Table of Contents
Scientific classification of Escherichia coli
| Domain | Bacteria |
| Phylum | Proteobacteria |
| Class | Gammaproteobacteria |
| Order | Enterobacterales |
| Family | Enterobacteriaceae |
| Genus | Escherichia |
| Species | E. coli |
| Binomial name | Escherichia coli |
Escherichia coli
- Theodor Escherich first discovered E. coli, in 1885 after isolating it from the feces of newborns.
- Escherichia coli also termed E. coli, which is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus Escherichia.
- It is usually found in the lower intestine of warm-blooded organisms (endotherms).
- Most E. coli strains are harmless, however, some serotypes (EPEC, ETEC etc.) are responsible for serious food poisoning in their hosts. They also occasionally cause food contamination incidents that prompt product recalls.
- The normal microbiota of the gut includes the harmless strains of E. coli. They provide benefits to their host cell by generating vitamin K2, (which helps blood to clot) and preventing colonization of the intestine with pathogenic bacteria, having a mutualistic relationship.
- In the environment, they are mainly found within fecal matter. In the fresh fecal matter they grow massively under aerobic conditions for 3 days, but its numbers decrease slowly afterward.
- Cells can survive outside of the body for a limited amount of time, which makes them potential indicator organisms to test environmental samples for fecal contamination.

E. coli Habitat
- The availability of the nutrients within the intestine of host organisms is the main factor on which the niche of E. coli depends.
- The gastrointestinal (GI) tract of humans and many other warm-blooded animals is the primary habitat of E. coli.
- They can be found in the mucus or the epithelium on the wall of the intestine. Commonly they are found in the colon of the large intestine.
- They can form a mutual relationship with its host.
- They constitute about 0.1% to 1% of GI tract bacteria.
- They are facultative aerobes.
- They are also found in human feces.
- At the outside of the intestinal tract/outside of a host body, they are able to survive only for a few hours.
- Outside of the host body, they are mainly found in faecally contaminated environments such as water or mud or sediments.
- If E. coli comes in contact with raw vegetables, it has the potential to attach itself to the leaves of the vegetable.
- They are also found at a higher temperature, such as on the edge of hot springs.
- They are found on ground meats due to slaughterhouse processing.
Geographical distribution of Escherichia coli
- Many strains of E. coli that cause diarrhoea largely affect populations in underdeveloped nations. Globally, the prevalence of these strains varies considerably.
- EPEC, EAEC, and DAEC are reported to be most prevalent in underdeveloped nations.
- ETEC is the leading cause of both traveler’s diarrhoea and infantile diarrhoea in impoverished and emerging nations.
- Each year, ETEC is responsible for over 600 million instances of diarrhoea and 700,000 fatalities in children under the age of five.
- EHEC is a growing source of foodborne illness, especially in the northern United States and Canada.
- In addition, deadly HUS outbreaks in children have been observed in several nations.
Reservoir, source, and transmission of infection
- With the exception of neonatal meningitis and gastroenteritis, the majority of E. coli infections are endogenous.
- The infection is produced by the usual microbial flora of the patient, which includes E. coli.
- These bacteria cause infections, such as a urinary tract infection (UTI), when a patient’s defences are weakened or when personal hygiene is inadequate.
- In places with low sanitation, E. coli-caused diarrhoea is prevalent, and infections are transmitted from outside the host.
- Food and water contaminated with human or bovine faeces are significant sources of E. coli that causes diarrhoea.
- Important causes of infection resulting from enterohemorrhagic E. coli include feces-contaminated ground beef, apple juice, and alfalfa sprouts (e.g., O157:H7).
- Infections are contracted through the consumption of contaminated food or drink.
- The typical source of E. coli in newborn infections is the mother’s gastrointestinal tract.
- The bacteria may also be acquired via nosocomial transmission, particularly in premature or ventilator-dependent newborns.
- Humans and animals (for E. coli O157:H7) are infection reservoirs.
E.coli Morphology and Arrangement

- E.coli is a gram-negative (-ve) bacteria.
- These are straight, rod shaped (bacillus) bacterium.
- They are arranged singly or in pairs.
- Their size is about 1–3 µm × 0.4–0.7 µm (micrometer).
- They are motile due to the presence of peritrichous flagella. Some strains of E. coli are non-motile.
- It is a non–sporing bacteria.
- Some of them may be fimbriated. The fimbriae are of type 1 (hemagglutinating & mannose-sensitive) and are present in both motile and non-motile strains.
- It has a polysaccharide capsule which can be easily demonstrated by using India ink preparation, appearing as a clear halo in a dark background.
- The cell wall is thin with only 1 or 2 layers of peptidoglycan.
- It is a facultative anaerobe.
- They can grow over a wide range of temperatures from 15-45°C.
Antigenic Structure of E.coli
Heat Stable Lipopolysaccharide (LPS) is the major cell wall antigen of E. coli. It contains 4 antigens such as H, O, K and F.
- H or Flagellar Antigen: These are Heat and alcohol labile protein. Mainly found on the flagella. They are Genus specific; Present as monophasic; 75 ‘H’ antigens have been recognized.
- O or Somatic Antigen: These are heat stable, resistant to boiling up to 2 hrs. 30 minutes. It is found on the surface of the outer membrane. An integral part of the cell wall. 173 ‘O’ antigens have been recognized.
- K or Capsular Antigen: These are Heat labile, acidic polysaccharide antigen present in the envelope. On Boiling removes the K antigen. They can Inhibit the phagocytosis. 103 ‘K’ antigens have been recognized
- F or Fimbrial Antigen: These are the Heat labile proteins, present in the fimbriae. K88, K99 antigens.
Biochemical reactions
E. coli exhibits the following responses:
- Lactose, glucose, mannitol, maltose, and numerous other sugars are fermented by E. coli, resulting in the formation of acid and gas. Sucrose is not fermented. Some E. coli strains are late lactose fermenters or nonlactose fermenters.
- They do not liquefy gelatin, do not create hydrogen sulphide (H2 S), or do not consume urea. Some mutant E. coli bacteria generate H2 S.
- The indole, methyl red (MR), Voges–Proskauer (VP), and citrate utilisation tests, also known as the “IMViC” tests, are four significant biochemical assays that are frequently used in the categorization of enterobacteria. E. coli is positive for indole and MR but negative for VP and citrate (IMViC++ – -).
- Some E. coli strains are late lactose fermenters or nonlactose fermenters. The capacity of E. coli to make H2 S (positive variations) and use citrate is governed by transmissible plasmids.
Susceptibility to physical and chemical agents
- In salt media used for isolating staphylococci, the inclusion of 7% sodium chloride inhibits the growth of E. coli.
- Sodium selenite in selenite broth, sodium tetrathionate in tetrathionate broth, and brilliant green in brilliant green tetrathionate broth also limit the growth of microorganisms.
Cell Wall Components and Antigenic Structure of Escherichia coli
Lipopolysaccharide (LPS)
- E. coli’s principal cell wall antigen is heat-stable lipopolysaccharide (LPS).
- The LPS is comprised of three parts: a genus-specific somatic O polysaccharide, a core polysaccharide shared by all Enterobacteriaceae (common antigen), and lipid A.
- There are four primary antigens found on E. coli organisms: H or flagellar antigen, O or somatic antigen, K or capsular antigen, and F or fimbrial antigens.
H or flagellar antigen
- On the flagella, the H antigens are heat- and alcohol-labile proteins.
- The H antigens are typically not shared with other enterobacteria as they are genus-specific.
- All H antigens exist as monophasic forms, but extremely rarely in diphasic forms.
- So far, a total of 75 “H” antigens have been identified.
O or somatic antigen
- O antigens are located on the surface of outer membranes and are determined by unique sugar sequences.
- O antigen is an LPS complex that is a fundamental component of the cell wall.
- It is resistant to boiling for up to two hours and thirty minutes.
- Up to this point, 173 (1, 2, 3, etc.) O antigens have been described.
- Somatic O polysaccharide antigen demonstrates cross-reactions with closely related taxa (Shigella, Salmonella, Yersinia, and Citrobacter) within the family Enterobacteraceae.
- Additionally, the O antigen exhibits cross-reactivity with individual E. coli O antigens. Using particular antibodies, the O antigens are identified via agglutination.
K or capsular antigen
- The heat-labile K antigen is the acidic polysaccharide antigen contained in the microcapsule or “envelope” (K for Kapsel, German for capsule) of the bacteria.
- The K antigen surrounds the O antigen and may impede the detection of O antigens. By boiling the bacterial suspension to eliminate the K antigens, this issue is resolved.
- Inhibiting phagocytosis may also contribute to the pathogenicity of K antigens. The K antigens are poor complement activators. 103 “K” antigens have been identified in total.
- There are two types of K antigens: I and II. The E. coli K I antigen exhibits cross-reactivity with the capsular antigens of Neisseria meningitidis and Haemophilus influenzae.
F or Fimbrial antigens
- These heat-labile proteins are found on the fimbriae as antigens.
- E. coli contains a number of filamentous protein structures resembling fimbriae.
- These are K88, K99 antigens in enterotoxigenic E. coli (ETEC) strains that cause diarrhoea in humans.
- These fimbrial antigens also contribute to the bacteria’s pathogenicity.
Antigenic typing
- E. coli serotyping is based on three major antigen groups: O antigens, K antigens, and H antigens.
- On the basis of O antigens, E. coli strains are originally categorised into a variety of O groups.
- On the basis of K antigens, each O group is subsequently subdivided into subgroups.
- Lastly, each subgroup contains strains with distinct H antigens.
- The antigenic pattern of a strain is determined by the quantity of a certain antigen it carries (e.g., E. coli O111:K58:H2).
- Humans contain different serotypes of E. coli in their normal intestines, none of which have K antigens.
- Normal colon strains are classified as “early” O groups.
- Intestinal-disease-causing enteropathogenic strains belong to “later” O groups (55, 86, 111, 112, etc.).
Virulence factors of Escherichia coli
E. coli produces a variety of virulence factors, including the following:
- Common factors of virulence associated with Entero bacteriaceae.
- Particularly associated E. coli virulence factors
Common virulence factors of Enterobacteriaceae include: These factors include (a) fimbriae, (b) endotoxin, (c) capsule, and (d) growth factor sequestration.
Fimbriae
- Fimbriae enhance the pathogenicity of E. coli and other Enterobacteriaceae members.
- The fimbriae come in two varieties:
- The first form is the most common and is encoded by chromosomes; however, it is unrelated to the virulence of the bacteria.
- The second type of fimbriae is rare and encoded by plasmids; nonetheless, it is strongly associated to the virulence of the bacteria.
- Some of them only exist as surface antigens and not as anatomically different structures.
- CFA in ETEC and K88 and K99 antigens in E. coli strains that cause diarrhoea in animals are examples of such fimbriae.
- The fimbriae play a crucial role in the pathogenesis of E. coli-caused UTIs.
Endotoxin
- Endotoxin is a significant virulence factor shared by all aerobic and certain anaerobic Gram-negative bacteria, including E. coli.
- Endotoxin is mostly responsible for the systemic symptoms of Gram-negative bacterial infections produced by E. coli.
- Endotoxin also protects the bacillus against phagocytosis and complement’s bactericidal actions.
Capsule
- E. coli is protected from phagocytosis by hydrophilic capsular K antigens, which reject the hydrophobic phagocytic cell surface.
- The capsular antigens prevent antibodies from attaching to the bacteria.
- However, the capsule is ineffective when O or K antigen antibodies are present.
- The majority of E. coli strains that cause newborn meningitis and septicemia include the KI envelope antigen, a virulence factor related to the meningococcal group B antigen.
- Frequently, E. coli and other intestinal bacteria that might cause systemic infections are resistant to serum killing. The bacterium’s capsule shields the organism from serum-induced death.
Sequestration of growth factors
- The ability of pathogenic bacteria to compete for resources in host cells is an important characteristic.
- Iron is essential for the growth of E. coli and other intestinal bacteria, which compete for it.
- The bacteria produce iron-chelating chemicals, such as siderophores, enterobactin, and aerobactin, which enhance the bacteria’s absorption of iron.
- Additionally, bacteria create hemolysins, which lyse host erythrocytes and liberate iron compounds for bacterial usage.
Specialized virulence factors associated specifically with E. coli
These include adhesins and exotoxins.
Adhesins
- E. coli organisms include a multitude of highly specific adhesins.
- Among these adhesins are (a) CFAs (CFA/I, CFA/II, CFA/III), (b) aggregative adherence fimbriae (AAF/I, AAF/II, AAF/III), (c) bundle-forming pili (Bfp), (d) intimin, (e) P pili (binds to P blood group antigens), (f) Ipa (invasion plasm (bind to Dr blood group antigens).
- All of these adhesins promote the solid adherence of E. coli to the mucosa of the gastrointestinal or urinary tract, preventing the germs from being removed by the flushing action of urination or intestinal motility.
Exotoxins
- Additionally, E. coli produces two forms of exotoxins: hemolysins (HlyA) and enterotoxins.
- Hemolysins are crucial to the pathophysiology of diseases induced by uropathogenic E. coli strains.
- Enterotoxins are essential E. coli virulence agents.
- There are three identified forms of E. coli enterotoxins.
- This consists of (a) Shiga toxins (Stx-1, Stx-2), (b) heat-stable toxins (STa and STb), and (c) heatlabile toxins (LT-I and LT-II).
Shiga toxins
- Shiga toxin (Stx) is so termed because it shares similar physical, antigenic, and biological features with Shigella dysenteriae type 1 toxin.
- The toxin is also known as verocytotoxin or verotoxin (VT) since its cytotoxic impact on Vero cells led to its discovery.
- Stx-1 and Stx-2 are the two kinds of Shiga toxins.
- Lysogenic bacteriophages code for both poisons.
- Both have one A and five B subunits.
- On the host cell, subunit B attaches to a particular glycolipid (globotriaosylceramide, GbJ).
- Although both toxins have the same biological function, they are antigenically distinct.
- Unlike Stx-1, Stx-2 is not neutralised by antibodies generated against Stx.
- Vero or HeLa cells display cytotoxic activity in response to Shiga toxins.
- The toxin exhibits enterotoxicity in the ileal loops of rabbits and paralytic mortality in mice.
Heat-stable toxin
- Low-molecular-weight proteins, heat-stable toxins (ST) are of two types: STa and STb.
- STa is related with human illness.
- STa is a tiny, methanol-soluble, monomeric toxin that acts in the intestine by activating cyclic guanosine monophosphate (cGMP).
- STa binds to guanylate cyclase, resulting in a rise in cGMP and a concomitant increase in fluid secretion.
- Within four hours of intragastric delivery, the toxin produces fluid accumulation in the intestines of infant mice, making the infant mouse a common animal model for demonstrating STa.
- The toxin induces fluid accumulation in the intestines of newborn piglets, but not weaned pigs.
- STb is not linked to any human disorders.
- STb unlike STa is not methanol soluble.
- STb’s precise mode of action is unknown.
- The toxin induces fluid buildup in the ligated intestinal loops of newborn piglets up to nine weeks of age, but not in infant mice.
Heat-labile toxin
- Heat-labile toxin (LT) is a protein that is heat-labile.
- In 1956, De and his colleagues discovered the toxin in E. coli isolated from diarrhoea cases in adults in Kolkata.
- They demonstrated the presence of the cholera enterotoxin in the bacteria using the rabbit ileal loop method, which is used to detect cholera enterotoxin.
- There are two types of LT: LT-I and LT-II. LT-I is associated with human diseases, but not LT-II. LT-I shares structural and antigenic similarities with the cholera toxin.
- The LT-I toxin is composed of one A subunit with a molecular weight of 25,000 Da and five identical B subunits with a molecular weight of 11,500 Da each.
- The B subunits bind to GM1 gangliosides, the same receptor as cholera toxin, and other surface glycoproteins on small intestinal epithelial cells.
- This binding facilitates the endocytosis-mediated entry of subunit A into the cell.
- By virtue of its ADP (adenosine diphosphate)-ribosyl transferase activity, the A subunit interacts with a membrane protein (Gs) that controls adenylate cyclase.
- This causes an increase in cyclic adenosine monophosphate (cAMP) levels, which leads to an increase in chloride secretion and a decrease in sodium and chloride absorption.
- This results in diarrhoea due to the hypersecretion of fluid into the gut lumen.
- In addition to causing fluid loss, the toxin stimulates the secretion of prostaglandin and the production of inflammatory cytokines.
- I transferable plasmid contains the genes for LT-I and STa, as well as the genes for adhesins (CFA/I, CFA/II, and CFA/III).
Pathogenesis of E. coli infections
The vast majority of infections, such as UTIs and sepsis, are endogenous and are caused by E. coli that is abundant in the gastrointestinal tract of the same host. Other E. coli infections, including gastroenteritis and neonatal meningitis, are exogenous, or acquired from the outside.
Urinary tract infections
- Urinary tract infections are frequently caused by E. coli serotypes that are typically found in faeces.
- UTI is an ascending infection in which intestinal bacteria contaminate the urethra, ascend into the bladder, and may spread to the kidney or prostate.
Nephritogenic strains
- Although the majority of E. coli strains can cause urinary tract infections (UTI), particular E. coli serogroups are more likely to cause illness.
- These are known as nephritogenic strains, and they include E. coli serotypes O1, O2, O4, O6, O7, and O18, among others.
- These serotypes are able to create adhesins (mainly P pili, AAF/I, AAF/III, and Dr) that adhere to cells lining the bladder and upper urinary tract, hence causing UTI.
- This inhibits the bacteria in empty urine from being eliminated.
- Typically, only one serotype is isolated from urine at a time; however, recurrences may be caused by many serotypes.
- In addition, they create hemolysin HlyA, which lyses erythrocytes and other cells, resulting in the production of cytokines and the promotion of an inflammatory response.
Gastroenteritis
- Exogenous infections obtained by polluted water, food, or vegetables are the cause of gastroenteritis.
- The strains of E. coli that cause gastroenteritis are divided into six categories. (a) enteropathogenic E. coli (EPEC), (b) enterotoxigenic E. coli (ETEC), (c) enteroinvasive E. coli (EIEC), (d) enterohemorrhagic E. coli (EHEC), (e) enteroaggregative E. coli (EAEC), and (f) diffusely adherent E. coli (DAEC).
Enteropathogenic E. coli
- In tropical regions, the most common cause of baby diarrhoea is EPEC.
- Disease is uncommon among adolescents and adults.
- EPEC strains include O26, O55, O86, O111, O114, O119, O125, O126, O12, O128, and O142.
- EPEC causes infection by attaching to the epithelial cells of the small intestine and then destroying the microvillus.
- The bacteria initially establish microcolonies on the epithelial cell surface, where they connect to the host cells using pedestals in the shape of cups.
- This connection is made possible by Bfp.
- This is followed by the secretion of proteins into the host epithelial cell by the bacterial type III secretion system.
- Translocated intimin receptor is introduced into the membrane of epithelial cells to serve as a receptor for intimin, an E. coli outer membrane adhesin.
- Consequently, the associated bacteria grow and destroy the microvilli, resulting in diarrhoea caused by malabsorption.
Enterotoxigenic E. coli
- Across underdeveloped nations, diarrhoea caused by ETEC is endemic in all age groups.
- This is also the cause of traveler’s diarrhoea, in which persons from industrialised nations who visit endemic regions frequently develop ETEC diarrhoea.
- Consumption of fecally contaminated food or water causes the sickness.
- Person-to-person transmission is not observed.
- Although plasmids containing enterotoxin genes can be found in any E. coli strain, diarrhoea is caused by certain ETEC serogroups (O6, O8, O15, O25, O27, O167).
- Due to their capacity to create heat-labile enterotoxins, these serotypes cause diarrhoea (LT-I, LT-II).
- LT-I, which is structurally identical to cholera toxin, causes diarrhoea similar to that of cholera in patients.
- The presence of adhesins that attach to intestinal mucosa (mainly P pili, AAF/I, AAF/III, and Dr) facilitates the progression of the disease.
Enteroinvasive E. coli
- EIEC strains resemble shigellae in a number of ways: (a) EIEC bacteria are nonmotile, (b) they do not ferment lactose or ferment late with acid generation alone, and (c) they do not decarboxylate lysine decarboxylase.
- These strains exhibit cross-reactivity with shigellae O antigen.
- These “atypical” E. coli strains were previously designated Shigella alkalescens and Shigella dispar under the “Alkalescens-Dispar Group” (resembling Shigella flexneri except in digesting dulcitol and generating alkali in litmus milk) (late lactose fermenter like Shigella sonnei but indole positive).
- These cells have been termed EIEC because they may enter interstitial epithelial cells and penetrate HeLa cells in tissue culture.
- Strains of EIEC are capable of invading and destroying the colonic epithelium, resulting in a condition characterised initially by diarrhoea with mucus.
- This capability of E. coli to invade cells is determined by a large plasmid that encodes outer membrane antigens known as “virulence marker antigens” (VMA).
- The bacteria lyse the phagocytes and proliferate in the cytoplasm of the cell.
- This ongoing process of epithelial cell loss and inflammatory infiltration results in the formation of intestinal ulcers.
- Commonly related with EIEC outbreaks include serogroups O28 ac, O112 ac, O124, O136, O143, O114, O152, and O154.
Enterohemorrhagic E. coli
- In affluent nations, EHEC strains are the leading cause of gastrointestinal illnesses.
- These bacteria can cause diarrhoea ranging from mild, simple diarrhoea to deadly, hemorrhagic colitis.
- Hemolytic uremic syndrome is a life-threatening condition that affects 10% of infected children younger than 10 years old.
- As few as 100 bacilli are sufficient to induce the sickness.
- EHEC sickness is particularly prevalent in children under 5 years of age and during the summer months.
- The condition is caused by the use of feces-contaminated water, unpasteurized milk or fruit juices, raw vegetables, and uncooked fruits.
- The disease is also transmitted by the intake of raw ground beef or other meat products.
- Serotypes O157:H7 and O26:H1 are the most common EHEC strains responsible for the sickness.
- These strains produce Shiga toxins (Stx-l, Stx-2, or both), which are the primary cause of diarrheal illnesses.
- HUS, a condition characterised by abrupt renal failure, thrombocytopenia, and microangiopathic hemolytic anaemia, is most frequently associated with Stx-2.
- Stx-2 destroys glomerular endothelial cells, leading to a decrease in glomerular filtration and acute renal failure.
- Additionally, the toxins induce the creation of tumour necrosis factor-alpha and interleukin-6, which contribute to the progression of the disease.
Enteroaggregative E. coli
- Due to their autoagglutination, EAEC strains exhibit a characteristic “stacked brick” configuration on Hep-2 cells or glass.
- The plasmid-carried bundle-forming fimbriae of the bacteria (such as AAF/I and AAF/II) mediate this process.
- These EAEC strains produce a heat-stable, low-molecular-weight enterotoxin known as enteroaggregative heat-stable enterotoxin-1 (EAST-1).
- EAEC promotes mucus secretion, which forms a coating over the small intestine’s epithelium.
- This layer of biofilm traps the bacteria in the small intestine epithelium.
- In animal tests, they cause microvilli shortening, mononuclear infiltration, and bleeding.
- These strains are linked to recurrent diarrhoea with dehydration in newborns, particularly in underdeveloped nations.
Diffusely adherent E. coli
- DAEC strains are mostly associated with diarrhoea in children aged 1 to 5 years.
- These strains are distinguished by their adherence to cultivated cells.
- They lengthen the microvilli by trapping bacteria within the cell membrane.
Septicemia
- Bloodstream invasion by E. coli may result in septicemia.
- E. coli strains linked to UTIs or intra-abdominal infections, such as peritonitis and abscesses following intestinal perforation, induce septicemia.
- High death rates are associated with E. coli septicemia in immunocompromised individuals and those with initial abdominal or central nervous system infections (CNS).
Neonatal meningitis
- E. coli and group B streptococci are the leading causes of CNS infections in infants aged one month.
- The condition is caused by E. coli strains with the KI capsular antigen, which are widely seen in the digestive tracts of pregnant women and newborns.
Cultural Characteristics of E. coli/E. coli colonies morphology
- Escherichia coli or E. coli can grow on ordinary media like Nutrient Agar medium (NAM).
- Commonly the NAM & MacConkey Agar medium is used for the cultivation of Escherichia coli in the Laboratory.
- The optimum temperature required for most of the E.coli strains is 37°C.
- E. coli can survive at 4.5– 9.5 pH but the optimum pH is 7.0.
- Escherichia coli (E. coli) is an aerobic bacterium which means it can grow best in the presence of oxygen and it is also a Facultative anaerobic organism which means it can also grow in a low oxygen environment.
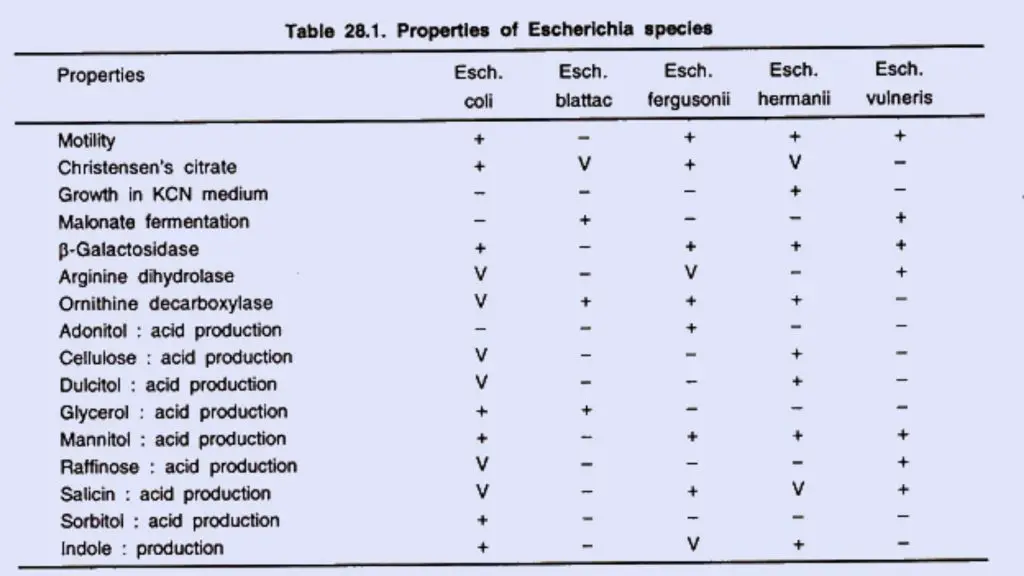
The following cultural media is used to grow E. coli.
- Nutrient Agar (NA): The E. coli colony on Nutrient agar media appears large, circular, low convex, grayish, white, moist, smooth, and opaque. There are two forms such as Smooth (S) form and Rough (R) form. The Smooth forms of E. coli are emulsifiable in saline. The smooth to rough variation (S-R variation) occurs due to repeated subculture.
- Blood Agar (BA): The Colonies of E. coli are big, circular, gray and moist. Beta (β) hemolytic colonies are formed.
- MacConkey Agar (MAC): The E. coli Colonies are circular, moist, smooth and of entire margin; appear flat and pink. These are lactose fermenting colonies.
- Mueller Hinton Agar (MHA): The E. coli Colonies are pale straw colored.
- Eosin Methylene Blue (EMB) Agar: Green Metallic sheen colonies are formed.
- m-ENDO Agar: The E. coli Colonies are green metallic sheen. Metabolize lactose with the production of aldehyde and acid.
- Violet Red Bile Agar (VRBA): The colonies are red (pink to red). Bluish fluorescence is seen around colonies under UV.
- Cystine Lactose Electrolyte-Deficient (CLED) Agar: They give lactose positive yellow colonies.
- Liquid Media: They exhibit homogenous turbid growth within 12-18 hours. R form agglutinates spontaneously, forming sediment on the bottom of the test tubes. After prolonged incubation (>72 hrs), pellicles are formed on the surface of liquid media. Heavy deposits are formed which disperses on shaking.
Culture characteristics of E. coli
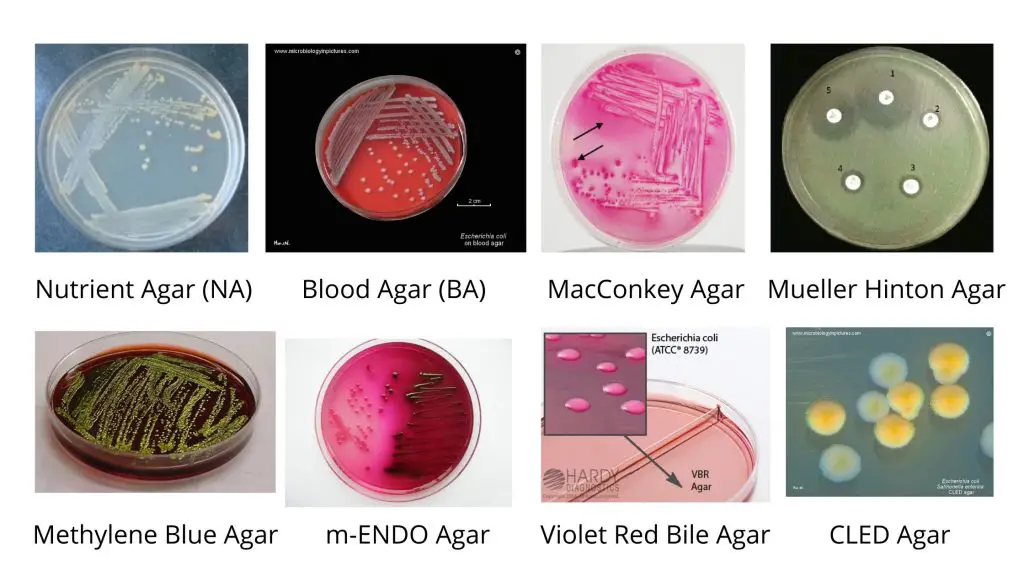
| Cultural Characteristics | Nutrient Agar Medium (NAM) | Eosin Methylene Blue (EMB) Agar medium | MacConkey Agar medium | Blood Agar Medium |
|---|---|---|---|---|
| Shape | Circular | Circular | Circular | Circular |
| Size | 1-3 mm | 2-3 mm | 2-3 mm | 1-3 mm |
| Elevation | Convex | Convex | Convex | Convex |
| Surface | Smooth (fresh isolation) ; Rough (repeated subculture) ; mucoid (capsulated strains) | Smooth (fresh isolation) ; Rough (repeated subculture) ; mucoid (capsulated strains) | Smooth (fresh isolation) ; Rough (repeated subculture) ; mucoid (capsulated strains) | Smooth (fresh isolation) ; Rough (repeated subculture) ; mucoid (capsulated strains) |
| Color | Greyish white | Green metallic sheen | Pink | Greyish white |
| Structure | Translucent –Opaque | Opaque | Opaque | Translucent –Opaque |
| Hemolysis | —– | —– | —– | β-Hemolysis (in some strains) |
| Emulsifiability | Smooth form – Easily emulsifiable; Rough forms – Autoagglutinable, hence do not emulsify easily | Smooth form – Easily emulsifiable; Rough forms – Autoagglutinable, hence do not emulsify easily | Smooth form – Easily emulsifiable; Rough forms – Autoagglutinable, hence do not emulsify easily | Smooth form – Easily emulsifiable; Rough forms – Autoagglutinable, hence do not emulsify easily |
E. coli colonies morphology
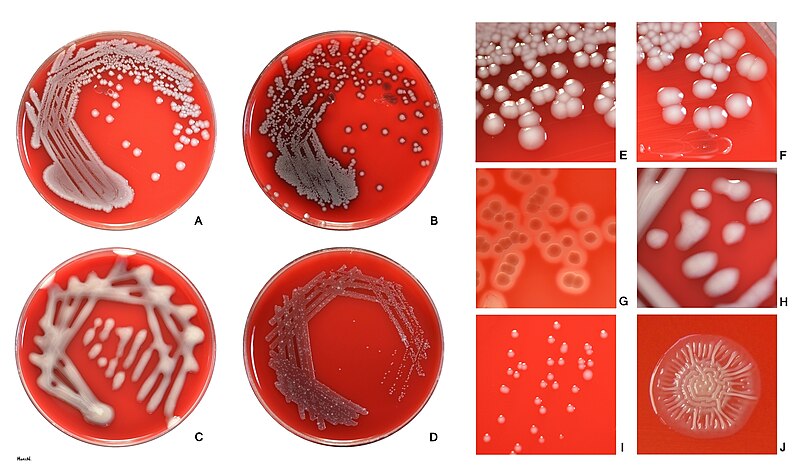
- Shape: E. coli colonies typically appear as circular or slightly irregular-shaped colonies.
- Color: The color of E. coli colonies is usually creamy or off-white, but it can vary depending on the growth medium and conditions. It is important to note that certain strains of E. coli, such as those containing plasmids with color genes, may exhibit unique colors like red, green, or blue.
- Size: E. coli colonies are generally small to medium-sized, ranging from 1 to 3 millimeters in diameter on standard agar plates. However, the size can vary depending on factors such as nutrient availability and incubation time.
- Texture: E. coli colonies typically have a smooth and shiny surface. However, certain strains may exhibit variations in texture, such as rough or mucoid colonies.
- Opacity: E. coli colonies are typically opaque, meaning they do not allow light to pass through them. This characteristic can be useful in differentiating E. coli from other bacterial species.
- Margin: The margin or edge of E. coli colonies is often well-defined and regular. It appears smooth and distinct from the surrounding agar.
- Elevation: E. coli colonies usually exhibit a convex or slightly raised elevation, forming a dome-shaped structure. However, the elevation can vary depending on the strain and growth conditions.
Toxin of E. coli
Except endotoxin associated with O antigen, some E. coli strains produce two types of exotoxin such as enterotoxin and haemolysin.
Enterotoxins
- Enterotoxins cause diarrhea, these are of two types such as heat labile (LT); heat stable (ST).
- The LT is similar to cholera enterotoxin which is similar antigenically and in its mechanism of action. It acts by stimulating the adenyl cyclase—cyclic adenosine monophosphate (cAMP) system to produce fluid accumulation in the intestinal lumen.
- ST stimulates fluid secretion into the gut by the mediation of cyclic guanosine monophosphate (cGMP) resulting in dehydration.
Haemolysin
- E. coli produced 3 types of hemolysins which are not related to pathogenesis.
- E. coli produces a part of normal intestinal flora of man and animals and the commensal strains belong to several O groups.
- There are many strains of E. coli which include commensal strains as well as strains with virulence determinants that cause a wide variety of infections of all age groups of men and animals.
Clinical infections of E. coli
The virulent form of E. coli found in the gut (enteritis) and of extra-intestinal sites (urinary tract infection, wound infection). The pathogenic form of E. coli is responsible for these following infections;
- Urinary tract infection (UTI)
- Septic infections of wound
- Diarrhoea
- Dysentery
- Septicaemia
- Pneumonia
- Neonatal meningitis
- Abscess in various organs.
There are four groups of E. coli which are responsible for diarrhoea in infants, children and adults are such as Enteropathogenic E. coli (EPEC); Enterotoxigenic E. coli (ETEC); Enteroinvasive E. coli (EIEC); Enterohaemorrhagic E. coli (EHEC).
Enteropathogenic E. coli (EPEC)
- EPEC causes infantile diarrhoea.
- They infect by adhering to the intestinal mucosa, and then cause the loss of microvilli and prevent the entry of bacteria into the mucosa.
- EPEC also produce a shigella-like toxin.
Enterotoxigenic E. coli (ETEC)
- The enterotoxins are now known to produce diarrhoea in children with dehydration, traveller’s diarrhoea in adults, and sometimes cholera infantum similar to cholera.
- ETEC also possesses colonisation factors (pili, K antigen) to enhance their virulence.
Enteroinvasive E. coli (EIEC)
- They do not produce enterotoxin but invade the intestinal mucosa like dysentery bacilli.
- Responsible for kerato-conjunctivitis on installation into the eyes of guinea pig (Sereny test) which is a diagnostic method for EIEC.
- Another diagnostic method is their invasion of HeLa cells in tissue culture.
- EIEC is a late lactose fermenter and may be anaerogenic.
- They have an antigenic relationship with shigella.
Enterohaemorrhagic E. coli (EHEC)
- EHEC has been very recently identified and is found to cause colitis with marked haemorrhage and absence of fever, producing Verotoxin (Cytotoxin) which affects the Vero cells in tissue culture.
Laboratory Diagnosis of Escherichia Coli
Diarrhoea
- To detect the EPEC, fresh diarrhoeal stool is plated directly on blood agar and MacConkey agar medium.
- After overnight incubation, E. coli colonies are emulsified in saline on a slide and tested by agglutination with polyvalent and monovalent O antiserum against entero-pathogenic serotypes and further identified by biochemical tests.
- Enzyme linked immuno-sorbent assay (ELISA) test is simplest and used to detect LT of ETEC.
- LT and cholera entero-toxin are antigenically similar. Sereney test is the only method available recently to demonstrate EIEC.
Urinary Tract Infection
- Most of the urine specimens are collected from adult patients by the clean-catch midstream technique.
- The detection of bacteria can be done microscopically by using Gram staining of uncentrifuged urine specimens, Gram staining of centrifuged specimens, or direct observation of bacteria in urine specimens.
- During the staining procedure the E coli appears as a non-spore-forming, Gram-negative rod-shaped bacterium.
- During the semi-quantitative method the routine urine cultures should be plated using calibrated loops.
- Only use blood agar and MacConkey’s agar for routine cultures.
- The culture must be incubated overnight at 35°C–37°C in ambient air before being read.
Other Test/ Reactions
- E. coli typically produces positive test results for indole, lysine decarboxylase, lactose, and mannitol fermentation and produces gas from glucose.
- An isolate from urine can be quickly identified as E. coli by its hemolysis on blood agar, typical colonial morphology with an iridescent “sheen” on differential media such as EMB agar, and a positive spot indole test result.
- More than 90% of E. coli isolates are positive for β-glucuronidase using the substrate 4-methylumbelliferyl-β-glucuronide (MUG).
Clinical Syndromes of Escherichia coli
E. coli causes (a) urinary tract infections, (b) gastroenteritis, (c) septicemia, (d) neonatal meningitis, and (e) other infections.
Urinary tract infections
- E. coli is the most prevalent bacteria responsible for more than 80% of all UTIs acquired in the community.
- E. coli causes a variety of urinary tract infections (UTIs), including urethritis or cystitis without complications, symptomatic cystitis, pyelonephritis, acute prostatitis, prostatic abscess, and urosepsis.
- Uropathogenic strains of E. coli predominately colonise sexually active females who develop simple cystitis.
- The periurethral region is subsequently colonised by E. coli due to faecal pollution, and the bacteria enter the urinary bladder through sexual contact.
Gastroenteritis
Diverse forms of diarrhoea are caused by distinct subtypes of E. coli.
- The pathogens EPEC, EAEC, and DAEC cause diarrhoea and dysentery. These conditions are more prevalent in underdeveloped nations. EPEC affects newborns and children predominantly. These strains induce acute, watery diarrhoea, which can lead to dehydration or become chronic and result in failure to thrive. EAEC and DAEC also cause diarrhoea comparable to that of EPEC.
- ETEC is widespread in regions with inadequate sanitation and is a pervasive contamination of food and water sources. Therefore, ETEC is the leading cause of both traveler’s diarrhoea and infantile diarrhoea in developing nations. Because local adult populations gain immunity to ETEC surface antigens, the sickness is limited to immunologically naive travellers and weaning infants. The incubation period is brief, ranging from one to three days. The disorder is characterised by the abrupt onset of diarrhoea that is free of blood, mucous, and faecal leukocytes. There may be vomiting, although the majority of individuals have no fever. This condition is self-limiting and lasts fewer than 5 days.
- EIEC causes diarrhoea and dysentery, comparable diseases to those brought on by Shigella species. These strains induce diarrhoea, dysentery, fever, vomiting, tenesmus, and abdominal cramps. Typically, stool contains blood and leukocytes.
- EHEC is a significant source of foodborne illness, especially in affluent nations like the United States and Canada. EHEC is a typical microbial flora of the cattle gut. Consequently, cattle are the principal reservoir for EHEC strains that cause diarrhoea in people. Consumption of beef products or foods contaminated with cattle faeces carrying EHEC strains results in illness.
By manufacturing two Shiga toxins, EHEC produce two different syndromes: hemorrhagic colitis and hemolytic uremic syndrome (HUS). The Stx is responsible for the infection’s systemic complications and symptoms.
Hemorrhagic colitis
- The incubation period ranges between 1 and 5 days.
- The symptoms range from diarrhoea to severe hemorrhagic colitis.
- Frequently accompanying watery diarrhoea are stomach cramps and vomiting.
- In the majority of patients, diarrhoea develops bloody after 1–2 days, but is not typically accompanied by faecal leukocytes. Fever is present in around one-third of patients.
- Typically, the illness lasts between 4 and 10 days.
- EHEC diarrhea’s most prevalent strain, E. coli O157:H7, has a low infectious dose (about 100 bacilli) and travels quickly from kid to child via the fecal–oral pathway.
Hemolytic uremic syndrome (HUS)
- 10–15% of children with EHEC diarrhoea have a serious, potentially fatal consequence.
- Microangiopathic hemolytic anaemia, thrombocytopenia, and renal insufficiency are significant symptoms.
- Typically arises during the second week of illness, after diarrhoea has been treated.
- Anemia, weakness, irritability, and oliguria or anuria are present.
- In 10% of HUS patients, chronic renal failure may develop.
- Mortality rate: around 3–5%.
- EHEC-positive stool cultures are always negative in patients with HUS.
Septicemia
- E. coli causes septicemia, which is commonly associated with UTI, particularly in cases of urinary blockage due to any reason.
- The bacteria’s endotoxin or LPSs create a systemic reaction that can result in disseminated intravascular coagulation and even death.
- Mortality and morbidity rates linked with E. coli septicemia are identical to those associated with other aerobic Gram-negative bacilli.
Neonatal meningitis
- Urinary tract infections (UTIs) are commonly associated with E. coli septicemia, particularly when the urinary system is obstructed.
- Endotoxin or lipopolysaccharides (LPSs) from bacteria can cause a systemic reaction that can lead to DIC and even death.
- The mortality and morbidity rates of septicemia induced by E. coli are identical to those caused by any other aerobic Gram-negative bacterium.
Other infections
- These include intra-abdominal infections caused by E. coli, which are frequently the result of a perforated appendix, diverticulum, intra-abdominal abscess, cholecystitis, and ascending cholangitis.
- E. coli can also cause septic arthritis, endophthalmitis, sinusitis, osteomyelitis, endocarditis, and infections of the skin and soft tissues.
Demonstration of toxins of diarrheagenic E. coli
- A laboratory diagnosis of diarrhoea caused by diarrheagenic E. coli can be made by culturing faeces and demonstrating the presence of bacilli.
- The patient’s faeces are collected in a sterile container and transported directly to the laboratory.
- The faeces are directly inoculated onto the MacConkey and blood agar media.
- As mentioned previously, the plates are incubated overnight at 37°C and examined for the characteristic lactose-fermenting colonies on MacConkey and betahemolytic colonies on blood agar.
- Since E. coli is present in the intestine as commensals and is therefore detectable in normal faeces, it is necessary to conduct a battery of diagnostic tests in order to classify it as a diarrheagenic pathogenic E. coli strain.
- These strains are distinguished by (a) serotyping, (b) animal inoculation, (c) cytopathic effects in cell cultures, or (d) genetic techniques.
Identification of EPEC
- Commonly related with EPEC outbreaks include the serogroups O26, O55, O86, O111, O114, O119, O125, O126, O12, O128, and O142 of E. coli.
- Consequently, colonies of E. coli isolated from faeces on MacConkey agar are recognised by agglutination assays with particular polyvalent and monovalent antisera.
- In this technique, a saline suspension of E. coli colonies is prepared on a microscope slide and then combined with a drop of particular polyvalent and monovalent antisera against EPEC serogroups.
- Minimum of 10 colonies per plate must be tested.
- If individual colonies are negative, confluent growth is emulsified and tested.
- If E. coli colonies exhibit agglutination with a specific serogroup (for example, O111) in a positive test, then the isolate is identified as E. coli of that serogroup (O111).
Identification of ETEC
Because toxin production is not related with specific E. coli serogroups, the diagnosis of ETEC diarrhoea rests on the presence of enterotoxin in E. coli isolates from stool. ETEC strains are capable of producing either LT or ST, or both. Numerous tests are available to demonstrate the LT or ST manufactured by ETEC. Therefore, these tests are utilised for the detection and identification of ETEC isolates from faeces.
- The presence of LT in E. coli isolates can be established by:
- Method demonstrating fluid buildup in the ileum of rabbits.
- Method demonstrating the permeability factor of the toxin in adult rabbit skin.
- Tissue culture tests (rounding of Y1 mouse adrenal cells and elongation of Chinese hamster ovary cells [CHO] cells due to intracellular increase of cAMP concentration).
- Serological examinations (agar gel diffusion, reverse passive hemagglutination, and enzyme-linked immunosorbent assay).
- Molecular probes These are offered for the detection of LT in E. coli isolates from stool cultures or in faeces directly.
- The existence of ST in E. coli isolates can be demonstrated by:
- The infant mouse test is still routinely used to identify ST in E. coli isolates.
- Genetic probes—for detecting ST in E. coli isolates from stool cultures or in faeces directly.
Identification of EIEC
A significant number of EIEC strains are unusual E. coli strains. They are non-motile and do not digest lactose, or they ferment it very slowly with acid generation but without generating gas. Additionally, they do not decarboxylate lysine. EIEC are distinguished by:
Sereny test
- By injecting isolated EIEC into the conjunctival sac of guinea pigs, the test is conducted.
- After 72 hours, the animal is checked for mucopurulent conjunctivitis and severe keratitis.
Cell culture test
- HeLa or HEP-2 cells can be used to demonstrate the presence of the toxin.
- The bacterial suspension is introduced to the cell monolayer.
- The cells are next checked for the presence of intracellular E. coli because, if present, EIEC will invade the cells and reproduce within them.
VMA enzyme-linked immunosorbent assay
- This is a serological test used to detect the plasmid that encodes the VMA outer membrane antigens in stool isolates of EIEC.
Identification of EHEC
- The most prevalent serotype associated with clinical disease caused by EHEC strains is E. coli O157:H7.
- Typically, the strain does not ferment sorbitol; hence, sorbitol MacConkey media is usually employed to cultivate the strain from stool. Methods for identifying EHEC strains include:
- EHEC’s cytotoxic effects on Vero or HeLa cells are demonstrated.
- Employing DNA probes for the VT1 and VT2 genes in EHEC directly from faeces or culture isolates.
Identification of other strains
- Using agglutination assays with particular antisera, EAEC strains are identified.
- The majority of them are typed by particular H antisera rather than O antisera.
Treatment of E. coli infections
- Different antibacterial medicines can be used such as sulfonamides, ampicillin, cephalosporins, fluoroquinolones, and aminoglycosides.
- E. coli meningitis needs antibiotics, like as third-generation cephalosporins (eg, ceftriaxone).
- Respiratory support, adequate oxygenation, and antibiotics, such as third-generation cephalosporins or fluoroquinolones are required for E. coli pneumonia.
- In case of diarrheal disease the patient should drink plenty of fluids to avoid dehydration and to get as much rest as possible. Avoid dairy products, these may induce temporary lactose intolerance, and therefore make diarrhea worse.
Prevention and Control of E. coli infections
- It is universally suggested that attention be observed in regard to food and drink in areas where environmental hygiene is inadequate and that early and brief treatment (eg, with ciprofloxacin or trimethoprim-sulfamethoxazole) be substituted for prophylaxis.
- We can control E. coli infections by handwashing, rigorous asepsis, sterilization of equipment, disinfection, restraint in intravenous therapy, and strict precautions in keeping the urinary tract sterile (ie, closed drainage).
Medical Importance of E. Coli
- E. coli is used to produce insulin vis adopting the genetic engineering technique.
- In intestine, it Produces certain vitamins.
- It is used as a parameter to determine the fecal contamination of drinking water.
- In bacterial genetics, it is used for the plasmid study.
References
- Ananthanarayan and Paniker. Textbook of Microbiology.
- Bailey and Scott’s Diagnostic Microbiology. Part 3. Section 7.
- Mackie and McCartney Practical Medical Microbiology.
- Murray, P. R., Rosenthal, K. S., & Pfaller, M. A. (2013). Medical microbiology. Philadelphia: Elsevier/Saunders
- Sastry A.S. & Bhat S.K. (2016). Essentials of Medical Microbiology.
- Scaletsky, I. C., Fabbricotti, S. H., Carvalho, R. L., Nunes, C. R., Maranhão, H. S., Morais, M. B., & Fagundes-Neto, U. (2002). Diffusely adherent Escherichia coli as a cause of acute diarrhea in young children in Northeast Brazil: a case-control study. Journal of clinical microbiology, 40(2), 645-8.
- Subhash Chandra Parija. Textbook of Microbiology & Immunology.
- Topley and Wilson’s Microbiology and Microbial Infection. Bacteriology Volume 2. Part VI. Organisms and their biology. Chapter 36. Escherichia, 1360.
- https://paramedicsworld.com/escherichia-coli/morphology-culture-characteristics-of-escherichia-coli/medical-paramedical-studynotes
- https://www.microbiologyinpictures.com/escherichia%20coli.html
- https://www.biologydiscussion.com/bacteriology/systematic-bacteriology/escherichia-coli-e-coli-meaning-morphology-and-characteristics/30821
- https://en.wikipedia.org/wiki/Escherichia_coli
