Table of Contents
What is Dendritic cell?
- Dendritic cells (DCs) are antigen-presenting cells generated from progenitors in bone marrow and are widely dispersed throughout the body.
- DCs perform immune-surveillance for external and endogenous antigens and the subsequent activation of naive T cells, which results in a variety of immune responses. Different growth factors and cytokines, including GM-CSF, M-CSF, Flt3, and TGF-, can affect the differentiation and function of DCs, resulting in a wide range of DCs with distinct functional capabilities.
- Thus, DCs are categorised as plasmacytoid DCs (pDCs), conventional DCs (cDCs), and monocyte-derived DCs (DCs) (mDCs).
- The cDCs can be split into two functional states: immature and mature. Mature DCs are experts in antigen presentation, whereas immature DCs are experts in antigen uptake and processing.
- Observations indicate that juvenile cDCs can generate immunological tolerance, whereas mature cDCs can induce Th2 or Th1 immune responses.
- It is important to note that distinct subpopulations of DCs might release distinct cytokine patterns, leading in the development of distinct immune responses.
- In addition, DCs are involved in the pathophysiology of a number of disorders, including contact hypersensitivity, autoimmune diseases, and cancer, but they can also be utilised as therapeutic agents in these conditions.
- Dendritic cells (DCs) are antigen-presenting cells with a unique shape and expression of CD11c and major histocompatibility complex class II molecules, among others (MHCII).
- In addition, DCs can identify infections, indications of tissue injury, and tumour antigens before migrating to secondary lymphoid organs, where they present antigens and activate T cells. DCs can stimulate the development of several immune responses, including Th1, Th2, Treg, and Th17.
- There is a wide range of DCs with distinct phenotypes and localizations that create a cellular system that is responsible for immune monitoring and is spread throughout the body.
- DCs are categorised as conventional DCs (cDCs), plasmacytoid DCs (pDCs), and monocyte-derived DCs (DCs) (mDCs).
Location of Dendritic cell
- There are numerous forms of DCs with distinct characteristics and locations. DCs are typically classified as conventional, plasmacytoid, or monocyte-derived.
- cDCs can be categorised as cDC1s and cDC2s. CD8+ CD103+ in mice and BDCA3+ (CD141+) in humans are defining characteristics of cDC1s.
- In mice, the phenotype of CDC2 is CD11b+ CD4+ CD8, but in humans it is BDCA1+ (CD1c+).
- In mice, plasmacytoid pDCs express B220, mPDCA1, and Siglec-h, whereas in humans, they express BDCA4 and BDCA2.
- mDCs are a subset of DCs produced from monocytes that only arise in response to inflammation.
- Langerhans cells, which are normal residents of the epidermis and epithelia, are not regarded to be of the same lineage as the DCs described above since they derive from precursor cells that moved to the skin before birth and developed into LCs during the first week of life.
- In terms of their origin, DCs are distinct from bone marrow progenitor cells, which express Flt3 and occasionally M-CSFR.
- DCs are abundant in lymphoid tissues and epithelia. Additionally, DCs can express different molecular markers based on their location.
- Consequently, pDCs, CD1s, and CD2s can be found in various tissues of the body. It is essential to consider the phenotype and location of DCs in relation to their function on a given tissue.
- DCs are sentinel cells responsible for the identification of pathogens and indications of tissue damage, which promotes their migration to lymphoid organs to activate various subsets of T, natural killer (NK), NKT, and B lymphocytes.
- Long-term research has also revealed that the inflammatory or tolerogenic milieu created by the cytokines present in tissues is crucial for determining the functions that DCs can perform.
- In addition to the cytokines involved in their activation, it is crucial to understand the diverse types of DCs found in lymphoid organs, skin, the gastrointestinal tract, and the blood.
DCs in lymphoid organs
Lymph nodes
- One of the subsets of DCs found in lymph nodes is CD103+ migrating cDCs from peripheral tissues, which exhibit a mature phenotype characterised by an increase in MHCII, CD80, CD86, and CD40.
- There are also two types of resident DCs: CD8+, CD4+, or CD11b+ cells with an immature phenotype, unless the lymph node contains an inflammatory environment.
Spleen
- All DCs in the spleen are CD8+ and comprise around 20% of all spleen cells. DCs are categorised into subgroups based on CD11b expression.
Thymus
- At least three groups of DCs are present in the thymus: CD8+ cDCs (50%) Sirp+ cDCs (20%) and pDCs (30%).
Blood
- Several cell lines, including granulocytes, monocytes, and lymphocytes, can be identified in the blood, and in order to examine blood DCs, several lineage markers (Lin), such as CD3, CD19, CD14, CD20, and CD56, are employed to segregate populations of DCs using flow cytometry assays. Thus, cDC and pDC populations may be recognised in blood because they are Lin.
Skin
- In the epidermis and dermis, various types of DCs are present. LCs comprise 2% to 4% of the epidermis and express high amounts of Langerin (CD207), CD45, and low concentrations of CD11c and MHCII.
Gut
- Intestinal DCs are found in the lamina propria of the intestinal mucosa, particularly in Peyer’s patches.
- These cells are often CD103+, CD8+, and CD207+, express modest levels of MHCII, and have been seen to proliferate when Flt3 levels are high.
- A second group of DCs also resides in the lamina propria, but expresses the markers CD103 and CD11b to a lesser extent than CD103.
- These DCs can also be seen in the muscle layer of the gastrointestinal tract, therefore they may be mistaken for CD11b+ macrophages.
Structure of Dendritic cells
- Dendritic cells are bigger antigen-presenting cells with comparable dendrite-like cytoplasmic extensions to nerve cell dendrites.
- The morphology of the cells is uneven, and they include phase-dense granules, an irregular nucleus, and a tiny nucleolus.
- The cell’s protrusions stretch in multiple directions from the cell body and are responsible for patrolling for invading infections.
- The development of unique dendrites by dendritic cells is essential for the morphological identification of DC in a blood sample.
- There are no filaments in the cytoplasm of dendritic cells, however organelles such as mitochondria and Golgi complex can be detected.
- In a similar manner, dendritic cells at various stages of maturity contain distinct types of granules. The size and distribution of granules in dendritic cells vary, although melanin granules are the most common granules seen in dendritic cells.
Dendritic cell maturation
- Derived from progenitor cells in the bone marrow, immature DCs travel to virtually all lymphoid and nonlymphoid tissues in the body, such as the skin, lungs, and intestines.
- On the road from common DC progenitors to mature DCs, numerous transcription factors, signalling molecules, growth factors, cytokines, chemokines, and adhesion receptors have been implicated.
- In addition, immature DCs receive and process additional maturation signals by identifying damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) in their local environments via a variety of surface pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and C-type lectin receptors (CLRs).
- This detection of damaged cells or infections enables DCs to protect the integrity of the body through their sentinel-like capabilities.
- In response to chemotactic signals, maturing tissue DCs modify their surface chemokine receptor and adhesion molecule profiles in accordance with microenvironmental cues and migrate to secondary lymphoid organs.
- Immature resident or entering nonresident DCs can be further activated and differentiated into mature, functional DCs within lymphoid tissues.
- Mature DCs are able to process and deliver antigens in the context of self-MHC antigens to CD4+ or CD8+ T cells that are not yet activated. This results in either the activation of primary immunological responses against foreign antigens or the downregulation of T cell reactivity against self antigens.
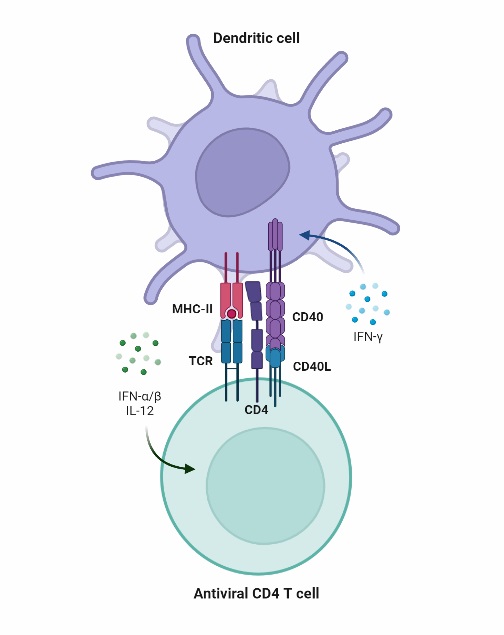
- Mature DCs stimulate naive T cells via their enhanced surface expression of peptide-loaded major histocompatibility complex (MHC) antigens, costimulatory (or coinhibitory) receptors and ligands, such as CD80 and CD86, and the production of cytokines such IL-6, IL-12p70 or interferons (IFNs).
- T cells can fine-tune the characteristics of mature DCs. Responding T cells may regulate DCs through CD40-CD40L interactions or T cell-derived cytokines such as IL-4 or IFN-γ, for example.
- Thus, T cells may additionally train professional APCs, which can induce various types of immunity or tolerance reliant on T cells.
Mechanism of Dendritic Cells in Immunity – How Dendritic cells work against pathogens? (Immunity)
- Dendritic cells serve an important role in the immune system. In our innate and adaptive immune processes, they function as phagocytes, antigen-presenting cells, and accessory cells (messengers and activators).
- One of three types of antigen-presenting cells, dendritic cells develop from precursor cells in bone marrow and lymph tissue.
- Antigen-presenting cells allow T lymphocytes to identify and eliminate antigens, which are foreign or dangerous substances.
- Without antigen-presenting cells, T lymphocytes would not respond to potentially harmful particles that enter the body or are created within it. Antigen-presenting cells (APCs) are composed of dendritic cells, macrophages, and B-cells.
- Dendritic cells are dispersed throughout the body and cluster in places that contribute to rapid immunological responses, including the lungs, gut, blood, and lymphoid tissues. These tissues must endure constant antigen assaults.
- Dendritic cells first arise in various tissues and organs as immature cells. Only after antigen capture do they mature; only after antigen capture are they referred to as antigen-presenting cells (APCs).
- As antigen-presenting cells, mature dendritic cells move to lymph nodes and present their acquired antigens to T lymphocytes.
- Dendritic cells play a crucial role in the innate immune system, where they perform antigen monitoring in the form of endogenous poisons and exogenous foreign chemicals.
- Antigens, or the surface proteins of antigens, stimulate an immune response. This is because certain molecular patterns on antigens or damaged cell membranes make them identifiable.
Dendritic Cell Function in Innate Immune System
- Before, at, or shortly after antigen exposure, the innate immune system provides a nonspecific mechanism of defence.
- Antigens include viruses and bacteria as well as substances generated by injured cells when they are burned, scraped, oxygen-deprived, or otherwise traumatised.
- The majority of vertebrates have several defences. These include the skin, stomach acid, mucus in the airways, the blood-brain barrier, perspiration, and tears. The innate immune system includes these structural barriers.
- If an antigen penetrates the body and survives these anatomical barriers, the subsequent phase of the innate immune system’s defence mechanism is activated.
- PAMPs (pathogen-associated molecular patterns) are common patterns of molecules found on the outer surfaces of all microorganisms.
- When our own cells are injured, dying, or dead, they contain DAMPs (damage-associated molecular patterns).
- The figure below depicts the invasion of pink antigen bacteria with LPS (lipopolysaccharide) PAMPs on their outer membranes.
- PAMPS are detected by LPS receptors on the surface of macrophages. In reaction to detection, the macrophage secretes cytokines. Cytokines alert other cells that an assault is imminent. Bacteria are captured and digested by the macrophage (phagocytosis).
- Numerous cell types, including dendritic cells, contain receptors for pattern recognition (PRRs). A single pattern recognition receptor can distinguish patterns associated with both pathogens and damage.
- The initial phase following PAMP or DAMP identification is the inflammatory response. By increasing blood flow to the region, more white blood cells are dispatched to eliminate the intruders.
- Many bacteria die at temperatures above the normal body temperature, therefore a fever is beneficial. Every day, coughing and sneezing rid us of innumerable diseases.
- Chemical agents such as histamine, prostaglandin, and bradykinin are released by white blood cells. These substances dilate nearby blood arteries and attract additional phagocytic cells.
- Phagocytic cells engulf and digest foreign particles and poisons. Like macrophages and neutrophils, dendritic cells are phagocytes.
- Macrophages and neutrophils scavenge and eliminate toxic or foreign particles and emit substances that attract other white blood cells.
- Dendritic cells engage in phagocytosis and store information from ingested particles, which can initiate adaptive (acquired) immune responses.
- Cytokines are molecules that send signals. This category contains interferons, which you may recognise as the active ingredient in certain antiviral medications.
- Cytokines induce or inhibit cellular proliferation, apoptosis, inflammation, differentiation, and migration.
- As autocrine messengers, they influence their own cell; as paracrine messengers, they influence neighbouring cells; and as bloodstream messengers, they influence distant destinations.
Dendritic Cells Function in Innate Immunity to Adaptive Immunity
- After antigen capture, the most critical function of dendritic cells is the activation of naive T lymphocytes.
- Similar to dendritic cells, T-lymphocytes (T cells) change their state when they encounter and recognise an antigen.
- The original form of a mature T-lymphocyte as it leaves the lymphoid organ of the thymus is characterised as nave.
- After contacting an antigen-presenting dendritic cell (or other APC type), a naive T-lymphocyte develops into an effector T cell.
- As an effector cell, it is capable of destroying the presenting antigen. Effector T cells are frequently referred to as cytotoxic T cells.
- The majority of effector T cells die once an antigen has been eliminated; however, a tiny percentage develops into memory T cells.
- Memory T-cells underpin our acquired (adaptive) immune response. They remember the antigen and will fight it if it re-enters the body.
Dendritic Cells in Cancer Therapy
- The application of dendritic cells in cancer therapy is the subject of extensive scrutiny. To move toward individualised cancer treatment in the form of a vaccine or even a prophylactic pill, we must have a far deeper understanding of how they function.
- Vaccination against cancer is a sort of immunotherapy. Relevant is the ability of dendritic cells to govern our immune responses, as somewhere along the cancer path our immune systems fail to recognise and destroy aberrant cells.
- As dendritic cells – and all antigen-presenting cells – are susceptible to being deceived by cancer cells, loading them with a tumour antigen outside the body before injecting them into the body could produce the desired immune response. No longer would cancer cells be allowed to flourish unchecked.
- Tumor cells inhibit the action of the immune system, including dendritic cell activity. Tumor cells conceal themselves behind their own anti-inflammatory cytokines.
- Not only are they difficult for APCs to identify, but they also inhibit the normal inflammatory response of healthy immune cells. Cancer cells can proliferate unimpeded and undetected.
- Dendritic cells are straightforward to cultivate in the laboratory. This makes them an attractive candidate for use in cancer vaccinations.
- Current research investigates whether preloading dendritic cells with specific cancer antigens in the laboratory and then injecting them into the body could one day serve as a cancer treatment.
- As the mechanism of immunity is not yet fully understood, and as individual physiological parameters react extremely differently to therapy, the findings of clinical trials are inconsistent.
Types of Dendritic Cell
There are three major dendritic cell types in humans. These are typical dendritic cells, plasmacytoid dendritic cells, and epidermal (dermal) dendritic cells.
1. Plasmacytoid Dendritic Cells
- Plasmacytoid dendritic cells are derived from lymphoid organs (lymph nodes, thymus, spleen, and tonsils) and bone marrow. Lymph vessels and nodes (green) travel along the same pathways as our blood vessels (blue and red).
- Plasmacytoid dendritic cells generate cytokines such as interferon type I and tumour necrosis factor. The generation of natural killer cells, B lymphocytes, and myeloid dendritic cells is stimulated by cytokines. They also possess exceptional antiviral effects.
2. Conventional Dendritic Cells
- Once in the bloodstream, conventional dendritic cells, also known as classical or myeloid dendritic cells, develop into three distinct cell types.
- These forms reach the lungs, digestive tract, liver, and kidneys.
3. Epidermal Dendritic Cells
- There are various types of epidermal and dermal dendritic cells on and within the skin. All epidermal dendritic cells stimulate epidermal and dermal T-cells.
- An illustration is the Langerhans cell (LC). Langerhans cells travel from the epidermis to the lymph nodes, carrying an antigen to present to a naive T cell.
- They accomplish this by promoting the differentiation of T cells into T helper cells. This differentiation takes place in glands near to the antigen location. T helper cells assist B cells in producing antibodies, as their name implies.
- Other types of epidermal dendritic cells are generated in the bone marrow and undergo differentiation in the blood and skin.
- The majority of our understanding of these cells is derived from rodent models, typically mice. We do not fully understand how these cells function in the human body.
4. Monocyte-DCs
- During inflammation, monocyte-derived dendritic cells create a new subpopulation of dendritic cells.
- Monocytes that develop into dendritic cells are CCR2-positive monocytes that move to the site of inflammation from the bone marrow.
- During bacterial infections, these monocyte-DCs provide protection to the cells of the innate immune system.
5. Migratory DCs
- Monocyte-derived dendritic cells generate a new subset of dendritic cells during inflammation.
- Monocytes that transform into dendritic cells are CCR2-positive monocytes that migrate from the bone marrow to the site of inflammation.
- During bacterial infections, these monocyte-DCs safeguard the innate immune cells.
What is blastic plasmacytoid dendritic cell neoplasm?
- Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive type of blood cancer that affects the plasmacytoid dendritic cells (PDCs). PDCs are a type of immune cell that play an important role in the body’s immune response to viruses and other pathogens.
- BPDCN is characterized by the abnormal growth and accumulation of malignant PDCs in the bone marrow, blood, and other tissues. The exact cause of BPDCN is not fully understood, but it is thought to arise from genetic mutations that lead to uncontrolled cell growth and proliferation.
- The symptoms of BPDCN can vary depending on the location and extent of the disease, but may include skin lesions, swollen lymph nodes, fever, fatigue, and bone pain. Diagnosis of BPDCN typically involves a combination of physical examination, blood tests, bone marrow biopsy, and imaging studies.
- Treatment options for BPDCN may include chemotherapy, stem cell transplantation, and targeted therapies. However, BPDCN is often difficult to treat and has a poor prognosis, with a high risk of relapse and progression.
- Due to its rarity and aggressive nature, BPDCN is an active area of research, with ongoing efforts aimed at developing new and more effective treatments for this devastating disease.
What is dendritic cell vaccine?
- A dendritic cell vaccine is a type of cancer immunotherapy that aims to harness the power of the body’s own immune system to fight cancer. Dendritic cells are a type of immune cell that play a key role in initiating and regulating the immune response.
- In a dendritic cell vaccine, dendritic cells are harvested from a patient’s blood or bone marrow and cultured in the laboratory. The dendritic cells are then loaded with tumor antigens, which are proteins found on the surface of cancer cells that can stimulate an immune response.
- Once the dendritic cells have been loaded with tumor antigens, they are injected back into the patient, where they migrate to the lymph nodes and activate other immune cells, such as T cells, to attack the cancer.
- Dendritic cell vaccines are still an experimental treatment and are not yet widely available. They are currently being tested in clinical trials for a variety of different types of cancer, including melanoma, prostate cancer, and glioblastoma (a type of brain cancer).
- While dendritic cell vaccines have shown promise in early clinical trials, more research is needed to fully understand their potential as a cancer treatment. Some challenges associated with dendritic cell vaccines include the difficulty of producing sufficient quantities of dendritic cells, as well as the potential for the immune system to become desensitized to the tumor antigens over time.
What is dendritic cell markers?
- Dendritic cells are a type of immune cell that play a crucial role in initiating and regulating immune responses. They express specific proteins or molecules on their surface, which are known as dendritic cell markers. These markers can be used to identify and distinguish dendritic cells from other cell types in the body.
- Some of the commonly used dendritic cell markers include CD11c, CD80, CD86, and MHC class II molecules. CD11c is a surface protein that is expressed on the majority of dendritic cells and is often used as a marker to identify these cells. CD80 and CD86 are co-stimulatory molecules that are expressed on the surface of activated dendritic cells and are important for initiating immune responses. MHC class II molecules are responsible for presenting antigens to T cells, and their expression on the surface of dendritic cells is critical for initiating T cell activation.
- Dendritic cell markers are often used in research to study the function and behavior of dendritic cells in different disease states or experimental conditions. Additionally, they can be used as targets for developing therapies that specifically target dendritic cells, such as vaccines or immunotherapies.
What is follicular dendritic cell sarcoma?
- Follicular dendritic cell sarcoma (FDC sarcoma) is a rare type of cancer that arises from follicular dendritic cells, which are specialized cells found in lymphoid tissues such as lymph nodes, tonsils, and spleen. FDC sarcoma can occur at any age, but it is more common in middle-aged adults.
- The exact cause of FDC sarcoma is not fully understood, but it is thought to arise from genetic mutations that cause the uncontrolled growth and division of follicular dendritic cells. The symptoms of FDC sarcoma can vary depending on the location of the tumor, but they often include swelling, pain, and other signs of inflammation in the affected area.
- Diagnosis of FDC sarcoma typically involves a combination of imaging tests, such as CT scans or MRI, and a biopsy to confirm the presence of cancerous cells. Treatment options for FDC sarcoma may include surgery to remove the tumor, radiation therapy, and chemotherapy. Because FDC sarcoma is a rare and complex disease, it is important to receive care from a specialized team of healthcare providers who have experience in treating this type of cancer.
What is dendritic cell therapy?
- Dendritic cell therapy is a type of immunotherapy that uses a patient’s own dendritic cells to stimulate and enhance the immune system’s ability to recognize and destroy cancer cells or infectious agents. Dendritic cells are a type of immune cell that plays a key role in initiating and regulating immune responses.
- In dendritic cell therapy, a patient’s dendritic cells are isolated from their blood or tissue sample and then exposed to cancer-specific or infectious agent-specific antigens in a laboratory. The activated dendritic cells are then infused back into the patient, where they can stimulate the patient’s immune system to recognize and attack cancer cells or infectious agents.
- Dendritic cell therapy is still an experimental treatment and is currently being tested in clinical trials for various types of cancer and infectious diseases. It is often used in combination with other treatments, such as chemotherapy or radiation therapy.
- The goal of dendritic cell therapy is to enhance the immune system’s ability to specifically target cancer cells or infectious agents while minimizing damage to healthy cells. While dendritic cell therapy has shown promise in early clinical trials, more research is needed to determine its safety and effectiveness as a cancer or infectious disease treatment.
What is epidermal dendritic cell?
- Epidermal dendritic cells, also known as Langerhans cells, are specialized immune cells found in the skin’s outermost layer, the epidermis. They are named after the German physician Paul Langerhans, who discovered them in the 19th century.
- Epidermal dendritic cells are part of the body’s first line of defense against pathogens that enter through the skin. They play a critical role in initiating and regulating immune responses to skin infections, allergic reactions, and other inflammatory skin conditions.
- Epidermal dendritic cells are characterized by the presence of specific proteins or molecules on their surface, such as CD1a and Langerin. These markers can be used to identify and distinguish epidermal dendritic cells from other cell types in the skin.
- When an epidermal dendritic cell encounters a foreign substance, such as a virus or bacteria, it takes up the antigen and migrates to nearby lymph nodes to present it to other immune cells, such as T cells and B cells. This process activates an immune response against the invading pathogen.
- Epidermal dendritic cells also play a role in the development of skin allergies and autoimmune skin disorders. In these conditions, epidermal dendritic cells can become activated and stimulate an immune response against harmless substances, leading to skin inflammation and damage.
- Research on epidermal dendritic cells is ongoing, and further understanding of their function and behavior may lead to new treatments for skin diseases and infections.
Functions of Dendritic cells
Dendritic cells play several crucial functions in the immune system. Here are some key roles of dendritic cells:
- Antigen Presentation: Dendritic cells are specialized antigen-presenting cells. They capture antigens from pathogens or other sources and process them. Dendritic cells then present these antigens on their cell surface, presenting them to other immune cells, such as T-cells, to initiate an immune response.
- Activation of T-Cells: Dendritic cells are responsible for activating T-cells, a type of white blood cell essential for adaptive immunity. Dendritic cells present antigens to T-cells, triggering their activation. This interaction is crucial for T-cells to recognize and respond effectively to specific pathogens or foreign substances.
- Differentiation of T-Helper Cells: Dendritic cells are involved in the differentiation of T-helper cells, a subset of T-cells. T-helper cells play a vital role in coordinating the immune response by releasing cytokines and activating other immune cells. Dendritic cells influence the differentiation of T-helper cells into specific subtypes, such as Th1, Th2, or Th17, depending on the nature of the antigen encountered.
- Activation of Natural Killer Cells: Dendritic cells contribute to the activation of natural killer (NK) cells, a type of cytotoxic lymphocyte in the innate immune system. Through the secretion of various cytokines, dendritic cells can stimulate NK cells, enhancing their ability to target and eliminate infected or cancerous cells.
- Regulation of Regulatory T Cells: Dendritic cells have been observed to play a role in the functional control of regulatory T cells (Tregs). Tregs are a subset of T-cells that help maintain immune homeostasis and prevent excessive immune responses. Dendritic cells can interact with Tregs, influencing their suppressive function and contributing to immune regulation.
Overall, dendritic cells are critical in initiating and coordinating immune responses. Their ability to capture, process, and present antigens, activate T-cells, influence T-helper cell differentiation, activate NK cells, and regulate Tregs makes them essential players in both innate and adaptive immunity.
FAQ
What is the result when a dendritic cell phagocytizes a microbe and processes it?
When a dendritic cell phagocytizes a microbe and processes it, the dendritic cell presents fragments of the microbe, called antigens, on its cell surface using a molecule called major histocompatibility complex (MHC). The dendritic cell then migrates to nearby lymph nodes, where it interacts with T cells and B cells to initiate an immune response against the microbe.
The presentation of antigens by dendritic cells is a crucial step in the activation of the adaptive immune response. T cells recognize and respond to the presented antigens by proliferating and differentiating into effector T cells, which can directly kill infected cells or secrete cytokines to activate other immune cells.
B cells can also interact with dendritic cells to produce antibodies against the presented antigens, which can neutralize or eliminate the microbe.
In summary, when a dendritic cell phagocytizes and processes a microbe, it plays a critical role in initiating and coordinating the immune response against the microbe.
Where in the lymph node is a dendritic cell most likely associated with a b or t lymphocyte?
Dendritic cells can interact with both B cells and T cells in different regions of the lymph node.
B cells are primarily found in the follicles of the lymph node, which are located in the outer cortex. Dendritic cells can be found in the interfollicular regions and the T cell zones surrounding the follicles.
T cells are primarily found in the paracortex of the lymph node, which is located between the cortex and the medulla. Dendritic cells are also present in the paracortex and can interact with T cells to initiate and coordinate the adaptive immune response.
Overall, dendritic cells can be found in various regions of the lymph node and can interact with both B cells and T cells to initiate and coordinate the immune response against invading pathogens.
What is the result when a dendritic cell phagocytizes a microbe and processes it?
When a dendritic cell phagocytizes a microbe and processes it, the dendritic cell presents fragments of the microbe, called antigens, on its cell surface using a molecule called major histocompatibility complex (MHC). The dendritic cell then migrates to nearby lymph nodes, where it interacts with T cells and B cells to initiate an immune response against the microbe.
The presentation of antigens by dendritic cells is a crucial step in the activation of the adaptive immune response. T cells recognize and respond to the presented antigens by proliferating and differentiating into effector T cells, which can directly kill infected cells or secrete cytokines to activate other immune cells.
B cells can also interact with dendritic cells to produce antibodies against the presented antigens, which can neutralize or eliminate the microbe.
In summary, when a dendritic cell phagocytizes and processes a microbe, it plays a critical role in initiating and coordinating the immune response against the microbe.
What is dendritic cell licensing?
Dendritic cell licensing refers to the process by which dendritic cells are activated and matured by signals from other immune cells or from the environment. The process of licensing enables dendritic cells to effectively stimulate T cells and initiate an adaptive immune response.
Dendritic cells that are not licensed, or immature dendritic cells, are not as effective at activating T cells and may even induce immune tolerance, which can be detrimental in the context of infectious diseases or cancer.
Licensing can occur through various mechanisms, such as the recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) on dendritic cells, or through the interaction of dendritic cells with activated T cells, which can provide a feedback loop to further enhance dendritic cell activation.
Once licensed, dendritic cells upregulate the expression of co-stimulatory molecules and cytokines, which are necessary for effective T cell activation and differentiation. In this way, dendritic cell licensing plays a critical role in initiating and coordinating the adaptive immune response against invading pathogens or cancer cells.
How big is a dendritic cell in nm?
The size of dendritic cells can vary depending on their subtype and activation state. Generally, dendritic cells are considered to be relatively large immune cells, with a typical diameter ranging from 10 to 30 micrometers (μm), which is equivalent to 10,000 to 30,000 nanometers (nm). However, dendritic cells can also exhibit processes or extensions that can increase their overall size and shape complexity. The size of dendritic cells may also change during activation or maturation, with increased surface area and complexity to better engage and communicate with other immune cells.
How dendritic cells determine whether antigens should activate or anergize a t cell?
Dendritic cells determine whether antigens should activate or anergize a T cell through a process known as antigen presentation, which involves the display of antigenic fragments on the dendritic cell surface in association with major histocompatibility complex (MHC) molecules.
If the antigenic fragment is recognized by the T cell receptor (TCR) on a T cell, and if the dendritic cell also provides co-stimulatory signals (such as B7-1/B7-2), then the T cell is activated and begins to proliferate and differentiate into effector T cells that can eliminate the pathogen or infected cells.
However, if the dendritic cell does not provide co-stimulatory signals or if it presents the antigenic fragment in the absence of inflammation or danger signals, then the T cell may become anergized or even deleted. This is an important mechanism of peripheral tolerance that helps prevent immune responses against self-antigens or harmless environmental antigens.
In addition, dendritic cells can also regulate T cell activation and differentiation through the secretion of cytokines or by expressing other molecules that can modulate T cell function. The ultimate outcome of dendritic cell-T cell interaction depends on multiple factors, including the nature of the antigen, the context of the immune response, and the activation status of both the dendritic cell and the T cell.
What are glioblastoma-dendritic cell (dc) vaccine?
Glioblastoma-dendritic cell (DC) vaccines are a type of immunotherapy that involves using dendritic cells, a type of immune cell, to stimulate the body’s immune system to attack glioblastoma, a type of aggressive brain cancer.
The process involves extracting dendritic cells from the patient’s blood, and then exposing them to antigens derived from glioblastoma cells in the laboratory. The dendritic cells are then activated and loaded with glioblastoma antigens, and then injected back into the patient’s body as a vaccine.
When the vaccinated dendritic cells encounter T cells in the patient’s body, they present the glioblastoma antigens to the T cells, which can then recognize and attack glioblastoma cells in the brain. The goal of the vaccine is to stimulate a specific and targeted immune response against the glioblastoma cells, while minimizing damage to healthy brain tissue.
Clinical trials of glioblastoma-DC vaccines have shown promising results in some patients, with increased survival and improved quality of life. However, further research is needed to fully understand the effectiveness and potential side effects of this type of immunotherapy.
What is the cause of blastic plasmacytoid dendritic cell neoplasm?
The cause of blastic plasmacytoid dendritic cell neoplasm (BPDCN) is not well understood. BPDCN is a rare and aggressive type of blood cancer that arises from immature plasmacytoid dendritic cells, which normally play a role in the body’s immune response.
Several genetic mutations have been identified in BPDCN cells, including abnormalities in genes that control cell growth, differentiation, and apoptosis (programmed cell death). These mutations may contribute to the uncontrolled proliferation and survival of BPDCN cells.
In addition, some studies have suggested that BPDCN may be associated with viral infections, such as human herpesvirus 6 (HHV-6) or human T-cell leukemia virus-1 (HTLV-1), although the exact nature of this relationship is unclear.
Other risk factors for BPDCN include advanced age, male gender, and exposure to environmental toxins or radiation.
Overall, the exact cause of BPDCN is not fully understood, and further research is needed to elucidate the underlying mechanisms and develop effective treatments for this rare and aggressive cancer.
Why you need fc block for dendritic cell flow cytometry?
When performing flow cytometry analysis of dendritic cells, it is often necessary to use an Fc receptor blocking agent, or “Fc block,” to minimize non-specific binding of antibodies to Fc receptors on the surface of cells. Fc receptors are proteins expressed on many cell types, including dendritic cells, that can bind to the Fc region of immunoglobulin (Ig) antibodies.
Without an Fc block, antibodies used for staining dendritic cells may bind to Fc receptors on the cell surface, leading to high background staining and potentially obscuring the specific signal of interest. By using an Fc block, the blocking agent binds to the Fc receptors on the cells, preventing non-specific binding of antibodies and improving the specificity and sensitivity of the flow cytometry analysis.
The most commonly used Fc block is a monoclonal antibody that recognizes and blocks Fc receptors, such as anti-CD16/CD32. It is important to optimize the concentration and incubation time of the Fc block to ensure effective blocking without causing cell damage or interfering with subsequent antibody staining.
In summary, Fc receptor blocking agents are important tools for minimizing non-specific antibody binding and improving the accuracy and specificity of flow cytometry analysis of dendritic cells.
What are dendritic cells, and what is their function?
Dendritic cells are a type of immune cell that play a critical role in initiating and regulating immune responses. Their main function is to capture and present antigens to other immune cells, such as T cells and B cells, to activate an immune response against foreign invaders.
Where are dendritic cells found in the body?
Dendritic cells are found in many tissues throughout the body, including the skin, lymph nodes, spleen, and mucosal surfaces of the respiratory and digestive tracts.
How are dendritic cells activated?
Dendritic cells can be activated by a variety of stimuli, including pathogens, inflammatory cytokines, and other immune cells. Once activated, dendritic cells migrate to lymph nodes to present antigens to other immune cells.
What is dendritic cell therapy, and how does it work?
Dendritic cell therapy is a type of immunotherapy that uses a patient’s own dendritic cells to stimulate and enhance the immune system’s ability to recognize and destroy cancer cells or infectious agents.
Can dendritic cells be used to treat cancer?
Yes, dendritic cell therapy is currently being studied as a potential treatment for various types of cancer, including melanoma, prostate cancer, and ovarian cancer.
What is the role of dendritic cells in autoimmune diseases?
Dendritic cells can contribute to the development of autoimmune diseases by activating autoreactive T cells that target self-antigens in the body.
What is the difference between myeloid and plasmacytoid dendritic cells?
Myeloid dendritic cells are specialized for initiating immune responses against pathogens, while plasmacytoid dendritic cells are specialized for producing type I interferons in response to viral infections.
What is the role of dendritic cells in vaccination?
Dendritic cells play a critical role in initiating and maintaining immune responses to vaccination by presenting vaccine antigens to other immune cells and activating a robust and long-lasting immune response.
Can dendritic cells be used for immunotherapy in infectious diseases?
Yes, dendritic cell therapy is being studied as a potential treatment for infectious diseases, such as HIV, hepatitis B and C, and COVID-19.
How can researchers study dendritic cells?
Researchers can study dendritic cells using a variety of techniques, including flow cytometry, microscopy, and genetic engineering to investigate dendritic cell function, behavior, and response to various stimuli.
References
- Judith A. Owen, Jenni Punt, Sharon A. Stranford (2013). Kuby Immunology. Seventh Edition. W. H. Freeman and Company.
- Martin-Gayo, E., & Yu, X. G. (2019). Role of Dendritic Cells in Natural Immune Control of HIV-1 Infection. Frontiers in Immunology, 10. doi:10.3389/fimmu.2019.01306
- Patente, T. A., Pinho, M. P., Oliveira, A. A., Evangelista, G. C. M., Bergami-Santos, P. C., & Barbuto, J. A. M. (2019). Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Frontiers in Immunology, 9. doi:10.3389/fimmu.2018.03176
- Cabeza-Cabrerizo, Mar & Cardoso, Ana & Minutti, Carlos & Costa, Mariana & Sousa, Caetano. (2021). Dendritic Cells Revisited. Annual Review of Immunology. 39. 10.1146/annurev-immunol-061020-053707.
- Pai, S., & Thomas, R. (2009). Dendritic Cells. Rheumatoid Arthritis, 116–123. doi:10.1016/b978-032305475-1.50021-5
- Luckashenak, N., & Eisenlohr, L. C. (2013). Dendritic Cells. Cancer Immunotherapy, 55–70. doi:10.1016/b978-0-12-394296-8.00005-1
- Song L, Dong G, Guo L, Graves DT. The function of dendritic cells in modulating the host response. Mol Oral Microbiol. 2018 Feb;33(1):13-21. doi: 10.1111/omi.12195. Epub 2017 Oct 9. PMID: 28845602; PMCID: PMC5771978.
- Song, L., Dong, G., Guo, L., & Graves, D. T. (2017). The function of dendritic cells in modulating the host response. Molecular Oral Microbiology, 33(1), 13–21. doi:10.1111/omi.12195
- https://www.beckman.com/resources/cell-types/blood-cells/leukocytes/dendritic-cells
- https://www.biolegend.com/de-at/dendritic-cells-pathway
- https://biologydictionary.net/dendritic-cells/
- https://www.bdbiosciences.com/en-us/learn/research/immunology/dendritic-cells#Overview
- https://www.irvinesci.com/primary-stem-cells/dendritic-cells.html
- https://www.thermofisher.com/in/en/home/life-science/cell-analysis/cell-analysis-learning-center/immunology-at-work/dendritic-cell-overview.html
- https://www.immunology.org/public-information/bitesized-immunology/cells/dendritic-cells
- https://en.wikipedia.org/wiki/Dendritic_cell
- https://www.news-medical.net/health/What-are-Dendritic-Cells.aspx
