Table of Contents
Confocal microscopy has various advantages over conventional optical microscopy, including a short depth of field, the elimination of out-of-focus glare, and the ability to collect optical slices serially from thick specimens. A prominent use of confocal microscopy in the biomedical sciences is the imaging of either fixed or live cells and tissues that have typically been labelled with one or more fluorescent probes.
Currently, the laser scanning confocal microscope (LSCM) is the most used confocal version for biomedical research. In this introduction, the LSCM is highlighted since it is the design most likely to be met by inexperienced users. In particular niches within the field of biological imaging, alternative equipment designs are preferred. The majority of specimen preparation processes can be applied, with slight modifications, to any of the confocal equipment versions and to other techniques for creating optical sections, such as deconvolution techniques and multiple-photon imaging.
What is a Confocal Microscope?
- A confocal microscope is a type of microscope that uses laser light to produce high-resolution images of samples at different depths within the sample. It is based on the principle of confocal laser scanning microscopy (CLSM), which uses a focused laser beam to scan the sample and detect the fluorescence emitted by the sample at different depths.
- Confocal microscopes are widely used in many different fields, including biology, medicine, and materials science, to study the structure and function of cells, tissues, and molecules at the microscopic level.
- One of the main advantages of confocal microscopy is its ability to produce high-resolution, three-dimensional images of samples. This is achieved by scanning the sample in a series of layers or optical sections and reconstructing the image using computer algorithms. Confocal microscopy can also be used to visualize multiple different fluorophores within a single sample, allowing for the simultaneous visualization of multiple processes or pathways.
- Overall, confocal microscopy is a powerful and versatile tool for studying the structure and function of cells, tissues, and molecules at the microscopic level.
- The concept of confocal microscopy was first proposed by Marvin Minsky in 1955. Minsky proposed using a focused laser beam to scan a sample and detect the fluorescence emitted by the sample at different depths, in order to produce high-resolution images of the sample.
- The first practical confocal microscope was developed by Marvin Minsky and a team of researchers at MIT in the 1970s. The first confocal microscope used a helium-neon laser and a photomultiplier tube to detect the fluorescence emitted by the sample.
- In the 1980s, the development of solid-state lasers and charge-coupled device (CCD) cameras led to the widespread adoption of confocal microscopy as a research tool. In the 1990s, the development of multi-photon excitation techniques and other advanced imaging techniques further expanded the capabilities of confocal microscopy.
- Today, confocal microscopy is a widely used technique in many different fields, including biology, medicine, and materials science, to study the structure and function of cells, tissues, and molecules at the microscopic level. It has played a significant role in the advancement of our understanding of the microscopic world and continues to be a powerful tool for scientific research.
Principle of Confocal Microscope
- Typically, a typical (wide-field) microscope uses different wavelengths from a light source to observe and illuminate a large area of a specimen, resulting in images that are hazy, unclear, and congested because cell sample images are collected from all directions without a focus point.
- To circumvent these problems, a confocal microscope is utilised. In wide-field and fluorescent microscopes, the entire specimen is illuminated, obtaining complete excitation and producing light that is detected by the microscope’s photodetector. However, the confocal microscope’s primary operating mechanism is point illumination.
- Examining a specimen that has been stained with fluorochrome. When a beam of light is focused on a specific area of a fluorochromatic specimen, the objective lens focuses the resulting illumination onto a plane above the objectives. On the focal plane above the objective is an aperture whose primary function is to block any stray light from reaching the specimen.
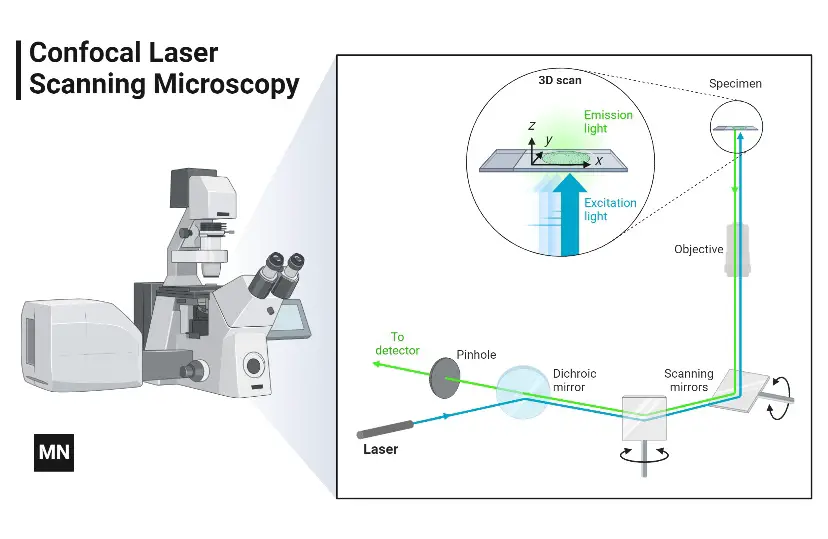
- The diameter of the illumination point, as determined by the numerical aperture of the objective, is between 0.25 and 0.8 microns, and its depth is between 0.5 and 1.5 microns at its brightest.
- The specimen typically rests on the plane of focus, which is the region between the camera lens and the perfect point of focus. Using the laser from the microscope, a plane on the specimen is scanned (beam scanning) or the stage is moved (stage scanning). The illumination is then measured by a detector, which produces an image of the optical section. Scannable optical portions are collected as data in a computer system to create a three-dimensional image. The image is measurable and quantifiable.
- Additionally, the aperture found above the objective, which blocks stray light, contributes to the favourable outcome.
- Regardless of the specimen’s thickness, the confocal microscope produces images with excellent contrast and resolution. In the high-resolution 3D image of cell complexes, including their structures, images are saved.
- The primary property of a confocal microscope is that it only detects what is in focus, while anything outside the focal point appears dark.
- Using two high-speed oscillating mirrors, the microscope scanner scans the focussed beam across a predetermined area to provide an image of the object. Their motion is made possible by galvanometer motors.
- The first mirror translates the beam along the lateral X-axis, while the second mirror translates it along the Y-axis. After an X-axis scan, the beam flies fast back to the origin to begin a new scan; this movement is known as flyback. During the flyback procedure, no data is gathered; hence, the laser scanner only illuminates the point of interest, which is the focal point.
Confocal microscopes employ fluorescence optics like widefield microscopes. Instead of illuminating the entire sample, laser light is focused on a fixed point at a specific depth. Fluorescent light is emitted here. The light detector only receives fluorescence signals from the lighted location because a pinhole in the optical channel blocks out-of-focus signals.
One optical plane is scanned by rastering the specimen. Scan numerous optical planes and use microscopy deconvolution software to visualise 3D objects (z-stack). Modern confocal microscopes with multiple lasers and emission/excitation filters can study multicolor immunofluorescence stainings.
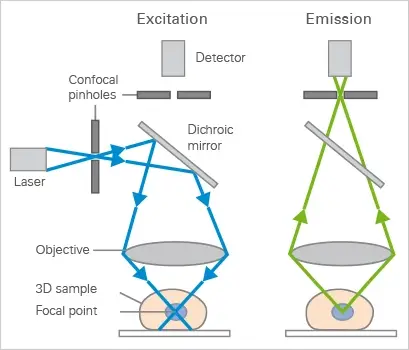
Mechanism of Confocal Microscope – How does a confocal microscope work?

- A confocal microscope uses laser beams instead of lights. The laser beams are released from their source and then focused onto a fluorescent stained sample.
- Neutral density filters and a set of scanning mirrors control the intensity of the laser light by moving them very precisely and quickly.
- One mirror tilts the beam within the X route, the opposite within the Y route. Together, they tilt the beam in a raster style.
- Then an objective lens focuses it onto the sample.
- The fluorochrome stained sample will be excited and then it will emit fluorescent lights.
- These fluorescent lights will travel back into the objective lens through the same path that the laser travels.
- The main effects of these scanning mirrors are on this light is to generate a spot of light which is not scanning, but standing still.
- Then a semi-transparent mirror reflects this fluorescent light away from the laser and toward the detection system.
- Before entering into the detection system, it passes through a pinhole. This pinhole allows only a small central portion of the light through to the light detectors.
- Confocal microscope produces a very low-intensity light, so the light is amplified by a photomultiplier tube (PMT).
- Photomultipliers have the ability to amplify a faint signal around one million times without introducing a single noise.
- After that, the PMT releases an electrical signal, which is then converted into an image by using a computer.
Characteristics features of Confocal Microscope
There are several characteristics and features of confocal microscopes that make them a powerful and versatile tool for scientific research:
- High resolution: Confocal microscopes can produce high-resolution images of samples, allowing for the visualization of fine details within the sample.
- Three-dimensional imaging: Confocal microscopes can produce three-dimensional images of samples by scanning the sample in a series of layers or optical sections and reconstructing the image using computer algorithms. This allows for the visualization of the structure and function of samples at different depths within the sample.
- Multiplexing: Confocal microscopes can be used to visualize multiple different fluorophores within a single sample, allowing for the simultaneous visualization of multiple processes or pathways.
- Specificity: Confocal microscopes can be used to selectively visualize specific molecules or structures within a sample, making it a specific technique for studying specific processes or pathways.
- Non-destructive: Confocal microscopy is a non-destructive technique, meaning that it does not damage the sample being imaged. This allows for the sample to be imaged multiple times or for other analyses to be performed on the same sample.
- Live cell imaging: Confocal microscopes can be used to visualize living cells over time, allowing for the study of dynamic processes within cells.
Overall, the high resolution, three-dimensional imaging capabilities, and multiplexing capabilities of confocal microscopes make them a powerful and versatile tool for studying the structure and function of cells, tissues, and molecules at the microscopic level.
Parts of Confocal Microscope
There are several key parts of a confocal microscope:
- Objective lens: The objective lens is a high-resolution lens that is used to collect and focus light from the sample.
- Laser light source: The laser light source is used to produce a focused beam of light that is used to scan the sample and excite the fluorophores within the sample. It can be chosen via a selection device and is matched with the fluorophores used in your experiment.
- Scanning system: The scanning system is responsible for moving the laser beam over the sample in a raster pattern to produce an image. It is typically based on a galvanometer or acousto-optic modulator.
- Beam splitter: It separates the excitation from the emitted light in the fluorescence beam path of the microscope.
- Detectors: The detectors are used to detect the fluorescence emitted by the sample at different depths. There are typically multiple detectors in a confocal microscope, including detectors for different wavelengths of fluorescence.
- Control and data acquisition system: The control and data acquisition system is responsible for controlling the various components of the microscope and acquiring and storing the data from the detectors. It typically includes a computer and specialized software for controlling the microscope and analyzing the data.
- Stage: The stage is a platform that holds the sample and allows it to be moved in order to scan the sample.
- Z-control: This allows the user to focus the laser beam on any focal plane within the specimen. The motorized Z-stepper allows us to move around the axial direction in small step sizes (approximately >10 nm) with high precision.
- Eyepieces: The eyepieces are used to view the image produced by the microscope.
- Pinhole: Pinhole is a type of adjustable iris. Pinhole allows the exclusion of most of the out-of-focus light from the acquired image and thus provides optical sectioning capacity. The size of the pinhole can be set by using the software on the user’s computer.
- Photomultiplier tube (PMT): It converts the photons into an electrical signal which is then used up by the computer to create an image of the specimen.
Overall, these parts work together to produce high-resolution, three-dimensional images of the sample using confocal laser scanning microscopy (CLSM).
You Might Like This Articles:
Types of Confocal Microscope
There are several types of confocal microscopes, including:
- Single-photon confocal microscopes: These microscopes use a single photon detector and a laser to produce high-resolution images of a sample.
- Two-photon confocal microscopes: These microscopes use a two-photon excitation process to produce high-resolution images of a sample. They are typically used to image deep within tissues, as the two-photon excitation process allows for deeper penetration of the sample.
- Multiphoton confocal microscopes: These microscopes use a multiphoton excitation process to produce high-resolution images of a sample. They are similar to two-photon confocal microscopes, but they are able to produce even higher-resolution images and can be used to image even deeper within tissues.
- Laser scanning confocal microscopes: These microscopes use a laser beam to scan over the sample and produce a high-resolution image. They are commonly used to image live cells and tissues.
- Fluorescence lifetime imaging microscopes (FLIM): These microscopes use the fluorescence lifetime of a sample to produce images. They are often used to study biochemical processes within cells and tissues.
- Stimulated emission depletion (STED) microscopes: These microscopes use stimulated emission to selectively deplete the fluorescence of a sample, resulting in high-resolution images. They are able to achieve a higher level of spatial resolution than other confocal microscopes.
- Superresolution microscopes: These microscopes use techniques such as STED, structured illumination microscopy, or single-molecule localization microscopy to achieve a spatial resolution that is beyond the diffraction limit of light. They are able to produce images with a higher level of detail than other confocal microscopes.
- Hybrid Scanning Confocal Microscopes: The slit-scanning confocal, which replaces the circular aperture with a rectangular slit to reject out-of-focus light, is an intermediate approach between single and multi-point scanning confocal microscopes. Slit-scanning devices cover a larger portion of the sample in a single field of view and greatly increase imaging speed at the expense of quick photobleaching and reduced resolution. 2002’s swept field confocal microscope (SFC) is yet another hybrid technique. The SFC can be operated in either a pinhole or slit scanning mode, in which the apertures stay immobile while galvanometer- and piezo-controlled mirrors sweep the image of the illuminated apertures across the sample. The emitted photons are directed to a CCD camera via a set of complementary pinholes or slits. The primary benefits of this method are its speed, increase in light collection efficiency, and decrease in artefacts caused by the apertures’ movement, as in a spinning disc system.
- Programmable array Microscope (PAM): This confocal microscope type employs a spatial light modulator (SLM – an object that imposes some form of spatially-varying modulation on a beam of light). The SLM is equipped with a series of moveable apertures (pinholes) and arrays of pixels with opacity, reflectivity, or optical rotation. In addition to microelectrochemical mirrors, the SLM is equipped with a charge-coupled device (CCD) camera for image capturing.
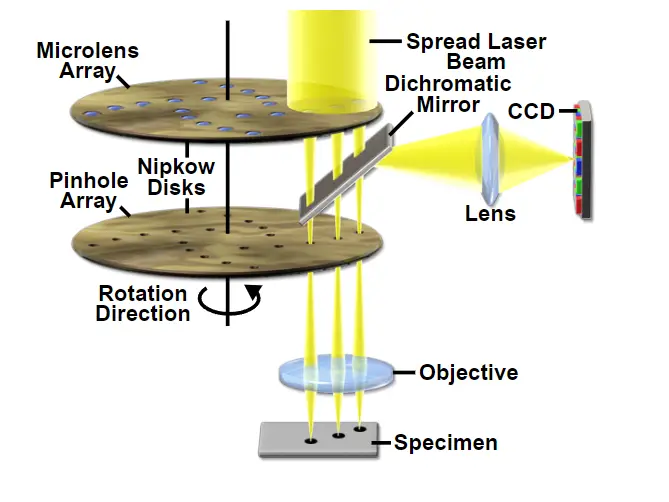
- Spinning disk: Spinning disc, also known as the Nipkow disc, is a form of confocal microscope that uses several moveable apertures (pinholes) on a disc to scan for light spots in a parallel way over a given plane for an extended length of time. Compared to a Confocal laser scanning microscope, the longer the exposure period, the less excitation energy is required for illumination. Reduced excitation energy minimises phototoxicity and photobleaching; hence, live cell imaging is its primary application.
- Dual spinning Disk: Dual spinning Disk or Microlens enhanced confocal Microscope -, it was designed by Yokogawa electric; it functions similarly to the spinning disc, with the exception that it has a second spinning disc with micro-lenses that is located before the spinning disc with pinholes. The microlenses capture a broad spectrum of light and focus it into each pinhole, so increasing the quantity of light directed into each pinhole and decreasing the amount of light obstructed by the spinning disc. These Confocal Microscopes with improved Microlenses are far more sensitive than spinning discs.
Important Things to remember
When developing experiments using confocal microscopy, there are various factors to consider, several of which are discussed here.
Objectives
- Modern objective lenses feature components to compensate for flatness of field and chromatic aberrations, which are essential for confocal microscopy since the laser beam must pass through portions of the objective lens that are located relatively far from the optical axis during scanning.
- To acquire the highest resolution, immersion objectives must be employed, and the refractive index must be matched to the mounting medium (Dean, 1998). Most goals are intended for use with 0.17 mm-thick cover glass to reduce artefacts caused by dispersion and varying thickness.
- Consequently, for confocal imaging targets specified for 0.17 cover glass, #1.5 coverslips should be utilised. Some objectives additionally include correction collars that enable for more exact calibration to the coverslip by altering the distance between elements in the objective barrel, which can aid in overcoming immersion-related aberrations.
- In addition to immersion medium and numerical aperture (NA), the working distance of the objective lens should be monitored carefully and matched to the tissue thickness being photographed.
Depth Penetration
- Imaging of deep tissue is in high demand, and techniques to maintain picture quality further from the coverslip are always being developed.
- Careful selection of high NA, long working distance objectives with refractive index matching to the medium can enhance the penetrability of a typical confocal microscope, although aberration-free imaging far from the coverslip is challenging.
- Recent developments in sample preparation include the use of commonly available chemicals to “clean” tissue of lipids and other highly scattering biological components.
Sample Size
- The overall size of the sample in a confocal microscope is directly proportional to the depth. Large samples can be examined using the 3D capabilities of a confocal microscope by scanning the beam across a volume to capture an image stack and then shifting the stage to successive fields-of-view.
- Software then stitches together these image stack “tiles” either during or after the experiment. While this has allowed for larger tissue slices, as noted above, sample thickness remains a limiting factor.
- In addition, picture artefacts can occur during the process of merging component tiles, and the imaging time on an LSCM grows dramatically with sample size because the beam must still be swept over each point.
Imaging Speed
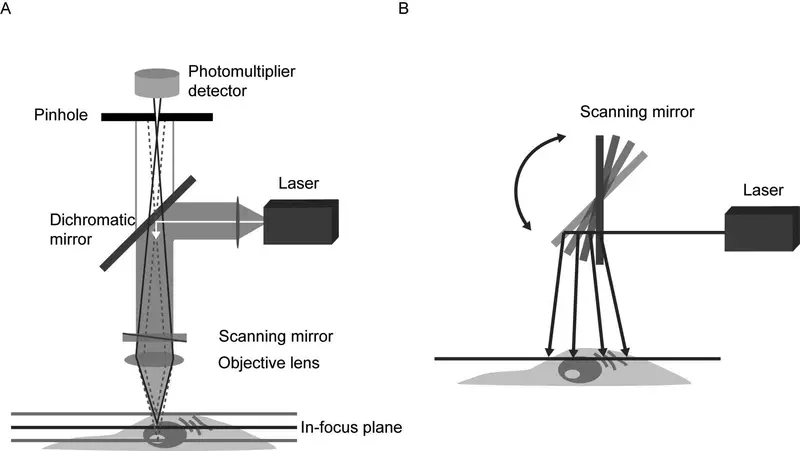
- Confocal microscopy experiments must consider acquisition speed. Confocal microscopy trades speed, resolution, and field-of-view. High magnification objectives can picture a single field of view faster, but bigger samples take longer to scan. Slit and spinning disc confocal microscopes speed imaging but photobleach.
- Resonant scanners, fixed-frequency mirrors, provide quick sample scanning in confocal microscopes (Figure 1B). Bidirectional resonant scanning speeds up imaging big samples, however mirror calibration must be done carefully to avoid artefacts.
Fluorophores
- Since the 1960s introduction of the green fluorescent protein, many fluorescent proteins (FPs) have been produced with different photophysical and spectral properties, expanding the palette of fluorescent probes for confocal imaging.
- Organic dyes are becoming brighter, smaller, and photostable. Confocal imaging with FPs, dyes, and a wide range of secondary antibodies has been effective for localising proteins and structures in whole cells and tissues and tracking fast dynamics in living cells.
- When choosing probes for imaging experiments, consider quantum efficiency, brightness, excitation and emission spectra, and optical filter tuning.
Sample Preparation
- Confocal microscopy resolution and sample shape depend on the mounting method for fixed tissues and the medium for living samples.
- Media without pH-indicator dyes like phenol red works best for live confocal imaging.
- To protect tissue when mounting fixed samples, use spacers between the coverslip and slide.
Application of Confocal Microscope
Confocal microscopes are used in a wide range of applications, including:
- Biology and medicine: Confocal microscopes are often used to study cells and tissues, including live cells. They are commonly used to study the structure and function of cells and tissues, as well as to identify and characterize specific proteins or other molecules within cells. Used for the examination of various eye diseases. Used for qualitative analysis, and quantification of endothelial cells of the cornea. Used for localizing of filamentary fungal elements in the corneal stroma in cases of keratomycosis.
- Materials science: Confocal microscopes are used to study the structure and properties of materials, including polymers, ceramics, and metals. Used to determine the age of the Magdalen papyrus.
- Microelectronics: Confocal microscopes are used to study the structure and properties of microelectronic devices, including transistors and integrated circuits.
- Art conservation: Confocal microscopes are used to study the structure and composition of artwork, including paintings and sculptures. It is also used for optical scanning and recovery of damaged historical audio.
- Geology: Confocal microscopes are used to study the structure and composition of geological samples, including minerals and rocks.
- Agriculture: Confocal microscopes are used to study the structure and function of plants, including their cells and tissues.
- Industrial inspection: Confocal microscopes are used to inspect the quality and consistency of industrial products, including food, pharmaceuticals, and consumer goods. It also widely used in the pharmaceutical industry to control the quality and uniformity of the drug distribution.
- Used in 3D optical data storage systems.
Overall, confocal microscopes are an important tool for researchers and scientists who need high-resolution images of small samples and structures.
Advantages of Confocal Microscopy
There are several advantages to using confocal microscopy, including:
- High spatial resolution: Confocal microscopes are able to produce high-resolution images of small samples and structures, making them ideal for studying cells, tissues, and other small samples.
- Increased depth of field: Confocal microscopes use a pinhole to block out-of-focus light, which increases the depth of field of the image. This allows for a clearer image of samples with varying depths, such as tissues.
- Reduced photobleaching: Confocal microscopes use a laser to excite the sample, which reduces the amount of light needed to produce an image. This reduces the photobleaching of samples, which can occur when samples are exposed to high levels of light for extended periods of time.
- Reduced background noise: The pinhole in a confocal microscope blocks out-of-focus light, which reduces the background noise in the image. This allows for a clearer, more detailed image of the sample.
- Live cell imaging: Confocal microscopes are often used to image live cells, as they do not produce a lot of heat and do not damage the sample.
- Multiplexing capabilities: Confocal microscopes can be used to study multiple samples or multiple fluorescence labels at the same time, allowing researchers to study multiple aspects of a sample simultaneously.
- Accuracy: Because the Confocal microscope analyses the image from one optical point to the next, there is no interference from scattered light from other portions of the specimen, resulting in a more accurate image.
- Living and Dead Cell: It can be used to examine both living and dead cells
- It can be used to collect optical portions in series.
- It illuminates the focus points consistently.
- Using a factor known as the zoom factor, they electronically adjust the magnification without changing the objectives.
- It produces 3D picture sets.
Overall, confocal microscopy is a powerful tool for producing high-resolution, detailed images of small samples and structures, and is widely used in a range of research and industrial applications.
Disadvantages of Confocal Microscopy
There are also some disadvantages to using confocal microscopy, including:
- Complexity and cost: Confocal microscopes are more complex and expensive than other types of microscopes, such as light microscopes. This can make them difficult for some researchers to access or afford. It is costly to create the UV light that Confocal Microscopes employ. They are also expensive to produce and acquire.
- Limited penetration depth: Confocal microscopes are not able to image very deep within tissues, as the laser beam used to excite the sample cannot penetrate very far into the sample.
- Low light sensitivity: Confocal microscopes are less sensitive to low levels of light than other types of microscopes, such as widefield microscopes. This can make it difficult to image samples with low levels of fluorescence.
- Sample preparation: Confocal microscopy requires the sample to be labeled with a fluorescent dye or protein, which can be time-consuming and may alter the sample’s properties.
- Limited field of view: Confocal microscopes have a small field of view compared to other types of microscopes, which can make it difficult to study large samples or structures.
Overall, while confocal microscopy is a powerful tool for producing high-resolution images of small samples and structures, it has some limitations that may make it less suitable for certain applications.
Confocal microscope cost/confocal microscope prices
The cost of a confocal microscope can vary widely depending on the specific features and capabilities of the instrument. Single-photon confocal microscopes can cost anywhere from a few thousand dollars to over $100,000, while two-photon and multiphoton confocal microscopes can cost significantly more. Superresolution microscopes, which use techniques such as STED or structured illumination microscopy to achieve a spatial resolution beyond the diffraction limit of light, can cost even more.
In general, the cost of a confocal microscope will depend on the level of detail and resolution required, as well as the complexity of the sample being studied. More advanced confocal microscopes with higher resolution and multiplexing capabilities will typically be more expensive. It is important to carefully consider the specific needs of your research or application before choosing a confocal microscope, as investing in a more expensive instrument may be necessary to obtain the desired level of detail and resolution.
What is Confocal Raman Microscopy?
Confocal Raman microscopy is a type of microscopy that uses the Raman effect to produce images of a sample. The Raman effect is the inelastic scattering of light that occurs when a photon interacts with a molecule, resulting in a change in the energy of the photon. This change in energy is characteristic of the molecule and can be used to identify and characterize the chemical makeup of the sample.
Confocal Raman microscopy combines the principles of confocal microscopy with Raman spectroscopy, which is a technique used to study the vibrational, rotational, and other low-frequency modes of a molecule. Confocal Raman microscopy uses a laser beam to scan over the sample and produce a high-resolution image, similar to a confocal microscope. However, rather than using fluorescence to produce the image, confocal Raman microscopy uses the Raman effect to create a “Raman map” of the sample. This map can be used to identify and characterize specific chemical components within the sample.
Confocal Raman microscopy is a powerful tool for studying the chemical makeup of small samples and structures, and is used in a wide range of applications, including biology, materials science, and industrial inspection.
Confocal microscope image
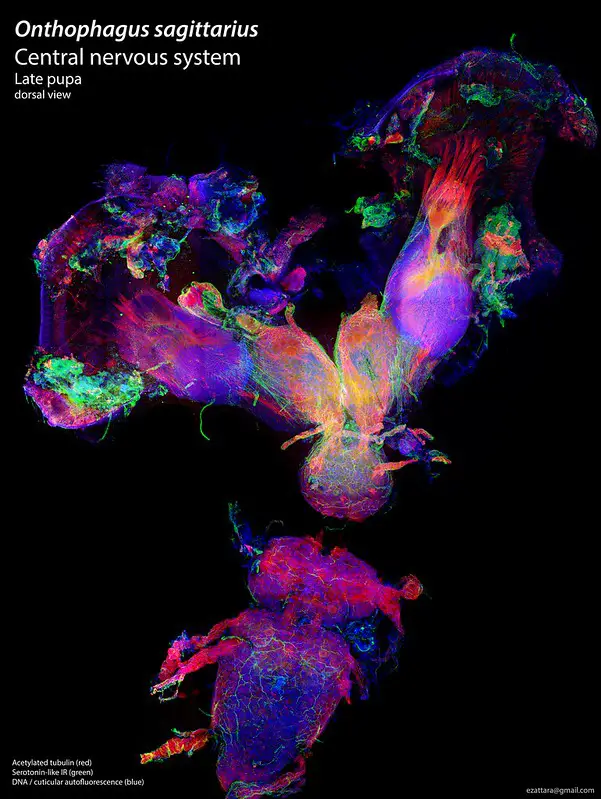
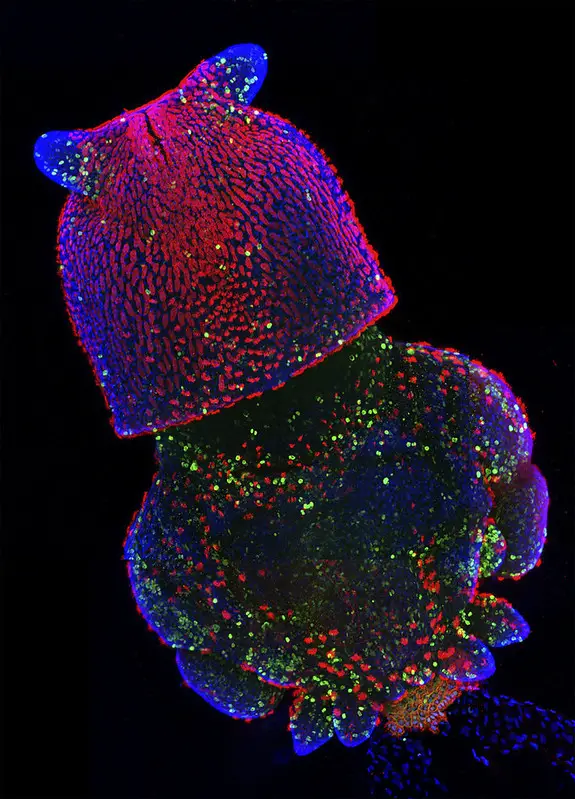
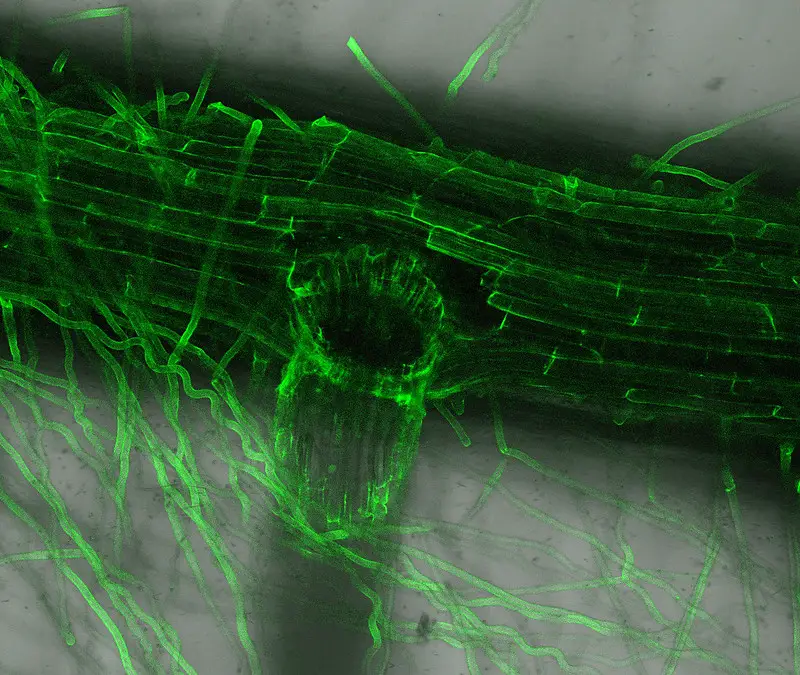
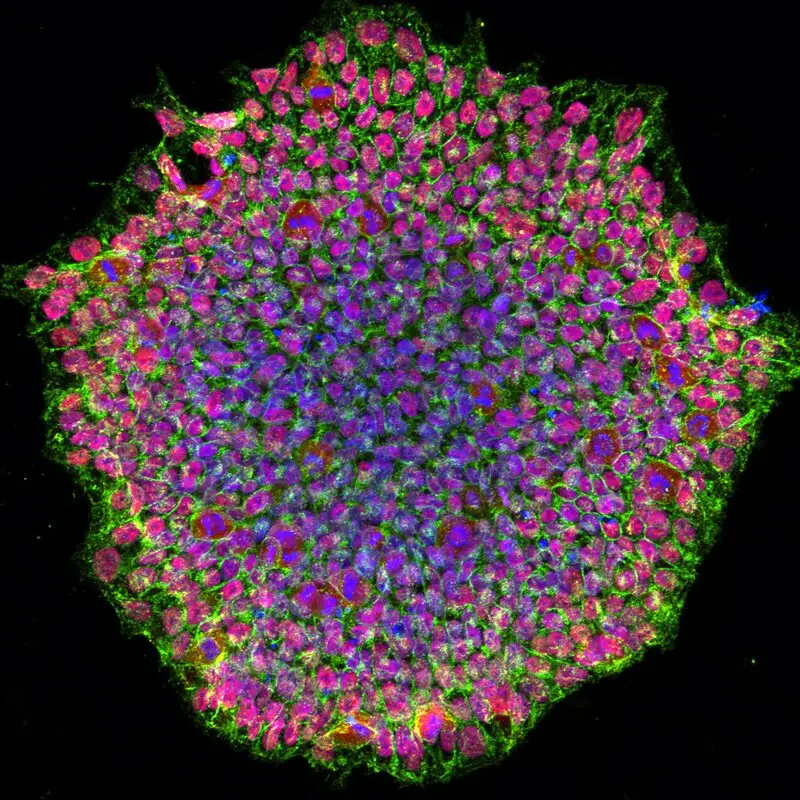
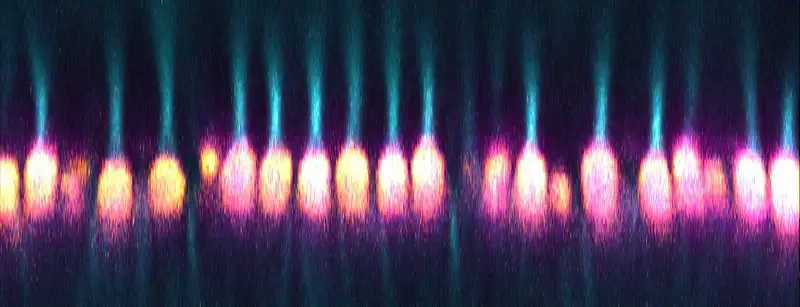
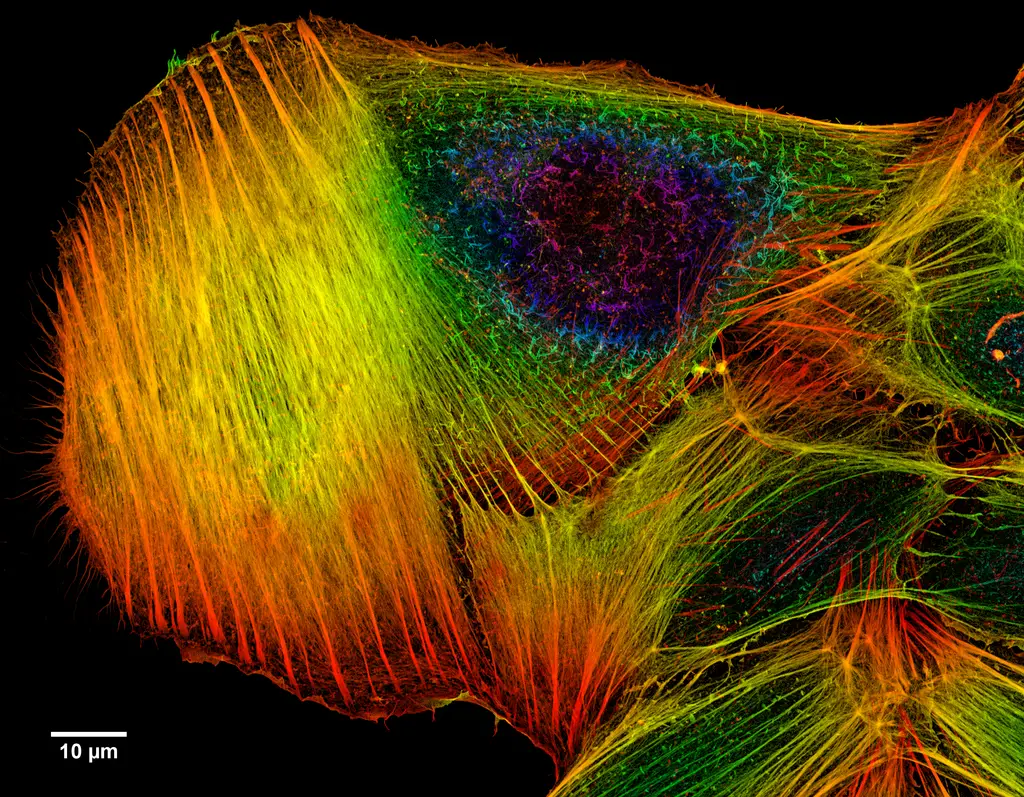
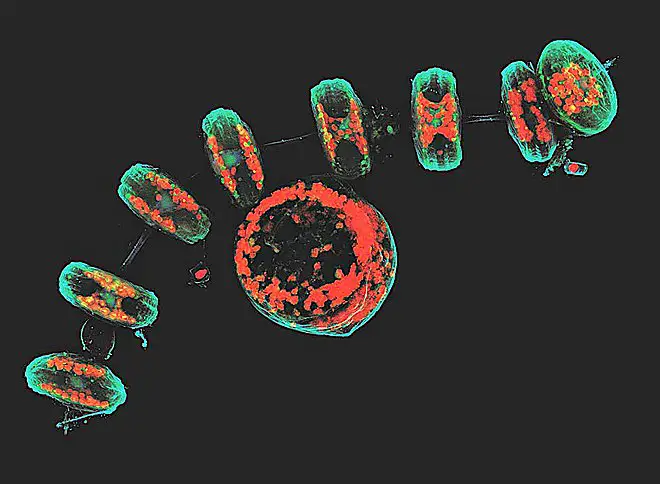
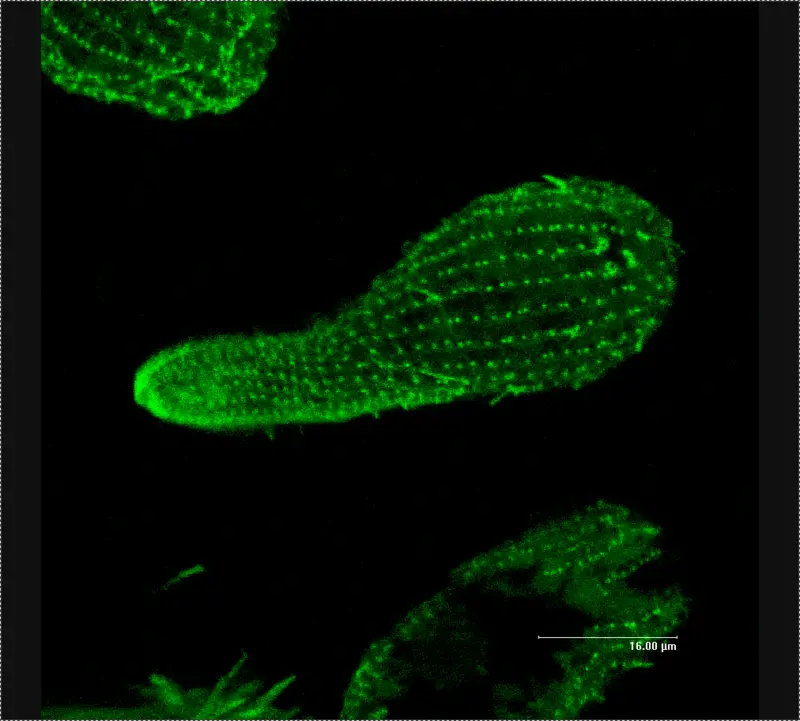
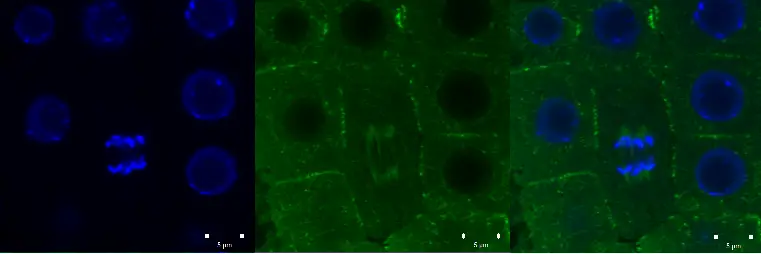
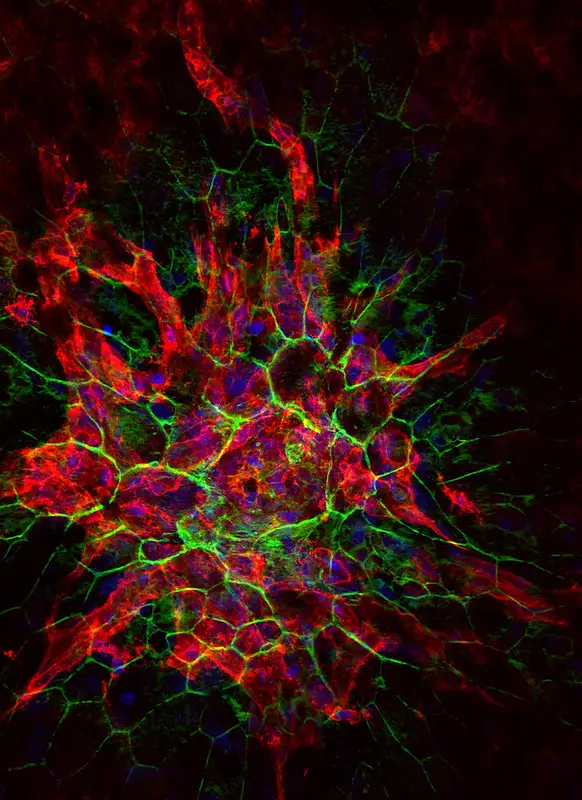
Check more Confocal microscope image – https://www.flickr.com/search/?text=Confocal%20microscope%20image
Reference
- Elliott AD. Confocal Microscopy: Principles and Modern Practices. Curr Protoc Cytom. 2020 Mar;92(1):e68. doi: 10.1002/cpcy.68. PMID: 31876974; PMCID: PMC6961134.
- https://bitesizebio.com/19958/what-is-confocal-laser-scanning-microscopy
- https://www.microscopemaster.com/confocal-microscope.html
- https://ibidi.com/content/216-confocal-microscopy
- http://www.physics.emory.edu/faculty/weeks//confocal/
- https://www.olympus-lifescience.com/en/microscope-resource/primer/techniques/confocal/confocalintro/
- https://www.future-science.com/doi/full/10.2144/000112089
- https://www.microscopyu.com/techniques/confocal/introductory-confocal-concepts
