Table of Contents
What is CLED Agar?
- CLED Agar, which stands for Cystine-Lactose-Electrolyte-Deficient Agar, is a commonly used culture medium in diagnostic routine urinary bacteriology. It was initially described by Sandys and later modified by Mackey and Sandys. CLED Agar is a non-selective medium designed to support the growth of most urinary pathogens while providing distinct colony morphology.
- The primary purpose of CLED Agar is to isolate and enumerate bacteria from urine samples. It serves as a differential medium that allows for the differentiation of various microorganisms present in the urine. The medium supports the growth of both urinary pathogens and contaminants.
- One of the distinguishing characteristics of CLED Agar is its lack of electrolytes. This feature helps prevent the excessive swarming of Proteus species, which are known for their ability to spread rapidly on culture media. By eliminating electrolytes, CLED Agar provides good colonial differentiation without promoting the extensive spread of Proteus species.
- CLED Agar not only promotes the growth of urinary pathogens but also allows for the quantitative determination of these pathogens, including Proteus species, when calibrated loops are used for inoculation. This means that the agar can be utilized for quantitative analysis to determine the bacterial load of specific pathogens in a urine sample.
- In summary, CLED Agar is a differential, non-selective culture medium used for the isolation, enumeration, and differentiation of microorganisms in urine. It supports the growth of urinary pathogens while preventing excessive swarming of Proteus species. It is commonly employed in diagnostic laboratories for routine urinary bacteriology.
Composition of CLED Agar
C.L.E.D. Agar w/Bromo Thymol Blue)
| Constituents | gm/Litre |
| Lactose | 10.0 |
| Enzymatic Digest of Gelatin | 4.0 |
| Enzymatic Digest of Casein | 4.0 |
| Beef extract | 3.0 |
| L-Cystine | 0.128 |
| Bromothymol Blue | 0.02 |
| Agar | 15.0 |
pH 7.3 +/- 0.2
C.L.E.D.Medium (with Andrade Indicator)
| Ingredients | Gms / Litre |
|---|---|
| Peptone | 4.000 |
| HM peptone B | 3.000 |
| Tryptone | 4.000 |
| Lactose | 10.000 |
| L-Cystine | 0.128 |
| Bromothymol blue | 0.020 |
| Andrade indicator | 0.100 |
| Agar | 15.000 |
| Final pH (at 25°C) | 7.5±0.2 |
Principle of CLED Agar
The principle of CLED Agar revolves around its composition and the specific reactions of microorganisms within the medium. Here’s a summary based on the information provided:
CLED Agar contains enzymatic digest of casein, enzymatic digest of gelatin, and beef extract, which provide essential nutrients, such as nitrogen, vitamins, minerals, and amino acids, required for bacterial growth. L-cystine is included to support the growth of cysteine-dependent dwarf coliform colonies.
Lactose, a fermentable carbohydrate, serves as a carbon and energy source. Bacteria capable of fermenting lactose will produce acid as a byproduct, leading to a decrease in pH. This acid production results in a color change of the medium from green to yellow. Bromothymol blue, a pH indicator, is used to detect this change.
The presence of cystine benefits organisms with specific cystine requirements, particularly coliforms, promoting their growth. The inclusion of lactose enables the differentiation of lactose-positive bacteria, which form yellow colonies due to lactose fermentation. Bacteria that decarboxylate L-cystine cause an alkaline reaction and form dark blue colonies.
Furthermore, CLED Agar is electrolyte-deficient, which helps limit the invasion of the environment by Proteus species. This characteristic reduces excessive swarming, preventing the spread of Proteus on the culture medium.
In summary, the principle of CLED Agar lies in its nutrient composition, use of L-cystine for specific bacterial growth requirements, detection of lactose fermentation through pH indicator, and electrolyte deficiency to control the spread of Proteus. These elements work together to support the growth of urinary pathogens while allowing for differentiation and identification based on colony color and characteristics.
Preparation of CLED Agar
C.L.E.D. Agar w/Bromo Thymol Blue)
To prepare CLED Agar with Bromo Thymol Blue, follow the steps below:
- Suspend 36.15 grams of CLED Agar in 1000 ml of distilled water. Make sure to mix the agar thoroughly to ensure even distribution.
- Heat the mixture to boiling, allowing the medium to dissolve completely. Continuously stir to prevent clumping or uneven heating.
- After the medium has dissolved, autoclave it at 15 pounds of pressure (121°C) for 15 minutes. Autoclaving sterilizes the agar, eliminating any potential contaminants.
- Allow the agar to cool down to a temperature of 45-50°C. Cooling it sufficiently is important to prevent the denaturation of any sensitive components.
- Mix the agar well to ensure that the components are thoroughly incorporated.
- Pour the prepared CLED Agar with Bromo Thymol Blue into sterile Petri plates. The plates should be sterile to prevent contamination during the pouring process.
Once poured into the plates, the CLED Agar is ready for use in the isolation, enumeration, and differentiation of urinary microorganisms. The agar’s composition and the addition of Bromo Thymol Blue indicator enable the observation of color changes that aid in the identification of specific bacterial characteristics.
C.L.E.D.Medium (with Andrade Indicator)
To prepare CLED Medium with Andrade Indicator, follow these steps:
- Suspend 36.25 grams of CLED Medium in 1000 ml of distilled water. Ensure that the medium is evenly dispersed throughout the water by mixing thoroughly.
- Heat the mixture to boiling, allowing the medium to dissolve completely. Stir continuously during heating to prevent clumping or uneven distribution of the medium.
- Autoclave the solution at 15 pounds of pressure (121°C) for 15 minutes. Autoclaving sterilizes the medium, eliminating any potential contaminants.
- After autoclaving, allow the medium to cool down to a temperature of 45-50°C. Cooling is necessary to avoid denaturation of sensitive components.
- Mix the medium well to ensure uniformity and proper incorporation of the components.
- Pour the prepared CLED Medium with Andrade Indicator into sterile Petri plates. Make sure the plates are sterile to prevent contamination during pouring.
Once poured into the plates, the CLED Medium with Andrade Indicator is ready for use in the isolation, enumeration, and differentiation of urinary microorganisms. The medium’s composition and the presence of the Andrade Indicator allow for the observation of specific color changes that aid in the identification of bacterial characteristics.
Test Procedure Using CLED Agar
- Collecting the urine sample:
- Collect an undiluted, well-mixed urine sample using a calibrated loop with a volume of either 0.01 ml or 0.001 ml. The calibrated loop ensures consistent and accurate inoculation.
- Loading the loop and inoculating the sample:
- Ensure the calibrated loop is properly loaded with the urine specimen.
- Inoculate the sample down the middle of the CLED Agar plate in a single streak.
- Perform additional spreading of the inoculum from the initial streak to ensure even distribution of the sample on the agar surface.
- Incubation:
- Place the inoculated CLED Agar plates in an incubator set at 35 ± 2°C.
- Incubate the plates in ambient air for a period of 24 to 48 hours.
- The incubation time allows for the growth and proliferation of microorganisms present in the urine sample.
Following the completion of the incubation period, the CLED Agar plates can be examined for colony growth, pigmentation, and other specific characteristics of the microorganisms present in the urine. These observations aid in the identification and enumeration of urinary tract pathogens.
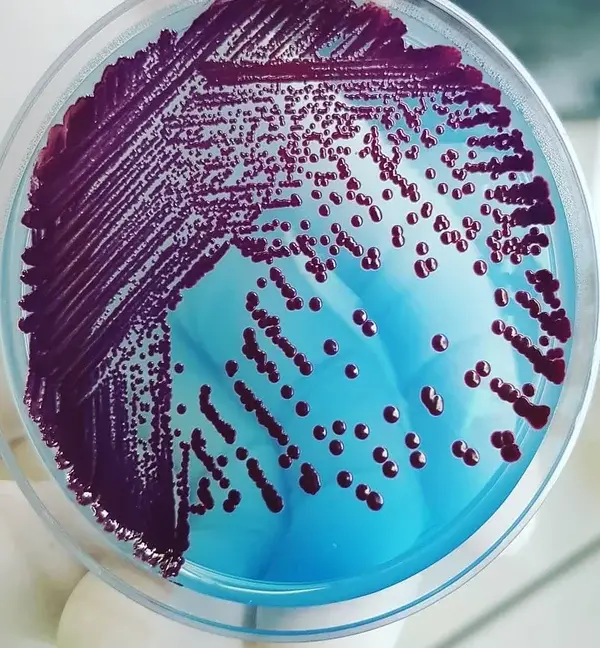
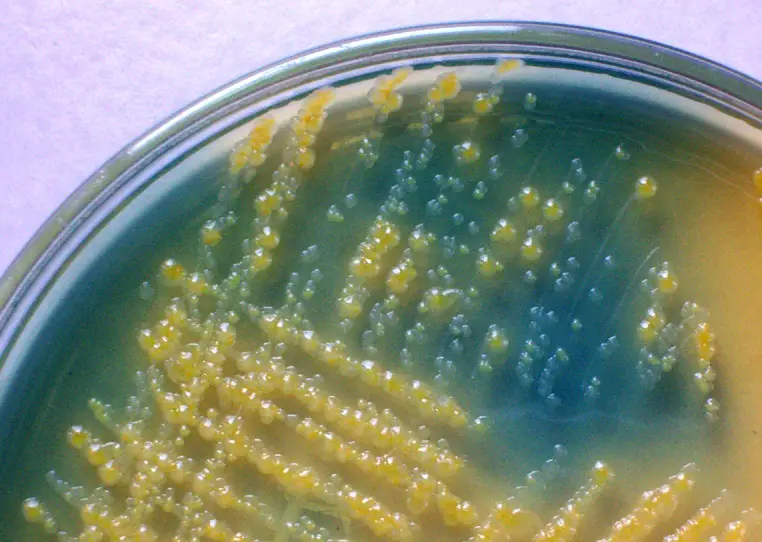
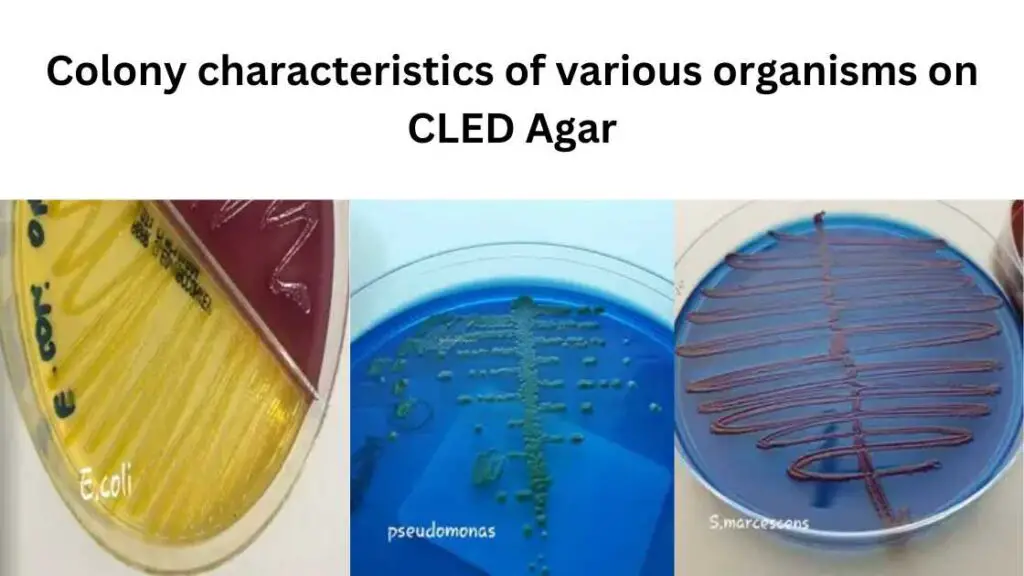
Colony characteristics of various organisms on CLED Agar
Different microorganisms can show different cultural responses to CLED Agar. These reactions are after incubation at the right temperature and atmosphere for 18-24 hours.
| Organism | Colony Morphology |
| Escherichia coli | Large, elevated, yellow, opaque colonies with a center more intense yellow; yellowish medium |
| Klebsiella species | extremely mucoid colonies varying in color from yellow to whitish-blue. yellowish medium. |
| Proteus species | translucent blue colonies are usually smaller than Escherichia coli. blue-green to blue medium. |
| Salmonella species | flat blue colonies |
| Pseudomonas aeruginosa | green colonies with a typical matte surface and rough periphery. “Sweet” odor. Blue-green agar |
| Enterococcus faecalis | yellow colonies about 0.5 mm in diameter. yellow medium |
| Staphylococcus aureus | deep yellow colonies about 0.75 mm diameter, uniform in color. yellow medium |
| Corynebacteria | very small grey colonies |
| Lactobacilli | similar to corynebacteria but with a rougher surface |
Quality Control of CLED Agar
The quality control of CLED Agar involves two important aspects: sterility testing and performance testing. Here’s an overview of each:
- Sterility testing:
- Incubate 3-5 plates of uninoculated CLED Agar at 37℃ for 18-24 hours.
- Any growth observed in the medium indicates a positive result, suggesting contamination.
- If growth is detected, the entire lot of CLED Agar should be discarded.
- Performance testing:
- Inoculate one or more standard strains onto the prepared CLED Agar medium.
- Incubate the plates at 35 ± 2°C in an aerobic atmosphere for 18-24 hours.
- Observe and evaluate the growth, pigmentation, colony size, and inhibition of Proteus swarming/spreading after overnight incubation.
Desired characteristics for specific standard strains include:
- Escherichia coli ATCC 25922: Luxuriant growth with yellow colonies and medium.
- Proteus vulgaris ATCC 8427: Good growth with colorless to blue colonies; some spreading is acceptable, but swarming should be inhibited.
- Enterococcus faecalis ATCC 29212: Growth with small colonies, ranging from colorless to yellow.
- Staphylococcus aureus ATCC 25923: Good growth with small, yellow to yellowish colonies.
- Uninoculated plates: The medium should exhibit a green to blue-green color.
By performing sterility testing and performance testing with appropriate standard strains, laboratories can ensure the quality and reliability of the CLED Agar. These quality control measures help identify and address any potential issues with the medium, ensuring accurate and consistent results in microbiological testing.
| Organism | Desired characteristics |
| Escherichia coli ATCC 25922 | Luxuriant Growth; colonies are yellow, medium yellow |
| Proteus vulgaris ATCC 8427 | Good growth; colonies are colorless to blue; swarming is inhibited; however, slight spreading is acceptable |
| Enterococcus faecalis ATCC 29212 | Growth; small colonies ,colorless to yellow |
| Staphylococcus aureus ATCC 25923 | Good growth; colonies small, yellow to yellowish |
| Uninoculated plates | Green to blue-green |
Application of CLED Agar
CLED Agar has several important uses in microbiology, particularly in routine urine culture and the growth and enumeration of urinary tract organisms. Here are the key uses of CLED Agar:
- Routine urine culture: CLED Agar is commonly employed for routine urine culture in clinical laboratories. It provides a suitable medium for the isolation and identification of microorganisms present in urine specimens.
- Differentiation of lactose fermenters and non-fermenters: CLED Agar enables the differentiation of bacteria based on their ability to ferment lactose. Lactose fermenters produce acid during lactose fermentation, leading to a color change in the medium from green to yellow, whereas lactose non-fermenters do not cause a change in color.
- Isolation and counting of urinary microorganisms: CLED Agar is suitable for the isolation and enumeration of various aerobically growing microorganisms from urine specimens. It supports the growth of Enterobacteriaceae, Pseudomonas, non-fermenting Gram-negative rods, enterococci, staphylococci, Candida species, and many others commonly encountered in urinary tract infections.
By providing a non-selective medium with specific differentiation properties, CLED Agar allows microbiologists to isolate, identify, and quantify microorganisms present in urine samples. It plays a vital role in diagnosing urinary tract infections and guiding appropriate treatment decisions.
Advantages of CLED Agar
CLED Agar offers several advantages in microbiology and urinary tract infection diagnosis. Here are the key advantages:
- Discrimination of gram-negative bacteria: CLED Agar allows for good discrimination of gram-negative bacteria based on their ability to ferment lactose. Lactose fermenters and non-fermenters can be differentiated by observing color changes in the medium. This aids in the preliminary identification of different groups of gram-negative bacteria.
- Inhibition of Proteus swarming: One significant advantage of CLED Agar is its ability to inhibit the swarming of Proteus species. Swarming, which is the rapid and extensive movement of Proteus bacteria across a solid medium, can be problematic in urinary tract infection cultures. CLED Agar helps prevent excessive swarming of Proteus mirabilis and Proteus vulgaris, which are frequently involved in urinary tract infections.
- Cost-effectiveness: Using CLED Agar for urine culture offers a cost-effective alternative compared to the combined use of blood agar and MacConkey agar. CLED Agar combines the necessary features of supporting the growth of urinary pathogens, providing differentiation based on lactose fermentation, and inhibiting Proteus swarming into a single medium. This eliminates the need for multiple agar plates, reducing costs and simplifying the laboratory workflow.
It is important to note that MacConkey agar, which contains bile salts, also inhibits Proteus swarming. However, CLED Agar provides additional advantages of lactose fermentation differentiation and cost-effectiveness, making it a preferred choice for routine urine culture in many laboratories.
Overall, the advantages of CLED Agar include its ability to discriminate gram-negative bacteria, inhibit Proteus swarming, and offer a cost-effective solution for urine culture. These features contribute to its widespread use in microbiology laboratories for the diagnosis of urinary tract infections.
Limitations of CLED Agar
CLED Agar, like any diagnostic medium, has its limitations. Here are some important limitations to consider:
- Insufficient recovery of streptococci and other blood-dependent organisms: Streptococci and certain other organisms that require blood or serum for optimal growth may not be adequately recovered on CLED Agar or may require extended incubation. If such organisms are expected to be present, it is recommended to also cultivate the specimen on a blood agar plate to enhance their recovery.
- Inability to support the growth of specific genitourinary pathogens: CLED Agar is not suitable for the growth of certain genitourinary pathogens, including Neisseria gonorrhoeae, Gardnerella vaginalis, Chlamydia, Ureaplasma, and other fastidious organisms. If these pathogens are suspected, alternative culture methods specific to these organisms should be employed.
- Serological tests required for complete identification: While CLED Agar provides a useful tool for the isolation and enumeration of microorganisms from urine, it does not provide definitive identification. Serological tests using pure cultures are necessary to fully identify the isolated organisms and determine their specific characteristics.
FAQ
What is CLED Agar used for?
CLED Agar is used for routine urine culture and the growth, enumeration, and differentiation of urinary tract microorganisms.
What does CLED stand for?
CLED stands for Cystine-Lactose-Electrolyte-Deficient, which reflects the composition and characteristics of the agar medium.
How does CLED Agar differentiate between lactose fermenters and non-fermenters?
Lactose fermenters produce acid during lactose fermentation, leading to a color change in the medium from green to yellow. Lactose non-fermenters do not cause a color change.
Does CLED Agar inhibit the swarming of Proteus species?
Yes, CLED Agar inhibits the swarming of Proteus species, such as Proteus mirabilis and Proteus vulgaris, which are frequently involved in urinary tract infections.
What are the advantages of using CLED Agar?
CLED Agar offers good discrimination of gram-negative bacteria, inhibits Proteus swarming, and is relatively cost-effective compared to using multiple agar plates for urine culture.
Can all urinary pathogens be grown on CLED Agar?
While CLED Agar supports the growth of most urinary pathogens, certain fastidious organisms such as Neisseria gonorrhoeae, Gardnerella vaginalis, and Chlamydia do not grow on this medium.
How can I perform quality control of CLED Agar?
Quality control involves sterility testing by incubating uninoculated plates and performance testing by inoculating standard strains to assess growth, pigmentation, colony size, and Proteus swarming inhibition.
Can CLED Agar be used for quantifying urinary pathogens?
Yes, CLED Agar allows for quantitative determination of urinary pathogens when calibrated loops are used for inoculation.
Is additional testing required for complete identification of microorganisms grown on CLED Agar?
Yes, serological tests using pure cultures are necessary for complete identification of the microorganisms isolated on CLED Agar.
Can CLED Agar be used as a standalone medium for urine culture?
CLED Agar can be used as a primary isolation medium for routine urine culture. However, additional tests and culture media may be required for comprehensive identification and characterization of microorganisms.
References
- https://www.usbio.net/protocols/cled-agar-bevis
- https://www.condalab.com/int/en/dehydrated-culture-media/1579-14838-cled-agar-cystine-lactose-electrolyte-deficient.html
- https://hardydiagnostics.com/media/assets/product/documents/CLEDAgar.pdf
- https://www.neogen.com/globalassets/pim/assets/original/10041/official_ncm0034_cystine-lactose-electrolyte-deficient-cled-agar_technical-specifications_en-us.pdf
- https://www.vetbact.org/?displayextinfo=50
- https://www.sigmaaldrich.com/IN/en/product/sial/55420
- https://www.bd.com/resource.aspx?IDX=8967
- https://exodocientifica.com.br/_technical-data/M352.pdf
- https://exodocientifica.com.br/_technical-data/M792.pdf
- https://microbiologie-clinique.com/cled-agar-principle-interpretation.html
