Table of Contents
Aseptic technique is a collection of routine procedures that are used to stop the sterile media stocks, cultures as well as other solutions from being affected by harmful microorganisms (i.e. sepsis). These measures are sometimes described as “sterile method,” the term “sterile technique” is appropriate only when it comes to preventing the introduction of any microorganisms into medical equipment or the reagents (e.g. for example, during surgical procedures).
Because the aim of biologists is to cultivate microorganisms or eukaryotic cells with no introduction of any extraneous organisms aseptic methods are essential for ensuring that experiments are conducted with precision and accuracy. Always keep in mind that a totally safe working environment for scientists does not exist.
There are however many easy, common sense practices to reduce the possibility of contaminations in the culture. Aseptic methods limit the possibility of contamination of the culture by microorganisms in the environment or contamination of the environment caused by the microorganisms handled.
Examples of aseptic technique
Aseptic techniques include:
- Cleansing and disinfecting surfaces in labs prior use
- Limiting the time that media or cultures are not capped and exposed to air
- Keeping petri dishes shut whenever it is
- effectively sterilize inoculating loops, as well as any other apparatus that comes in contact with media or cultures as well as
- Avoid breathing in the presence of the sterile instruments or cultures.
Rules to follow for any aseptic technique
There are general guidelines to follow when using any aseptic procedure.
- Close the doors and windows to stop draughts and to avoid sudden movements that can cause disturbance to the air.
- Make the transfers on an area that has been disinfected. Ethanol disinfection is recommended due to due to its speedy action. If the bench’s surfaces are difficult to clean Cover it with piece of tough material that is easier to disinfect.
- Begin the work only after all the equipment and all materials are readily accessible.
- Finish all the tasks as quickly as you can, but in any pressure.
- Vessels should be accessible for the maximum amount of time that is possible.
- When vessels are in use All work should be performed near to the Bunsen burner flame , where air flows upwards.
- After opening the test tube or bottle the neck should be immediately warmed up by burning (see below) while holding the bottle close to the horizontal position as feasible and so that any air movement will be outwards of the vessel.
- In the course of manipulations using an Petri dish, avoid the exposure of the sterile inner surfaces to contamination by air.
- The sterilized pipettes that will be placed into cultures or sterile vessels should not be placed in contact with or come in contact with non-sterile materials, like clothing or the surface of the work space, or the outer of the test tubes/bottles.
- Everything that comes in contact with microorganisms should be sterilized prior to and following every exposure. This can be done performed by technical staff in preparation for and cleaning up following an activity (for instance when it comes to glassware that is to be employed) or by the employee during the process of doing the work (for example, when they are the case of flaming the wire loop).
Specific Aseptic Techniques
1. Sterile Handling
Always clean your hands and areas of work with 70 percent Ethanol. It is advised wearing gloves. This prevents foreign particles from coming into contact with customers and the samples during testing. If gloves aren’t used it is recommended to wash your hands before and after the test.
Clean the exterior of the containers bottles, flasks, plates and dishes using 70% ethanol prior placing them into the culture hood for cells. Do not pour reagents and media directly into flasks or bottles. Utilize sterile glass pipettes, disposable plastic pipettes as well as a pipettor work with liquids and only use each pipette one time to keep out cross-contamination. Don’t open sterilized pipettes until they are to be utilized. Make sure to keep pipettes within the area of work.
Always seal the flasks and bottles after use. Seal multi-well plates by tape or put them in bags that can be resealed to stop airborne pollutants and microorganisms from entering. Never uncover a sterile flask, bottle, Petri dish, etc. until you are ready to use it . And never let it out into the outside world. Cover it back when you are completed. If you have removed the cover or cap and need to put it back on the floor, put the cap on the work surface with the opening in the direction of downwards.
Make sure to use only sterile glassware as well as other tools. Make sure not to speak or sing when performing sterile procedures. Do your experiments as quickly as you can to reduce the risk of the risk of contamination.
2. Inoculating agar plates, slopes and cultures
Transfer the cultures as swiftly as is possible using tubes and plates opened to air for the least amount of time. The usual practice is to remove agar plates from the body, but without completely removing the lid away from its base.
If you are concerned that the lid on the Petri dish has to be removed for longer than usual, you should work near to the Bunsen burner flame to lessen the chance of contamination. If you are experiencing frequent contamination of your plates by fungal spores, you can reduce the possibility of draughts even more Consider inoculating plates below, with the agar’s surface looking downwards. This way, there is likely to be less risk of spores being deposited on the plate via air.
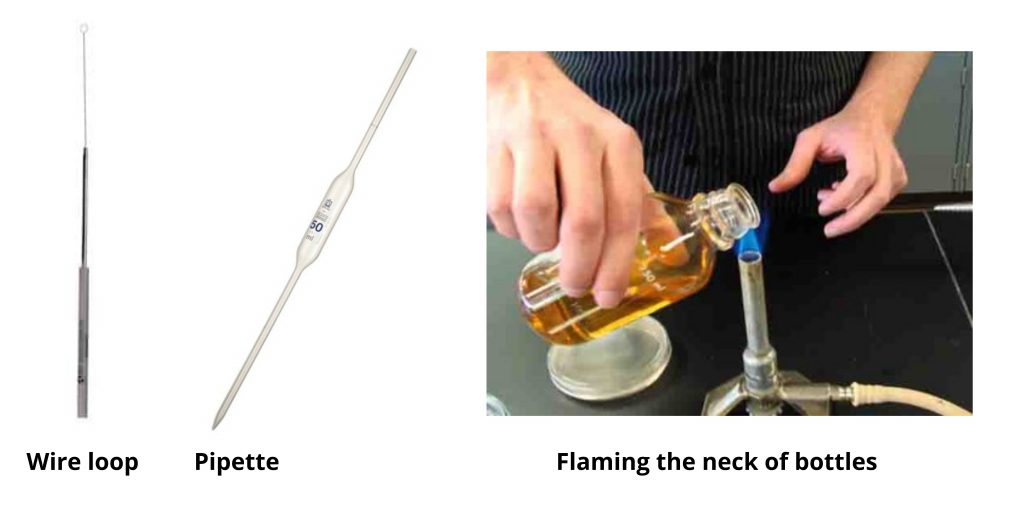
3. Using a wire loop
When making use of a wire loop use its handle loop to the top, just as one would grip a pencil at an angle that is nearly vertical. This will leave the finger free to grab hold on the capor cotton wool plug in the test tube or bottle. This also guarantees that any liquid cultures in the loop will be able to flow through the flame.
Sterilise wire loops by heating it to red hot in the roaring blue Bunsen burner flame prior to and after usage. This will ensure that bacteria that are contaminating organisms and spores will be destroyed. The process of flaming must heat the point of the loop slowly. This is because , after use, it will be a reservoir for culture that could splutter upon rapid heating and release small pieces of the culture that form an aerosol.
Place the handle end of the wire into the blue light cone of the flame. It is the coolest region in the fire. The rest of the wire upwards , slowly, to the hotter part of the flame – directly over the cone of blue.
Keep it there until it’s red hot. Make sure that the entire width of the wire gets adequate heat. Let it cool for a few minutes in the air, and then begin using it immediately. Do not set the loop in the ground or move it around. After use, you must re-sterilise the loop. the use.
4. Using a pipette
Sterile dropped or graduated (Pasteur) pipettes may be utilized to transfer cultures or media that are sterile and solutions. Take the pipette out of the wrapper/ container at the end, which is the cotton wool plug making sure to touch the pipette only as much as it is necessary to hold an firmly grip. Place the teat. It can be useful to first dip the teat in sterile fluid to make it lubricate.
The pipette barrel should be held like pen, however, don’t grasp the teat. Your little finger is free to grasp the capor cotton wool plug from an empty bottle or test tube and your thumb to manage the teat. Press the teat gently and drink up a quantity of fluid that is sufficient to the amount needed however it does not go beyond and soak the plug of cotton.
The teat’s pipette tip under the surface of the liquid creates air bubbles, which can lead to “spitting” and, as a result the formation of aerosols. Be sure to squeeze the teat before putting the tip in the liquid. After that, release the pressure gently until the amount of liquid has been drawn up. Then remove the tip of the pipette from the fluid.
Return any excess. After use, put the contaminated pipette in an adjacent pot to be discarded of disinfectant. Only remove the teat once the pipette is in the pot to be discarded, otherwise droplets of culture can be a source of contamination for the work surface.
5. Flaming the neck of bottles and test tubes
This makes sure that no microorganisms get into the vessel’s mouth to infect the culture, as well as the media. The process of passing into the container’s mouth by the flame generates convection that is able to escape the opening and assists in preventing contamination. The hot portion of the flame is situated above the cone, which is a bright blue. the bottle must be moved across the flame and not fixed in position.
- Remove the cap on the bottle to ensure that it can be taken off easily.
- Lift the bottleor test tube using the left side of your hand.
- Take off the cap/ cotton wool plug from the test tube or bottle by curling your finger toward the palm of your left hand. (Turn the bottle but away from to remove the cap.)
- Don’t put the cap or cotton wool plug in the capor the cotton wool cap.
- Burn the neck on the bottleor test tube by moving the neck upwards and back through an extremely warm Bunsen burning flame.
- After completing the procedure that is required, such as taking out the culture then replace the cap/ cotton wool plug in the test tube or bottle with your finger. Be careful! The bottle is likely to be hot. (Turn the bottle over, but not over the cap.)
- If the plugs of cotton wool have lost the shape they once had, then they may be re-directed to the neck of the vessel by gently turning the vessel’s mouth while the plug is pulled downwards.
6. Disinfecting surfaces
For technicians who work with ethanol, it is recommended to use ethanol disinfection due to its quick process (around five minutes). Technicians will be more knowledgeable and are able to manage the fire risks that are associated with working with alcohol.
Tools Used for Maintaining Aseptic Conditions
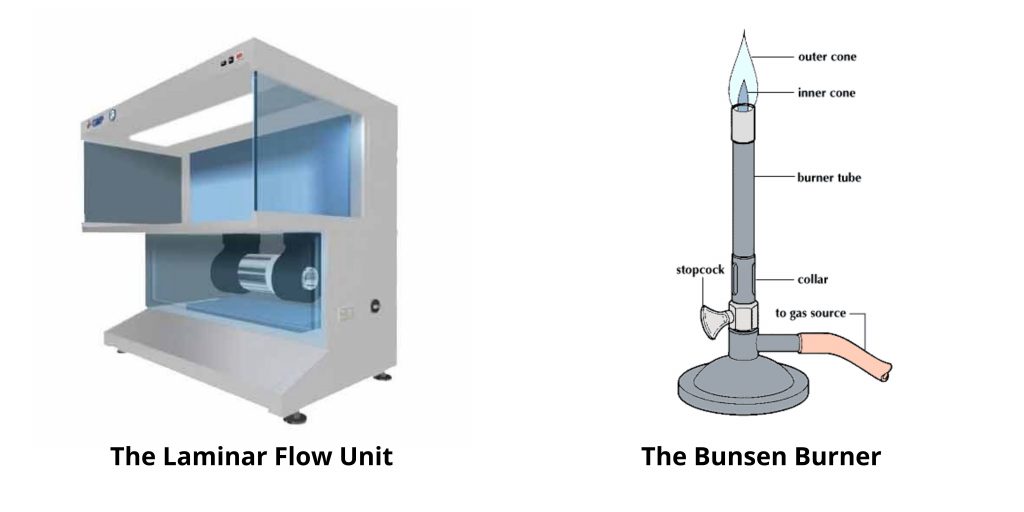
1. The Bunsen Burner
Perhaps the most effective method of creating a safe and sterile bench in the lab is to use the simple gas powered burner. This piece of equipment produces a continuous stream of an flammable gas, usually natural gas (methane)–based on a concept developed around 150 years back by German scientist Robert Wilhelm Bunsen (1811-1899).
The primary function of the open flame used in aseptic techniques is to form the appearance of a hot air cone over and around the bench in order to limit the possibility of organisms residing in the suspended particles of dust. The capacity to use Bunsen burner flame Bunsen burn flame warm things extremely quickly makes it an excellent choice for sterilizing the inoculating loops and warm glass necks of bottles or for igniting alcohol on the spreaders of culture.
A Bunsen burner is not suitable in certain situations, e.g., within an airflow unit, in which the heat can interfere with the flow of air. Microincinerators can be utilized in lieu. It’s an element of heating that is circular. By putting an inoculating loop or needle inside the ring will rapidly steam sterilize and heat the loop or needle.
A microincinerator can not offer the other aseptic benefits from a Bunsen burner.
2. The Laminar Flow Unit
Laminar flow units (or the hood) is a sophisticated device which can also help to prevent contamination of reagents as well as biological culture. When used correctly, it can provide the area of work with ultra-clean, clean air. It also blocks air from rooms from entering the work space and also removes airborne pollutants that are introduced into the work space by employees.
The most essential component of an hood with laminar flow is a high-efficiency, bacteria-retentive filter, i.e., the HEPA (high-efficiency particulate air) filter. An approved HEPA filter should be able to remove at least 99.97 percent of pollen, dust bacteria, mold as well as any airborne particle with an area of >0.3 millimeters when operating at 85 milliliters per minute. First HEPA filters were created during the late 1940s, through the U.S.A.Atomic Energy Commission as part of the Manhattan Project (the development of the Atomic bomb) to offer a reliable effective and efficient method to remove radioactive particles.
HEPA filters were classified following World War II, allowing the development of extensive research and commercial applications. Laminar flow hoods are vital elements of many biosafety level (BSL)-2 labs, and aid in preventing the spreading of infections caused by viruses and bacteria.
