Table of Contents
Differential centrifugation (also called the differential velocity method) is a common process in the fields of biochemistry and cell biology it is utilized to differentiate organelles and other subcellular particles by their sedimentation rates. Although it is commonly used in biochemical analysis it is a general method that is useful for the crude elimination of non-living suspended particles (e.g. nanoparticlesor colloidal particles viruses). In a typical situation that differential centrifugation is utilized to study cell-biological processes (e.g. organelle distribution) A tissue specimen is very first de-lysed in order to cut the cell membranes, and let out organelles and the cytosol. The lysate then is subjected to repeated centrifugations. particles that are able to dissolve with a certain centrifugal force for a certain period of time, form an elongated “pellet” in the base in the tube that is centrifugal.
Following each centrifugation, the supernatant (non-pelleted solution) is removed from the tube and centrifuged again with a higher centrifugal force and/or length. Differential centrifugation is a good choice for the separation of crude particles just on the sedimentation rate However, more fine-grained purifications can be made using density by using equilibrium centrifugation using density gradients. This means that the differential centrifugation process is the continual collection of particles from previous supernatantusing larger centrifugation forces. Cellular organelles isolated by differential centrifugation can maintain an incredibly high level of normal function, so long as they’re not exposed to denaturing conditions in isolation.
Principle of Differential centrifugation
Differential centrifugation relies on the different sedimentation rates of biological particles that vary in dimensions and densities. When the greater force of centrifugal forces is applied, the initial sedimentation of larger molecules occurs. More particles settle down based on the speed and length of the individual centrifugation processes as well as the density and relative sizes of each particle. The largest group of particles form a solid in the inside of the tube leaving smaller-sized structures in the supernatant. Therefore, larger molecules settle rapidly and have lower centrifugal force, whereas smaller molecules require more time and greater forces. When particles that are not as dense as the media, they tend to flounder instead of sinking.
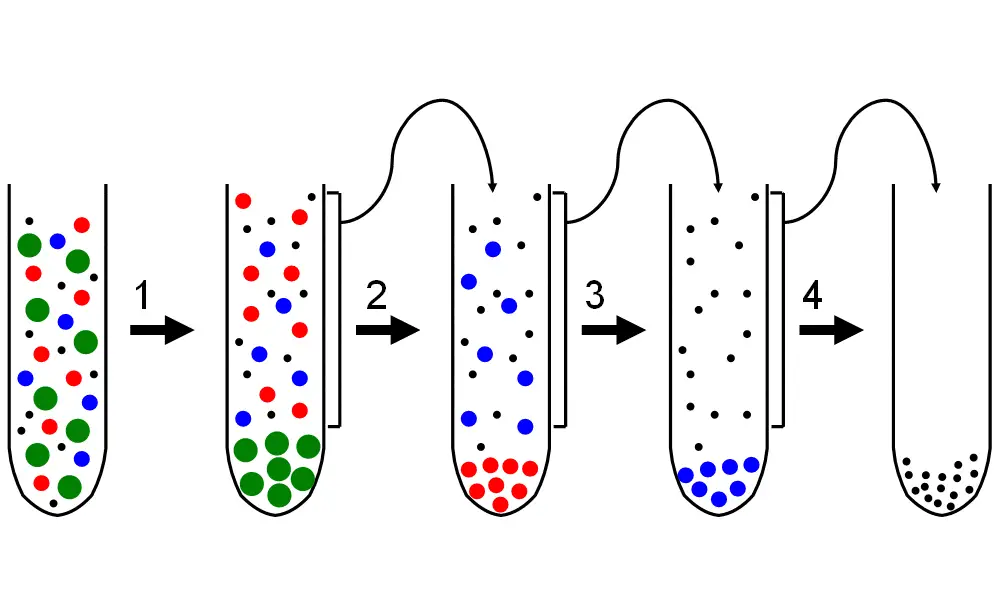
Differential centrifugation Protocol
The organelles of sub-cellular origin (nucleus mitochondria, lysosomes, nucleus microsomes) in the homogenate of a tissue liver can be isolated using these techniques of differential centrifugation. The procedure consists of these steps
- Preparation of homogenate from the liver – 10 percent solution in 0.25 milliliters of sucrose.
- A centrifugation of 1000 g for 10 mins.
- The pellet is separated from the sedimented that is the nucleus.
- The supernatant that is decanted by the step (c) is then subjected to centrifugation at 3300 grams for 10 min.
- Separation of the sedimented pellet that is containing mitochondria.
- The supernatant emitted by the step (e) is then subjected to centrifugation at 16300 grams for 20 minutes.
- Separation of the pellet that has been sedimented that contains Lysosomes.
- The supernatant that is decanted by the step (g) is subjected centrifugation at 105000 grams for 60 minutes.
- The pellet is separated from the sediment that contains microsomes.
- The supernatant resulting from the final stage is the cell-free cytosol.
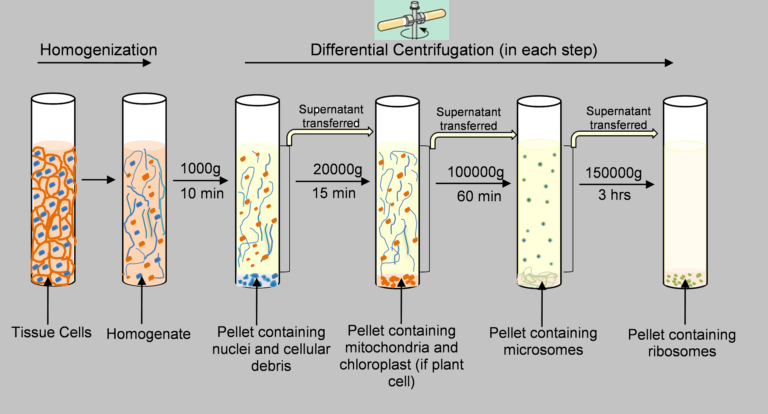
The isolation of organelles in sub-cellular form is a fundamental procedure in numerous biochemical research labs through this use of different centrifugation methods. A diagrammatic representation of steps-wise separation of sub-cellular organelles using an homogenate from the liver is provided in the following figure.
Fractionation by differential centrifugation
For a typical homogenate of cells for a typical cell homogenate, a 10-minute. spin at a slow speed (400-500 x grams) produces a pellet comprised of unbroken tissue, entire cells, cell nuclei and large pieces of debris. The pellet that is low speed is often referred to as”the nuclear pellet. It is a 10 minute. spin at a moderately rapid speed that produces forces of between 10,000 and 220,000 x g, can destroy mitochondria as well as lysosomes and peroxisomes. Thus, the second part of the conventional cell fractionation system is known as the mitochondrial pellet.
Cell fractionation further through differential centrifugation is a requirement for an ultracentrifuge. The instrument is constructed to spin rotors with high speeds, which generate extremely high forces of g. The air needs to be removed from the chamber to keep heat from building up caused by friction with the air. Indeed, many rotors designed to be used in an ultracentrifuge were not designed aerodynamically since they spin in the vacuum. A one-hour high-speed ultracentrifuge operation that generates an force in the range of 80,000 x g produces microsomal pellets. Microsomes contain fragments of membrane which include cell membranes as well as the endoplasmic reticulum. Membrane fragments create vesicles when ruptured in an aqueous media and a close examination will uncover a myriad of membrane vesicles of different dimensions. Vesicles can be classified based on density, due to the different levels of protein. However, that’s the subject of another article.
You can spin for a few hours with 150,000xg, or around and you will be able to bring down ribosomes, and possibly the biggest macromolecules. The remaining supernatant is composed of soluble elements of the cells, such as salts, small macromolecules , precursor molecules, as well as gases that have been dissolved.
Sample preparation for Differential centrifugation
Before differential centrifugation is used to separate the distinct cells from one another the sample of tissue must first be homogenized. For this, the blender, which is usually made of porous porcelain with the same size and shape as the container is utilized. The container is in the majority of instances an insulated ceramic boiling vessel.
It is crushed before the buffer solution is then added, creating an liquid suspension from the crushed tissue samples. This buffer is extremely dense, inert Aqueous solution designed to keep samples in liquid media, without harming it by chemical reactions or the process of osmosis. The majority of the time the solution used is sucrose, however in some cases, brine may be utilized. Then, the blender linked to a rotor with a high speed is placed into the container housing the sample. It presses the sample to the walls in the vessel.
Once the rotator is on the tissue sample will be crushed by the pores of porcelain and the container’s wall into tiny pieces. This process breaks the membranes of the tissue sample, leaving the individual organisms suspended inside the liquid. This process is referred to as homogenization. Some cells remain intact following grinding, and certain organelles could be damaged. This will be addressed in the last stage of the centrifugation process.
What is Equilibrium (isopycnic) sedimentation?
Equilibrium sedimentation is the use of an inclination of a solution like Cesium Chloride as well as Sucrose to segregate particles based on their densities (mass/volume). It can be used as an emulsifying procedure to achieve differential centrifugation. The solution is made by placing the largest part of the gradient in the lowest point. The particles to be separated are placed in the gradient and then centrifuged. Each particle moves (either upwards or downwards) until it has reached an environment with a similar density.
The density gradient can be continuous or created in a gradual way. For example, when using sucrose for the preparation of density gradients, you can delicately float a solution of 40% sucrose over an overcoat of 45% sucrose , and then add layers of less dense over. The homogenate, which is prepared using a buffer dilute, and quickly centrifuged to get rid of damaged cells and tissue before being layered over the over. After centrifugation for about an hour at around 100,000 x mg, it’s possible to observe the cellular components in disks on the shift in density between the layers and the next. By carefully altering the densities of layers to match the type of cell it is possible to enrich for certain cell components. Caesium chloride permits more precise separation of particles with similar density. Indeed, by using a caesium chloride gradient DNA particles that contain high-isomers (13C or 15N, for instance) are separated from DNA particles that don’t have heavy isotopes.
Differential centrifugation vs density gradient centrifugation
There are two principal methods for separating particles using centrifugation. They are differential centrifugation and the density-gradient centrifugation. Differential centrifugation is based on the differing rates of sedimentation for particles of different density. The more dense, larger particles exhibit a greater percentage of their sedimentation. Density gradient centrifugation results in the best distinction of the particles than DI centrifugation, by using a density matrix to allow particles to pass through.
The process of differential centrifugation involves multiple centrifugation stages of gradually increasing centrifugal force. The most dense and largest particles with the highest amount of sedimentation are the pellet in its initial lower-force spin, while smaller less dense particles will remain inside the supernatant. The supernatant and pellet are then separated and the supernatant returned to the centrifuge using a greater centrifugal force in order to draw out the next set of particles with a lesser amount of sedimentation.
This process can be repeated at least as many times as is necessary to separate each desired group of particles. For instance cells are dissolving in a buffer that is detergent-free and all membrane bound proteins will remain bound to their particular membranes. After centrifugation, the soluble cytosolic proteins are placed in the supernatant whereas membrane-associated proteins will be found in the more dense pellet. To further enhance proteins from an organelle in the cellular system the use of differential centrifugation utilized. Nuclei will form a granule when they are centrifuged at 600 g in 10 minutes.
The supernatant could be centrifuged at 15,000g for 10 minutes in order to get mitochondria and lysosomes to the pellet. The supernatant generated from this procedure can be re-centrifuged at 100,000 g over 10 minutes to make a microsomal pellet. The diverse nature of biological particles renders isolates that undergo differential centrifugation more prone to contamination , and lower recovery. This problem can be resolved through washing of the sample, repeating the centrifugation process in addition to further filtering the sample.
Density gradient centrifugation is based on an insulated tube filled with a substance that creates an increasing density gradient and viscosity. Different types of media can be utilized for separation of the density gradient such as polysaccharides, polyhydric alcohols inorganic salts, silica. The kind of matrix is determined by the molecule that is targeted. A density gradient matrix permits better separation of particles while reducing contamination. The particles travel through the matrix with different rate of sedimentation and then settle into clear bands.
What is the difference between centrifugation and differential centrifugation?
Centrifugation and differential centrifugation are both techniques used to separate components of a biological sample based on their physical characteristics. However, they differ in the specific physical characteristic that is used to separate the components:
- Centrifugation: Centrifugation is a general term that refers to the process of separating components of a sample based on their weight or mass. It involves subjecting the sample to high levels of centrifugal force, which causes heavier components to sediment to the bottom of the tube, while lighter components remain suspended in the supernatant. Centrifugation can be used to separate cells, organelles, proteins, and other subcellular structures.
- Differential centrifugation: Differential centrifugation is a specific type of centrifugation that separates components based on their size, density, and shape. It involves subjecting the sample to high levels of centrifugal force, which causes larger, denser components to sediment to the bottom of the tube, while smaller, less dense components remain suspended in the supernatant. Differential centrifugation can be used to isolate specific cell types, organelles, proteins, or other subcellular structures from a complex mixture.
Overall, both centrifugation and differential centrifugation involve separating components of a biological sample based on their physical characteristics, but differential centrifugation is a more specific technique that separates components based on their size, density, and shape. Both techniques can be used to isolate specific components of a sample for further study or analysis.
Application of Differential centrifugation
Differential centrifugation is a technique used to separate different components of a mixture based on their size, shape, and density. It involves spinning the mixture at high speeds using a centrifuge, which generates a centrifugal force that separates the components of the mixture based on their physical characteristics.
Some common applications of differential centrifugation include:
- Isolating cells and organelles from tissues: Differential centrifugation can be used to separate cells from tissues, or to isolate specific organelles (such as nuclei or mitochondria) from cells.
- Purifying proteins: Differential centrifugation can be used to separate proteins based on their size and shape, allowing researchers to purify specific proteins for further study.
- Separating viruses and bacteria: Differential centrifugation can be used to separate viruses and bacteria from other components of a mixture, such as cells or tissues.
- Analyzing the composition of samples: Differential centrifugation can be used to analyze the composition of a sample, such as the types and proportions of different cell types or organelles present.
Overall, differential centrifugation is a widely used technique in a variety of fields, including biology, biochemistry, and medicine, and has many important applications in research and analysis.
Advantages of Differential centrifugation
Differential centrifugation is a technique used to separate different components of a biological sample based on their size, density, and shape. It involves subjecting the sample to high levels of centrifugal force, which separates the components based on their physical characteristics. Some of the advantages of differential centrifugation include:
- Efficiency: Differential centrifugation is a fast and efficient way to separate different components of a biological sample. It can be used to isolate specific cell types, organelles, or other subcellular structures from complex mixtures.
- High-throughput: Differential centrifugation can be used to process large volumes of sample quickly, making it a useful technique in high-throughput studies.
- Versatility: Differential centrifugation can be used to separate a wide range of biological components, including cells, organelles, proteins, and nucleic acids.
- Reliability: Differential centrifugation is a reliable method for separating biological components, as it is based on physical characteristics that are relatively stable and do not depend on biochemical or genetic changes in the sample.
- Cost-effective: Differential centrifugation is a relatively inexpensive method for separating biological components, especially when compared to more complex techniques such as chromatography or electrophoresis.
Overall, differential centrifugation is a useful technique for isolating specific components of a biological sample for further study or analysis.
Disadvantages of Differential centrifugation
There are a few disadvantages to using differential centrifugation as a method for separating biological components:
- Limited resolution: Differential centrifugation can only separate components based on their size, density, and shape. It may not be able to distinguish between components that are very similar in these physical characteristics.
- Damage to cells or organelles: The high levels of centrifugal force used in differential centrifugation can cause damage to delicate cells or organelles, which may affect their viability or function.
- Contamination: Differential centrifugation can result in contamination of the separated components with other materials present in the sample. For example, if the sample contains bacteria, they may be present in the final preparation of the separated component.
- Difficulty in handling large volumes: Differential centrifugation requires the use of a centrifuge, which can be difficult to operate with large volumes of sample.
- Time-consuming: Differential centrifugation can be a time-consuming process, especially if multiple rounds of centrifugation are required to obtain a pure preparation of the desired component.
Overall, while differential centrifugation is a useful technique for separating biological components, it is not without limitations and may not be suitable for all applications.
How to increase orgenlle purity after differential centrifugation?
There are several strategies that can be used to increase the purity of an organelle preparation obtained through differential centrifugation:
- Use multiple rounds of centrifugation: Repeating the differential centrifugation process multiple times can help to further purify the organelle preparation. For example, a sample can be centrifuged at different speeds or for different periods of time to separate different components.
- Use selective lysis: Before undergoing differential centrifugation, the sample can be treated with enzymes or chemicals that selectively lyse or disrupt certain cell types or organelles. This can help to reduce the presence of contaminants in the final organelle preparation.
- Use density gradient centrifugation: In addition to differential centrifugation, the sample can be subjected to density gradient centrifugation, which separates components based on their density. This can help to further purify the organelle preparation by separating it from other components with similar size and shape characteristics.
- Use biochemical methods: After differential centrifugation, the organelle preparation can be further purified using biochemical methods such as chromatography or immunoprecipitation. These methods can help to remove contaminants that may not have been separated during the centrifugation process.
Overall, using a combination of these strategies can help to increase the purity of an organelle preparation obtained through differential centrifugation.
FAQ
What does the process of differential centrifugation achieve?
The process of differential centrifugation separates different components of a biological sample based on their size, density, and shape. It involves subjecting the sample to high levels of centrifugal force, which causes larger, denser components to sediment to the bottom of the tube, while smaller, less dense components remain suspended in the supernatant. The process of differential centrifugation can be used to isolate specific cell types, organelles, proteins, or other subcellular structures from a complex mixture.
Differential centrifugation is a useful technique for purifying and isolating specific components of a biological sample for further study or analysis. It can be used to prepare samples for a wide range of applications, including microscopy, biochemistry, molecular biology, and cell biology. Differential centrifugation is a relatively simple and cost-effective method that is widely used in research and other scientific applications.
What is left in the supernatant of differential centrifugation?
During the process of differential centrifugation, larger, denser components of a biological sample sediment to the bottom of the tube, while smaller, less dense components remain suspended in the supernatant. The specific components that remain in the supernatant will depend on the specific sample being processed and the conditions used for the centrifugation.
In general, the supernatant of differential centrifugation may contain a variety of components, including cells, organelles, proteins, and other small molecules. The components that remain in the supernatant may be further separated and purified using additional techniques, such as density gradient centrifugation, chromatography, or immunoprecipitation.
Overall, the supernatant of differential centrifugation contains a mixture of smaller, less dense components that have not sedimented during the centrifugation process. These components may be of interest for further study or analysis, and can be further purified using additional techniques as needed.
