a visible marker, specific chemical substances or reagents can be applied to the TLC plate. These substances can react with the separated components to produce visible colored spots or bands, which can be observed under normal room light or under UV light.
- The positions of the separated molecules on the chromatogram can be determined by measuring the distances traveled by the molecules and the solvent front. This measurement is known as the relative mobility (Rf) value and is calculated as the ratio of the distance traveled by a specific molecule to the distance traveled by the solvent front. The Rf value is a useful qualitative parameter for describing the mobility and relative positions of the molecules on the TLC plate.
- Thin-layer chromatography is a versatile and widely used technique in various fields such as pharmaceuticals, food analysis, forensics, and environmental sciences. It allows for rapid separation and analysis of complex mixtures, as well as identification of individual components based on their Rf values and visual markers. TLC offers a cost-effective and efficient method for qualitative analysis and can serve as a preliminary step in more advanced chromatographic techniques.
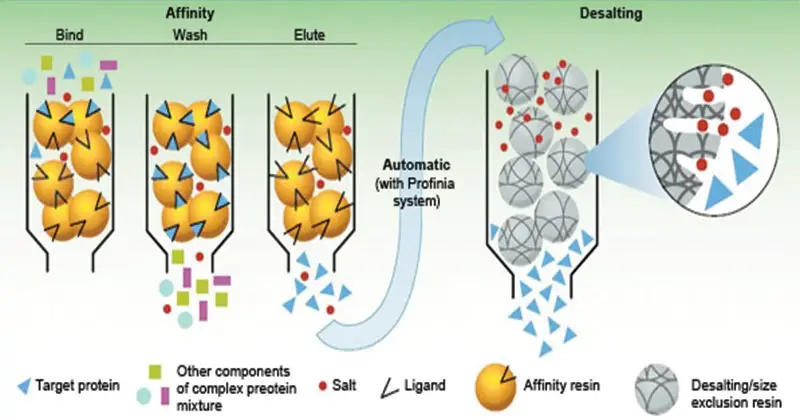
Affinity chromatography
- Affinity chromatography is a powerful technique used for the purification of various biomolecules, including enzymes, hormones, antibodies, nucleic acids, and specific proteins. It exploits the specific binding affinity between a target molecule (such as a protein) and a ligand immobilized on the solid support within the column.
- In affinity chromatography, the filling material of the column contains a ligand that has the ability to form a complex with the specific protein of interest. This ligand can be made of materials such as dextran, polyacrylamide, or cellulose. The ligand is immobilized onto the solid support, which is typically a matrix material.
- When a sample mixture containing the target protein is applied to the affinity column, the specific protein selectively binds to the ligand, forming a complex. This interaction between the target protein and the ligand allows for its retention within the column, while other proteins and impurities pass through and are eluted.
- Once the specific protein is bound to the column, it can be eluted by altering the conditions of the buffer solution. This can be achieved by changing the pH or by adding a salt solution, which modifies the ionic strength of the buffer. These changes disrupt the binding between the ligand and the target protein, causing the bound protein to dissociate from the column.
- The elution of the bound protein is typically performed in a step-wise manner, where the elution buffer is adjusted gradually to optimize the release of the target protein. The eluted protein can then be collected and further analyzed or utilized for various applications.
- Affinity chromatography provides a highly specific and efficient method for purifying biomolecules based on their affinity for specific ligands. It enables the isolation of target proteins from complex mixtures with high selectivity and purity. This technique plays a crucial role in various fields, including biochemistry, biotechnology, and pharmaceutical research.
- In summary, affinity chromatography utilizes specific ligands immobilized on a solid support to selectively bind and retain target proteins within the column. By modulating the buffer conditions, the bound protein can be eluted, allowing for its purification and isolation. Affinity chromatography is widely employed for the purification of enzymes, hormones, antibodies, nucleic acids, and other specific proteins.
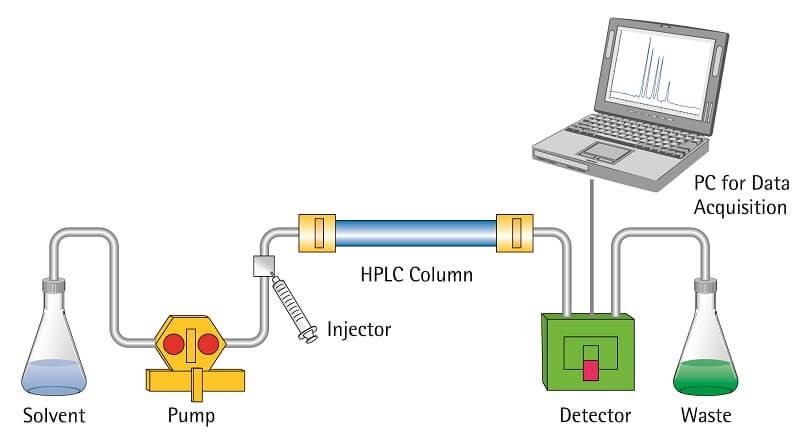
High-prssure liquid chromatography (HPLC)
- High-pressure liquid chromatography (HPLC) is a powerful chromatographic technique used for the separation, identification, and purification of various molecules. It offers efficient and rapid analysis of compounds such as amino acids, carbohydrates, lipids, nucleic acids, proteins, steroids, and other biologically active substances.
- In HPLC, the mobile phase, which is a solvent or solvent mixture, is forced through a column under high pressure ranging from 10 to 400 atmospheric pressure. The flow rate of the mobile phase is relatively high, typically between 0.1 and 5 cm/sec. This technique utilizes small particles as the stationary phase in the column, and the application of high pressure enhances the separation efficiency and allows for faster analysis.
- The main components of an HPLC system include a solvent reservoir or depot, a high-pressure pump, a commercially prepared column, a detector, and a recorder. The solvent reservoir holds the mobile phase, which is pumped through the column by the high-pressure pump. The column contains the stationary phase, which interacts with the sample components to achieve separation. The detector monitors the eluting compounds, and the recorder records the signals generated by the detector.
- One of the key advantages of HPLC is its ability to perform rapid and accurate structural and functional analysis of various molecules. The high separation power of HPLC, achieved through the use of small particle sizes and high pressure, allows for efficient separation and identification of complex mixtures. The analysis is typically completed within a short period of time, making HPLC a valuable tool in research, quality control, and analytical laboratories.
- HPLC systems are often equipped with computerized control and data analysis capabilities, allowing for precise control of separation parameters and automated data processing. This enhances the accuracy and reproducibility of the analysis. The computerized system can also be used to monitor the duration of the separation process and control the collection of separated components.
- In summary, high-pressure liquid chromatography (HPLC) is a versatile and widely used analytical technique for the separation, identification, and purification of various molecules. Its high separation power, fast analysis time, and compatibility with a wide range of compounds make it a valuable tool in many scientific and industrial applications.
Hydrophobic interaction chromatography (HIC)
- Hydrophobic interaction chromatography (HIC) is a chromatographic technique that relies on the hydrophobic interactions between the hydrophobic regions of proteins or biomolecules and the hydrophobic ligands present on the chromatography matrix. It is commonly used for the separation and purification of proteins, peptides, and other biomolecules.
- In HIC, the stationary phase consists of a hydrophobic adsorbent material, typically a resin or beads, that is functionalized with ligands containing hydrophobic side chains. These hydrophobic ligands interact with the hydrophobic regions of the target molecules, leading to their binding and retention on the column.
- The hydrophobic interactions in HIC are driven by the tendency of hydrophobic molecules or regions to minimize their exposure to the surrounding aqueous environment. When a sample containing the target biomolecules is applied to the HIC column, the hydrophobic regions of the biomolecules interact with the hydrophobic ligands on the stationary phase, causing them to bind to the column.
- To elute the bound biomolecules from the HIC column, a gradient of decreasing hydrophobicity is typically applied. This can be achieved by reducing the concentration of organic solvent or increasing the concentration of salts in the mobile phase. As the hydrophobic interactions between the biomolecules and the stationary phase weaken, the bound molecules are gradually released and eluted from the column.
- HIC offers several advantages in protein purification. It allows for the separation and purification of proteins based on their hydrophobicity, complementing other chromatographic techniques that rely on different principles of separation. HIC can be particularly useful for separating proteins with similar charges or sizes but different hydrophobic characteristics.
- Furthermore, HIC can be performed under mild, non-denaturing conditions, preserving the native structure and activity of the target proteins. This makes HIC suitable for purifying and isolating biologically active proteins that are sensitive to denaturation or conformational changes.
- Overall, hydrophobic interaction chromatography (HIC) is a powerful technique in the field of protein purification and separation. By utilizing the hydrophobic interactions between the target biomolecules and the hydrophobic ligands on the stationary phase, HIC enables the selective capture and purification of proteins based on their hydrophobic characteristics. It finds applications in various areas of research, biotechnology, and pharmaceutical development.
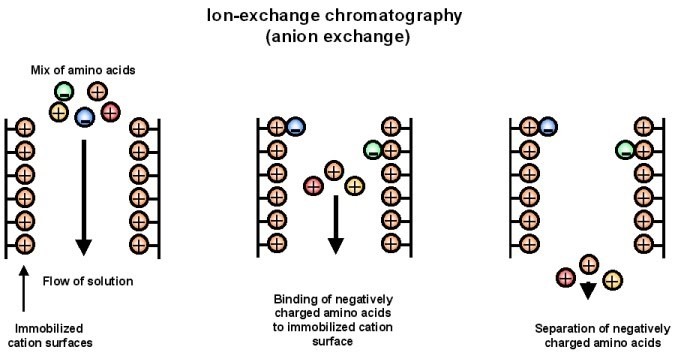
Ion exchange chromatography
- Ion-exchange chromatography is a chromatographic technique that exploits the electrostatic interactions between charged protein groups and a solid support material, known as the matrix. The matrix possesses an ion load that is opposite to the charge of the proteins to be separated, allowing for the formation of ionic ties between the matrix and the proteins.
- In ion-exchange chromatography, the matrix can be either positively charged or negatively charged, depending on the type of proteins being targeted. If the matrix is positively charged, it is referred to as an anion-exchange matrix, and it adsorbs negatively charged proteins. On the other hand, if the matrix is bound with negatively charged groups, it is known as a cation-exchange matrix, and it adsorbs positively charged proteins.
- To separate proteins using ion-exchange chromatography, the sample mixture containing the proteins is applied to the column packed with the appropriate ion-exchange matrix. The proteins with charges opposite to that of the matrix will bind to the matrix through electrostatic interactions. This binding allows for the separation of the target proteins from other components in the mixture.
- The elution or release of the bound proteins from the ion-exchange column is achieved by modifying the conditions of the buffer solution. This can be done by changing the pH, the concentration of ion salts, or the ionic strength of the buffer solution. By adjusting these parameters, the electrostatic interactions between the proteins and the matrix can be disrupted, leading to the release of the proteins of interest.
- Ion-exchange chromatography provides a highly selective method for separating proteins based on their charge properties. It is commonly used in protein purification processes, as it allows for the isolation and purification of proteins with specific charge characteristics from complex mixtures. This technique plays a crucial role in various fields, including biochemistry, biotechnology, and pharmaceutical research.
- In summary, ion-exchange chromatography relies on electrostatic interactions between charged protein groups and a matrix with an opposite charge. By exploiting these interactions, proteins can be selectively adsorbed and subsequently eluted by modifying the buffer conditions. This technique offers a valuable tool for the separation and purification of proteins based on their charge properties.
Dye- ligand chromatography
- Dye-ligand chromatography is a chromatographic technique that utilizes the binding affinity between certain enzymes or proteins and specific dye molecules. The development of this technique was driven by the observation that many enzymes have the ability to bind purine nucleotides, such as Cibacron Blue F3GA dye.
- Cibacron Blue F3GA dye possesses a planar ring structure with negatively charged groups, which resembles the structure of NAD (nicotinamide adenine dinucleotide). It has been found that the dye can bind to the adenine and ribose binding sites of NAD, functioning as an analog of ADP-ribose. This binding capacity of dye-ligand adsorbents is remarkably strong, approximately 10-20 times stronger than other adsorbents used in affinity chromatography.
- In dye-ligand chromatography, the stationary phase consists of an adsorbent material, typically a solid support, which is functionalized with the specific dye ligand. The dye ligand interacts selectively with the target enzyme or protein, facilitating its binding and retention on the adsorbent material within the chromatography column.
- To separate the bound proteins from the column, elution is performed under appropriate pH conditions using high-ionic strength solutions. This takes advantage of the ion-exchange properties of the adsorbent material. By altering the pH and ionic strength, the binding interactions between the dye ligand and the target proteins can be disrupted, leading to their release from the column.
- Dye-ligand chromatography offers several advantages in protein purification. The strong binding affinity between the dye ligand and the target proteins allows for efficient capture and purification. The selectivity of the dye ligand ensures the specific isolation of the desired proteins, while other contaminants are removed. Additionally, dye-ligand chromatography can be applied to a wide range of proteins, enzymes, and nucleic acids, making it a versatile technique in various biochemical and biotechnological applications.
- Overall, dye-ligand chromatography provides an effective and robust method for the purification of proteins and other biomolecules based on their specific binding interactions with dye ligands. This technique has contributed significantly to the field of protein purification and continues to find applications in various research and industrial settings.
Pseudoaffinity chromatography
- Pseudoaffinity chromatography is a chromatographic technique that utilizes compounds with high affinity for specific enzymes or proteins to selectively capture and purify target molecules. One example of pseudoaffinity chromatography is immobilized metal affinity chromatography (IMAC), which is widely used in protein purification.
- In pseudoaffinity chromatography, specific ligands are immobilized onto a solid support, such as beads or resin, and these ligands exhibit affinity towards certain enzymes or proteins. In the case of IMAC, metal ions such as nickel, cobalt, or zinc are commonly used as the immobilized ligands.
- The affinity of the ligands in pseudoaffinity chromatography is based on specific interactions with the target enzymes or proteins. For example, anthraquinone dyes and azo-dyes are known to have affinity for enzymes such as dehydrogenases, kinases, transferases, and reductases.
- During the chromatographic process, the sample containing the mixture of molecules is applied to the pseudoaffinity column. The target enzymes or proteins with affinity for the immobilized ligands selectively bind to the ligands, while non-target molecules pass through the column.
- To elute the bound target molecules, specific elution conditions are employed. These conditions can involve changes in pH, ionic strength, or the addition of competing ligands that disrupt the affinity between the target molecules and the immobilized ligands.
- Pseudoaffinity chromatography provides a powerful tool for the purification and isolation of enzymes or proteins with high selectivity and specificity. It allows for the separation of target molecules from complex mixtures based on their affinity towards specific ligands immobilized on the solid support.
- The immobilized metal affinity chromatography (IMAC) variant of pseudoaffinity chromatography, using metal ions as the immobilized ligands, has gained significant popularity in protein purification. Metal ions such as nickel, cobalt, or zinc have high affinity for certain amino acid residues, such as histidine, present in many proteins. This enables the selective binding and purification of histidine-tagged proteins, which are commonly used in recombinant protein expression and purification systems.
- In summary, pseudoaffinity chromatography, including techniques like immobilized metal affinity chromatography (IMAC), utilizes specific ligands with high affinity for target enzymes or proteins to achieve selective capture and purification. By exploiting the specific interactions between the ligands and the target molecules, pseudoaffinity chromatography provides a valuable tool for protein purification and isolation in various research and biotechnological applications.
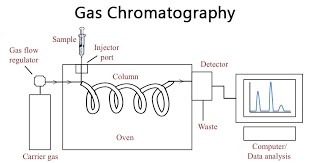
Gas chromatography
- Gas chromatography is a widely used chromatographic technique that operates on the principle of “gas-liquid” chromatography. In this method, the stationary phase is a column, which is inserted into the instrument and contains a liquid stationary phase that is adsorbed onto the surface of an inert solid support. The mobile phase, known as the carrier gas, consists of gases such as helium (He) or nitrogen (N2).
- The process of gas chromatography involves the passage of the mobile phase, an inert gas, through the column under high pressure. The sample to be analyzed is first vaporized and then introduced into the gaseous mobile phase. Within the column, the components present in the sample become dispersed between the mobile phase and the stationary phase on the solid support.
- Gas chromatography offers several advantages, including simplicity, versatility, high sensitivity, and fast analysis times. It is particularly suitable for the separation and analysis of small molecules, even in trace amounts. The technique is widely employed in various scientific fields, including chemistry, pharmaceuticals, environmental analysis, and forensic sciences.
- Gas chromatography is known for its excellent separation capabilities, allowing for the detection and quantification of individual components in complex mixtures. The separation is achieved based on differences in the affinity of the sample components for the stationary phase and their volatility. Components with stronger interactions with the stationary phase will spend more time in the column and elute later, while those with weaker interactions will elute earlier.
- The separated components are detected by a detector, which can be a thermal conductivity detector (TCD), flame ionization detector (FID), or other types of detectors depending on the specific analytical requirements. The detector produces a signal that is recorded and analyzed, generating a chromatogram that represents the distribution of components in the sample.
- Gas chromatography is a highly efficient technique that allows for precise quantitative analysis and identification of individual compounds within a mixture. It is frequently used for quality control, research, and routine analysis in various industries. The versatility and sensitivity of gas chromatography make it an indispensable tool in the field of analytical chemistry.
Commonly employed chromatography techniques include
| Technique | Stationary phase | Mobile phase | The basis of separation | Notes |
|---|---|---|---|---|
| *Paper Chromatography | solid (cellulose) | liquid | Polarity of molecules | compound directly on cellulose paper |
| *Thin Layer Chromatography (TLC) | Solid (silica or Alumina) | liquid | Polarity of molecules | glass is coated with a thin layer of silica. On it is seen the chemical |
| *Liquid column chromatography | Solid (silica or Alumina) | liquid | Polarity of molecules | Glass column is filled with silica-slurry |
| Size exclusion chromatography | Solid (microporous pieces of silica) | liquid | the size of molecules | small molecules get stuck in tiny pores within the stationary phase while larger molecules move through the spaces between the beads and have very short retention time. Thus, larger molecules emerge first. In this form of chromatography there isn’t any interplay whether chemical or physical, between the analyte and stationary phase. |
| Ion-exchange chromatography | (cationic or anionic resin) (cationic or anionic resin) | liquid | The charge of the ions in molecules | molecules with the same charge to resins will be able to bind strongly to the resin. molecules with similar charges to the resin will move through the column, and then elute out first. |
| Affinity Chromatography | Solid (agarose as well as porous glass beads which immobilized molecules are like antibodies and enzymes) | liquid | the binding affinity of the chemical analyte to the molecule that is immobilized on the stationary phase | If the molecule acts as an enzyme’s substrate that it binds to the enzyme, and the non-bound analytes will move through the mobile phase and then elute out of the column leaving the substrate attached to the enzyme. It is then removed of the stationary part and then eluted from the column using the appropriate solvent. |
| Gas chromatography | Solid or liquid support | gas (inert gas such as Helium or argon) | the boiling point of molecules | The samples are dissolved and the sample with the lowest boiling point is taken out first. The molecule that has the highest boiling point exits the column last. |
Applications of Chromatography
Chromatography finds extensive applications across various industries and scientific disciplines. Here are some notable applications of chromatography:
- Pharmaceutical Sector: Chromatography plays a crucial role in drug development and quality control in the pharmaceutical industry. It is used for identifying and analyzing samples to detect trace elements or chemicals. Chromatography techniques help separate compounds based on their molecular weight and element composition, allowing for the determination of unknown compounds and assessing the purity of mixtures.
- Chemical Industry: Chromatography is utilized in the chemical industry for a range of purposes. It is employed in testing water samples and assessing air quality. High-performance liquid chromatography (HPLC) and gas chromatography (GC) are commonly used to detect contaminants such as polychlorinated biphenyls (PCBs) in pesticides and oils.
- Food Industry: Chromatography is extensively applied in the food industry. It is used for detecting food spoilage and additives, ensuring food safety and quality. Chromatographic techniques help determine the nutritional composition and quality of food products.
- Forensic Science: Chromatography plays a vital role in forensic science, particularly in forensic pathology and crime scene analysis. It is utilized in the analysis of blood and hair samples collected from crime scenes, aiding in the identification of potential suspects and providing evidence for investigations.
- Molecular Biology Studies: Chromatography techniques are widely employed in molecular biology research. Various hyphenated techniques, such as electrochemical liquid chromatography-mass spectrometry (EC-LC-MS), are utilized in metabolomics, proteomics, and nucleic acid studies. HPLC is commonly used in protein separation, including the purification of insulin, plasma fractionation, and enzyme purification.
- Fuel Industry, Biotechnology, and Biochemical Processes: Chromatography has diverse applications in the fuel industry, biotechnology, and biochemical processes. It is employed for analyzing and purifying various compounds and biomolecules, facilitating research, development, and production processes.
Overall, chromatography plays a critical role in scientific research, quality control, and analytical testing across multiple industries, contributing to advancements in medicine, agriculture, environmental sciences, and more.
FAQ
What is chromatography?
Chromatography is a technique used for separating and analyzing complex mixtures into their individual components. It involves the separation of substances based on their different affinities to a stationary phase and a mobile phase.
What are the different types of chromatography?
There are several types of chromatography, including gas chromatography (GC), high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), ion-exchange chromatography, affinity chromatography, and more.
What is the principle behind chromatography?
Chromatography relies on the differential interactions of the components in a mixture with the stationary phase and the mobile phase. Components that have stronger interactions with the stationary phase will elute more slowly, resulting in separation.
What are the applications of chromatography?
Chromatography has a wide range of applications, including pharmaceutical analysis, environmental monitoring, food analysis, forensic investigations, biochemistry research, quality control in various industries, and more.
What is the difference between HPLC and GC?
HPLC is a liquid chromatography technique that uses a liquid mobile phase, while GC is a gas chromatography technique that uses a gas mobile phase. HPLC is typically used for analyzing compounds that are soluble in liquid, while GC is used for volatile and gaseous compounds.
How does chromatography separate components in a mixture?
Chromatography separates components based on their distribution between a stationary phase (solid or liquid) and a mobile phase (liquid or gas). The components that interact more with the stationary phase will have a slower migration and elute later, resulting in separation.
What is the role of the stationary phase in chromatography?
The stationary phase provides a surface for the components in the mixture to interact with. It can be a solid material (such as silica gel or a resin) or a liquid immobilized on a solid support.
What is the role of the mobile phase in chromatography?
The mobile phase carries the sample through the stationary phase. It can be a liquid or a gas and is chosen based on the type of chromatography and the properties of the components being separated.
How is the separated mixture detected in chromatography?
Various detection techniques are used in chromatography, including UV-Vis spectroscopy, fluorescence, mass spectrometry, refractive index measurement, and electrochemical detection. The choice of detection method depends on the nature of the analytes and the specific chromatographic technique.
How can I optimize chromatography separations?
Optimizing chromatography separations involves adjusting parameters such as the choice of stationary and mobile phases, column dimensions, flow rates, temperature, and detection conditions. Method development and optimization can be achieved through experimental exploration and systematic adjustments to achieve the desired separation goals.
