Table of Contents
What is Nitrification?
Nitrification is the biological process that involves the oxidation of ammonia to Nitrite, followed by the conversion of the nitrite to the nitrate.
Nitrification is one of the phases of the nitrogen cycle in which living organisms oxidize ammonia in soil to useful nitrogen forms that is then consumed by different species of organisms. The nitrogen cycle is where nitrogen is transformed into a variety of forms that are then transported from the soil to the atmosphere, to living organisms, and then returned to the air. Nitrification can be described as an aerobic procedure that is carried out in soils by a variety of aerobic microorganisms, including archaea and bacteria. The bacteria that oxidize ammonia are known as ammonia-oxidizing bacterium (AOB) and the archaea that oxidizes archaea is referred to as ammonia oxidizing archaea (AOA).
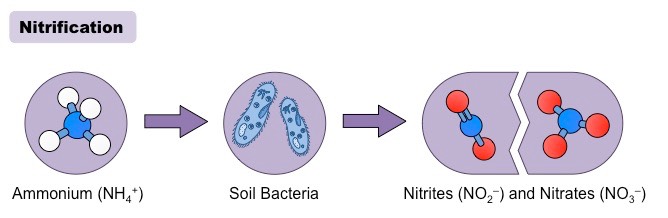
The reactions that occur during Nitrification include:
2NH4+ + 3O2 – 2NO2- + 2H+ + 2H2O
2NO2- + O2 – 2NO3-
Since this process is comprised of two steps of reaction that are two distinct kinds of microorganisms participate in the process of nitrification. The first step in the ammonia oxidation is caused by microbes in the soil , which include bacteria belonging to the genera Nitrosomonas and Nitrosococcus and arceae such as Nitrosopumilus maritimus as well as Nitrososphaera viennensis. The conversion of ammonia to Nitrite is the primary rate-limiting process of the process of nitrification. This second process is carried out by bacteria belonging to that genus Nitrobacter or Nitrospira. Each of the microorganisms are chemoautotrophs and utilize the energy produced by the reaction to create organic carbon molecules. Nitrification is a crucial process for many organisms since it is the sole means that can provide nitrogen to certain microorganisms found in soil.
The organisms convert ammonia to the nitrates that are more water-soluble than ammonia and therefore can be absorbed into the system with greater ease. In addition, it is essential in agriculture systems where ammonia can be used as fertilizer. Ammonia is converted into nitrate , which aids in nitrogen leakage into plants. Nitrifying bacteria contribute to the treatment of wastewater, where various nitrogen compounds are transformed into nitrogen and nitrates after which the gas is removed from the water. Nitrification will be controlled by a range of factors , such as oxygen availability as well as soil moisture and the amount of ammonia available. The bacteria that nitrify also decreases under conditions of acidity and when temperatures are that is higher than 35 degrees Celsius.
What is Denitrification?
Denitrification is a process in biology of reducing nitrate into the nitrite that is followed by the conversion of nitrate to nitrogen gas, which generally results in the removal of nitrogen gas from the air.
Denitrification is similar to nitrification. It is an microbial process performed by various kinds of microorganisms. It is also a crucial element of the nitrogen cycle, where nitrogen is released into the atmosphere from below. In this instance the oxidized substances of nitrogen are transformed into gaseous forms, including nitrogenus oxide (N2O) and nitrogen gas (N2). Denitrification, in contrast to nitrification is carried out by facultative anaerobes which carry out denitrification through anaerobic respiration in order to transform oxidized compounds to gases. Denitrification happens at 10% to less of organic carbon and oxygen compounds.
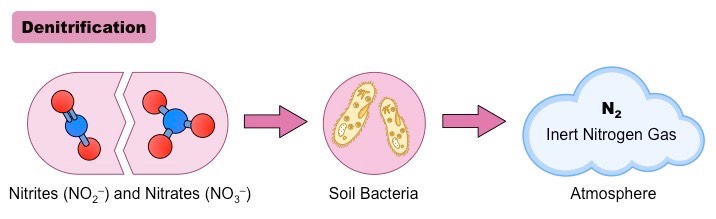
Denitrification is accomplished through a sequence of half-reactions. These include:
NO3- + 2H+ + 2e- – NO2- + H2O
NO2 + 2H + e- NO + H2O
2NO + 2 H+ + 2 e- – N2O + H2O
N2O + 2 H+ + 2 e- – N2 + H2O
The reaction in general can be represented in terms of:
2 NO3- + 10 e- + 12 H+ – N2 + 6 H2O
This process is performed primarily by heterotrophic bacteria, such as Paracoccus denitrificans, and a few species of Pseudomonas However, autotrophic denitrifiers, such as Thiobacillus denitrificans are also found. Denitrification is a crucial microbiological procedure that happens naturally in marine and terrestrial environments. In addition, it occurs in wastewater treatment processes to convert nitrogen-rich compounds into nitrogen gas prior to release into the air. However, it is possible for denitrification to be detrimental by removing NO3- in the soil, decreasing the amount of leaching. Denitrification is controlled through a range of variables, including the concentration of carbon and oxygen, even though certain aerobic bacteria belonging to the Genus Proteobacteria can aid in denitrification, even when oxygen is present.
Differences Between Nitrification and Denitrification – Nitrification vs Denitrification
| Base for comparison | Nitrification | Denitrification |
| Definition | Nitrification is the biological process that involves the oxidation of ammonia to Nitrite, followed by the conversion of Nitrite into the nitrate. | Denitrification is a process in biology that reduces nitrate to Nitrite. This is followed by the transformation of nitrite into nitrogen gas, which typically results in the elimination of nitrogen gas into air. |
| Reaction | The general reaction of nitrificationNH4+ – NO2– – NO3– | The general reaction of denitrification is:2 NO3- + 10 e- + 12 H+ – N2 + 6 H2O |
| Steps | Nitrification is a process that consists of two reactions. | The process of denitrification takes place through a series of half-reactions. |
| Process | Nitrification is a method of chemical reactions that cause oxidation. | Denitrification is the process that is made up of reaction reduction. |
| Nitrogen cycle | Nitrification is the next step in the cycle of nitrogen. | Denitrification is the final step of the nitrogen cycle. |
| Involves | Nitrification is the process of converting nitrogen compounds that are reduced into the oxidized form. | Denitrification is the process of converting nitrogen compounds that have been oxidized to reduced versions. |
| The end product | The final product of nitrification will be the nitrate (NO3 –). | The product that is created by denitrification is either the nitrous oxide (NO2) or nitrogen gas (N2). |
| Substrate | The primary or the starting compound for the nitrification process is ammonia. | The primary compounds, or substrates for denitrification are nitrates as well as Nitrites. |
| Oxygen concentration | Different microorganisms are able to nitrify with a high amount of oxygen. | Different microorganisms are involved in denitrification under an oxygen concentration that is low. |
| Microorganisms are involved | Common microorganisms that nitrify include the Nitrosomonas, Nitrosococcus. Nitrobacter, Nitrospira, Nitrosopumilus maritimus as well as Nitrososphaera vinnensis. | Common denitrifying microorganisms include Paracoccus denitrificans, Thiobacillus denitrificans, Proteobacteria. |
| Nutrition | Most microbes involved with Nitrification are chemically autotrophic. | The majority of microbes involved in Nitrification are heterotrophic. |
| Required pH | Nitrification thrives within the pH range of 6.5 to 8. | Denitrification is a thriving process within the pH range 7 to 9. |
| Temperature | The process is most likely to occur in temperatures between 20 and 30 degrees Celsius. | The process typically takes place at temperatures ranging from 26 degC to 38 degC. |
| Inhibitors | Certain inhibitors that are associated with the process of nitrification include sulfur-containing compounds as well as N-heterocyclic substances. | Some inhibitors involved in denitrification include acetylene and Cyanide, and some pesticides, such as Vapam. |
| Importance | Nitrification is a vital process that helps supply nitrates to plants that are a source for nitrogen. | Denitrification is an important step which ensures the cyclic movement of nitrogen out from within the air to soil, plants and then back in the atmospheric. Additionally, it is a key element in the wastewater treatment. |
