Table of Contents
What is Denitrification?
The nitrogen cycle concludes with denitrification. The nitrogen cycle consists of living organisms fixing atmospheric nitrogen and then releasing it back into the atmosphere. Denitrification is the process of releasing nitrogen from living organisms into the atmosphere.
- By converting nitrate (NO3-) to nitrogen gas, the nitrogen component is returned to the atmosphere through the process of denitrification (N).
- Thiobacillus species and Pseudomonas bacteria present in the soil carry out the denitrification process in the absence of oxygen.
- Gram-negative bacteria breakdown nitrate molecules in soil and aquatic systems into nitrous oxide (N2O) and nitrogen gas, which are subsequently discharged into the atmosphere.
- This process involves a wide variety of microorganisms; consequently, it is also known as the microbial process.
- This biogeochemical process is one of the primary environmental responses to alterations in the oxygen (O2) concentration.
- Denitrification is a universal process that occurs naturally in regulated ecosystems – marine and freshwater habitats, tropical and temperate soils, wastewater treatment plants, aquifers, manure storage facilities, etc.
Denitrification Process
Denitrification is the last step in the nitrogen cycle. It is a naturally occurring, microbially mediated process, where nitrate is used as a form of energy for denitrifiers.
In this process, soil bacteria convert plant-available soil nitrate (NO3–) into nitrogen (N) gases that are lost from the soil. Denitrification produces several gases: nitric oxide (NO), nitrous oxide (N2O) and dinitrogen (N2).
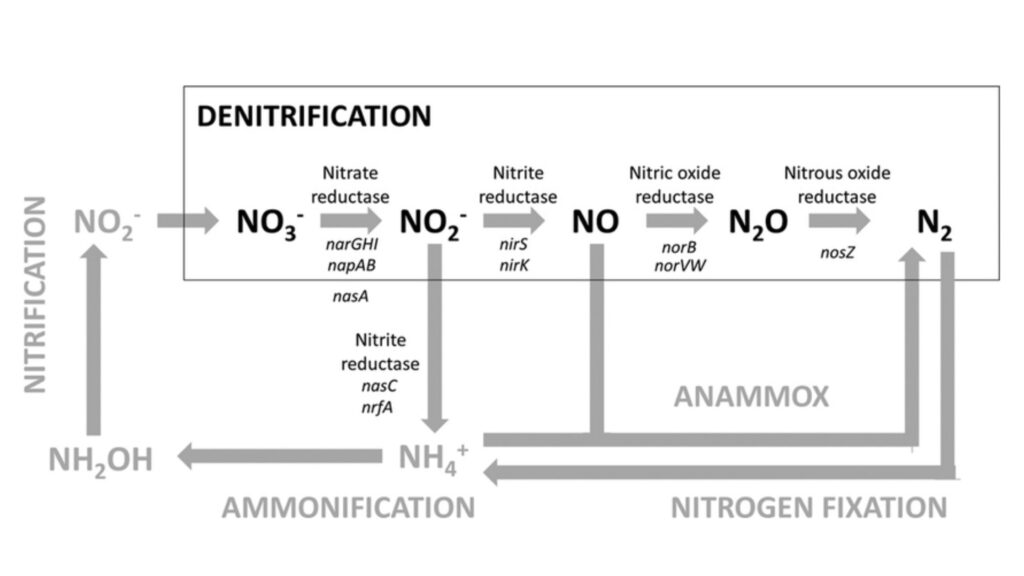
The flowchart of the denitrification process is:
Nitrite → Nitric Oxide → Nitrous oxide → Nitrogen gas.
Denitrification is a microbiological process that removes beneficial nitrogen from soil and releases the greenhouse gas nitrous oxide (N2O) and tropospheric pollutant nitric oxide (NO). The biological cycle of denitrification involves an enzyme cascade that converts nitrate to dinitrogen.
When the soil’s oxygen (O2) supply becomes depleted, a variety of microorganisms use oxygen for respiration instead of nitrate. Denitrification happens most frequently in wet, moist, or flooded soil when the oxygen supply for respiration is limited or restricted. Some fungi are capable of denitrification, however they are regarded insignificant.
Denitrification is more active in locations where the percentage of water-filled soil pores reaches 60 percent. The end-product gas is contingent on the conditions of the soil and the microbial community. As the oxygen deficiency grows, bacteria fulfil their jobs by converting an increasing amount of nitrate to dinitrogen (N2) gas. Denitrification occurs in the loss of valuable nitrogen (N) for the purposes of nutrient management, but the effects on the atmosphere will vary.
Organisms Involved in Denitrification
Bacillus, Enterobacter, Micrococcus, Pseudomonas, Spirillum, Proteus, Aerobacter, and Flavobacterium are among the many bacterial groups that comprise denitrifying microorganisms.
Control of Denitrification
Control of denitrification in terrestrial systems
Nitrogen
- Regular applications of mineral N or organic fertilisers (farmyard manure, sewage sludge) as well as intercropping or crop rotations with leguminous N-fixing plants are made to agricultural soils.
- The use of nitrogen fertilisers promotes nitrification and denitrification.
Oxygen
- The fundamental regulator of denitrification is oxygen. Denitrification is only effective when the soil atmospheric O2 content is between 4 and 17 percent.
- Rainfall, drainage, soil compaction, soil texture, and soil respiration are the primary variables governing the soil O2 content.
- Rainfall, inadequate drainage, and compaction all decrease the soil’s O2 content and promote denitrification.
Carbon
- The primary requirement for heterotrophic denitrifiers is a readily available carbon source, which serves two functions: (1) simple organic carbon compounds provide a carbon source for the growth of heterotrophic denitrifiers, and (2) in an aerobic environment, labile organic carbon stimulates O2 respiration, thereby reducing the O2 concentration surrounding respiring bacteria.
Control of denitrification in aquatic systems
- The conditions for denitrification in aquatic environments are identical to those in terrestrial systems: a nitrogen and carbon supply and low O2 concentrations.
- The principal byproduct of denitrification in aquatic systems is N2, however surface layers of the water column are frequently enriched in N2O relative to the atmosphere, resulting in the emission of N2O from the water.
- Denitrification occurs predominantly in the upper few millimetres of sediments, and denitrification rates are dependent on organic C and N contents.
- Expect the highest denitrification rates in the most contaminated rivers, lakes, and estuaries, followed by the coastal shelves, and the lowest rates in the open oceans.
- Drainage and runoff from agriculturally managed areas and effluents from sewage farms and businesses are the primary sources of NO3. For rivers, denitrification rates were shown to be inversely proportional to channel width, which would control the NO3’s residence period.
- Due to strong rates of sedimentation and upwelling, estuaries have a comparatively high concentration of organic carbon compared to many other aquatic environments.
- They can denitrify between 20% and 50% (and in some circumstances even more) of the NO3 contributed by rivers, thereby preventing the export of terrestrial N pollution to open oceans.
- Denitrification appears independent of salinity throughout the range of 1 to 13 parts per trillion (ppt), but directly reliant on nitrate content.
- Away from the euphotic surface layer and coastal shelves, nitrification and denitrification rates in the interior of oceans are principally determined by the mineralization of nitrogen-rich organic matter produced by N2 fixing organisms.
- The requirements for anaerobic environments are identical to those for terrestrial systems. It has been demonstrated that denitrification rates in benthic sediments are particularly responsive to concentrations of labile organic carbon at the sediment–water interface and increase with nitrate concentrations.
- The thermocline of the Arabian Sea and the eastern tropical South and North Pacific are the principal regions with low O2 levels. Numerous oceanic locations do not denitrify or fix nitrogen.
- In the subtropical North Pacific Ocean, measurements of the isotope ratios 14/15N and 16/18O of N2O coming from surface and deep waters have found substantial discrepancies.
- Compared to air and deep water N2O, surface water N2O was depleted in 15N and 18O. This suggests that nitrification, not denitrification, is likely the primary source of surface water N2O in the open oceans.
Factors affecting the denitrification process
The entire denitrification process is affected by the following variables:
The soil’s organic matter is the most influential factor in the denitrification process. The sole source of sustenance for the bacteria is the organic stuff present in the soil. Therefore, soil bacteria require a source of readily available organic matter, whether it comes from plants, the soil, or other sources.
Other variables include
- Soil pH.
- Soil texture.
- Temperature.
- Soil oxygen concentration.
- The soil’s moisture content
- Nitrate concentration in the soil.
References
- Skiba, U. (2008). Denitrification. Encyclopedia of Ecology, 866–871. doi:10.1016/b978-008045405-4.00264-0
- Martens, D. A. (2005). DENITRIFICATION. Encyclopedia of Soils in the Environment, 378–382. doi:10.1016/b0-12-348530-4/00138-7
- https://www.biologyonline.com/dictionary/denitrification
- https://www.biotecharticles.com/Agriculture-Article/Importance-of-Denitrification-3188.html

🙂 Excellent Article, Excellent Blog , Excellent Site ✅✅✅