Table of Contents
The technique of fluorescence spectrophotometry measures the intensity of light emitted by a substance after it has been excited by a certain wavelength of light. This method is employed to investigate the characteristics of molecules and detect the presence of specific chemicals in a sample.
The origins of fluorescence spectrophotometry can be traced back to the early 20th century, when scientists began to investigate the phenomena of fluorescence. In the 1920s and 1930s, scientists such as George Van Nevel and Robert Boyle began to develop methods for measuring the fluorescence intensity, providing the groundwork for the present approach of fluorescence spectrophotometry.
Fluorescence spectrophotometry is an essential instrument in numerous disciplines, such as chemistry, biology, and medicine. It is used to research the characteristics of molecules and identify unknown substances in chemistry. It is used to examine the structure and function of proteins and other biomolecules in biology. In the medical field, it is used to detect the presence of disease indicators and investigate the interactions between medications and biological substances.
Fluorescence spectrophotometry is also utilised in numerous industrial and environmental applications, including water analysis, food safety and quality management, and forensic science.
Overall, fluorescence spectrophotometry is a potent technology that permits scientists to investigate the properties of molecules and detect the presence of certain compounds with a high degree of sensitivity and specificity.
What is fluorescence spectrometry?
- Fluorescence spectrometry, commonly referred to as fluorimetry or spectrofluorometry, is a sophisticated branch of electromagnetic spectroscopy dedicated to the analysis of fluorescence emitted from a specimen.
- This technique employs a specific light beam, predominantly in the ultraviolet range, to stimulate the electrons present in certain molecular compounds. Upon excitation, these electrons release light, which is often within the visible spectrum, though not exclusively.
- This method stands distinct from absorption spectroscopy, another approach that investigates the light absorption properties of molecules. However, in the unique context of single molecule fluorescence spectroscopy, the technique delves deeper, capturing the intensity fluctuations of emitted light from individual fluorophores or their pairs.
- Fluorescence spectrometry’s core strength lies in its ability to swiftly and economically determine an analyte’s concentration in a solution, leveraging the fluorescent attributes of the analyte. When the nature of the compound under investigation is established, this method facilitates quantitative analysis, enabling the determination of analyte concentrations with relative ease. Typically, this modality is employed for analyzing substances in liquid form.
- The operational mechanism of fluorescence spectroscopy involves directing a light beam, with wavelengths ranging from 180 to 800 nm, through a cuvette containing the solution of interest. Subsequently, the emitted light from the sample is measured at a specific angle.
- This technique permits the capture of both the excitation spectrum (light absorbed) and the emission spectrum (light emitted) from the sample. The emission intensity directly correlates with the analyte’s concentration and is influenced by various factors, including the excitation wavelength, solvent concentration, cuvette path length, and sample’s inherent properties.
- A pivotal component in fluorescence spectroscopy is the fluorophore, a molecular entity responsible for the fluorescence phenomenon. Predominantly, fluorophores comprise molecules with aromatic structures, such as Tryptophan, Tyrosine, and Fluorescein.
- Luminescence, a broader category encompassing fluorescence, is the emission of light from a substance not attributed to heat. This emission can arise from various sources, including chemical reactions, electrical stimuli, or even stress in crystals. For luminescence to manifest, the material in question must be a semiconductor with a non-zero band gap and must receive external energy to initiate the process.
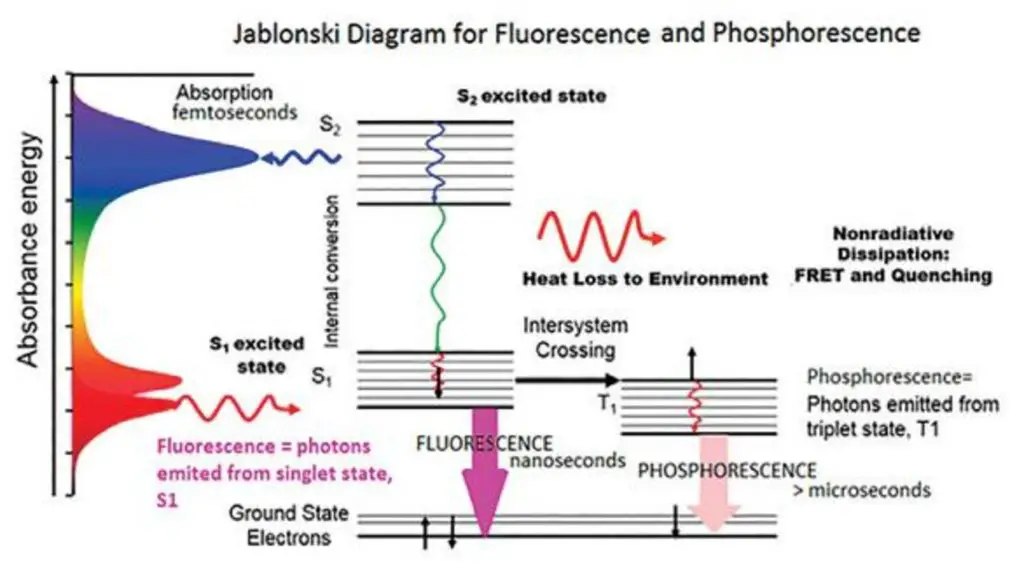
Principle of fluorescence spectroscopy
Fluorescence spectroscopy is a scientific technique that delves into the study of molecular and atomic interactions by analyzing the fluorescence emitted from a sample. The fundamental principle underpinning this method is the radiative emission that arises when a molecule absorbs energy at a specific wavelength where it exhibits a transition dipole moment. Upon absorbing this energy, the molecule’s photons are elevated to an excited singlet state. These photons then undergo a relaxation process, transitioning to the lowest vibrational energy level of the excited state. As they revert to the molecule’s ground state, they emit photons, producing fluorescence.
Three primary nonradiative relaxation mechanisms exist for fluorescent molecules wherein the excitation energy isn’t converted into photons: internal conversion, external conversion, and intersystem crossing. Internal conversion transpires when there’s a minimal energy gap between two electronic states, leading to a shift of electrons from a higher to a lower energy state. This energy is then channeled into the vibrational modes of the electronic state. External conversion results in energy loss due to the fluorophore’s interactions with surrounding solute molecules. Intersystem crossing is observed when energy levels of the excited singlet and triplet states overlap, causing electrons to transition between these states. The photon emission resulting from electrons reverting to their ground state from the triplet state is termed phosphorescence. Notably, phosphorescence peaks manifest at longer wavelengths compared to fluorescence peaks due to the triplet state’s reduced energy. Given the forbidden nature of these transitions, phosphorescence has a more extended duration relative to fluorescence.
Fluorescence intensity is directly proportional to the excitation light’s intensity and can be represented by the equation:
F=2.303×K×I0×ϵ×b×c
Where:
- F is the fluorescence intensity
- K is a constant specific to the instrument’s geometry
- I0 denotes the excitation light’s intensity
- ϵ is the molar absorptivity of the fluorophore
- b represents the pathlength
- c is the concentration of the sample
Fluorescence spectroscopy offers insights into the electronic and vibrational states of molecules. Typically, the species under study has a ground electronic state and an excited electronic state. Upon fluorescence, the species absorbs a photon, transitioning from its ground state to one of the excited electronic state’s vibrational states. This transition and the subsequent photon emissions provide valuable information about the structure of the vibrational levels. In atomic species, resonance fluorescence is observed, where the emitted photon often has the same wavelength as the absorbed one.
In practice, fluorescence measurements can be categorized into emission and excitation measurements. In emission measurements, while the excitation wavelength remains constant, the detection wavelength varies. Conversely, in excitation measurements, the detection wavelength is fixed, and the excitation wavelength is altered. An emission map, a three-dimensional data representation, is constructed by collating emission spectra from various excitation wavelengths, offering a comprehensive view of emission intensity as a function of both excitation and emission wavelengths.
Fluorescence Phenomenon
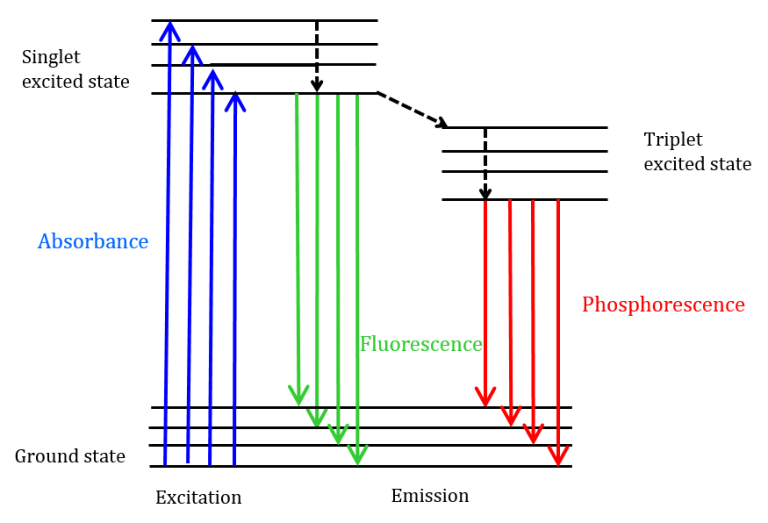
The phenomenon of fluorescence is rooted in the intricate energy levels inherent to every molecule. When a molecule absorbs a photon, an electron within it is elevated from its foundational state, termed the ground state (S0), to one of the multiple available excited singlet states (S1, S2, and so forth).
Molecules that are particularly predisposed to such transitions often possess intricate atomic structures. These structures may include atoms with unpaired electron sets, such as oxygen (O) and nitrogen (N), or they might have aromatic and aliphatic conjugated unsaturated systems that facilitate extensive electron delocalization.
Following this excitation, the electron descends to the lowest vibrational level of the primary excited state, S1. This descent releases energy through a process termed “internal conversion.”
The excited state then undergoes further relaxation to its foundational vibrational level. From the S1 state, which typically persists for approximately 10-9 seconds, the molecule can employ various pathways to revert to its ground state (S0). Among these pathways are:
- Fluorescence: This is a radiative process where the energy lost during internal conversion leads to the emission of photons. Notably, these emitted photons possess lesser energy compared to the initially absorbed ones.
- Nonradiative Processes: These encompass several mechanisms, including: a. Internal Conversion: This involves relaxation either via internal vibrations or through collisions with surrounding solvent or solute molecules. b. Energy Transfer: Here, energy is transferred to another chemical entity, instigating a photochemical reaction or leading to the creation of an excited-state dimer (referred to as an excimer) or an excited-state complex (known as an exciplex). These structures emit photons at wavelengths that are more extended than those of fluorescence. c. Intersystem Crossover: This process transitions the molecule to a triplet state, T1, which has a significantly prolonged lifespan (often exceeding 10-5 seconds) when compared to its S1 predecessor. Subsequently, this state can either emit energy through a delayed mechanism known as phosphorescence or undergo relaxation via internal conversion methods.
In essence, the fluorescence phenomenon is a manifestation of the intricate dance of electrons within molecular structures, transitioning between various energy states and employing multiple pathways to return to equilibrium.
The Electronic Excited State
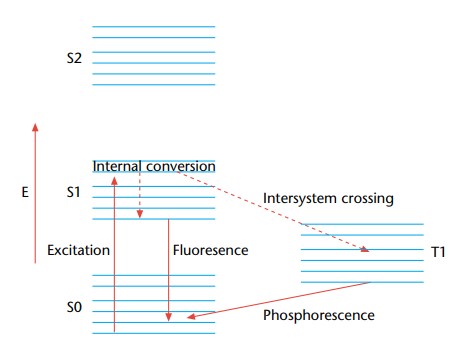
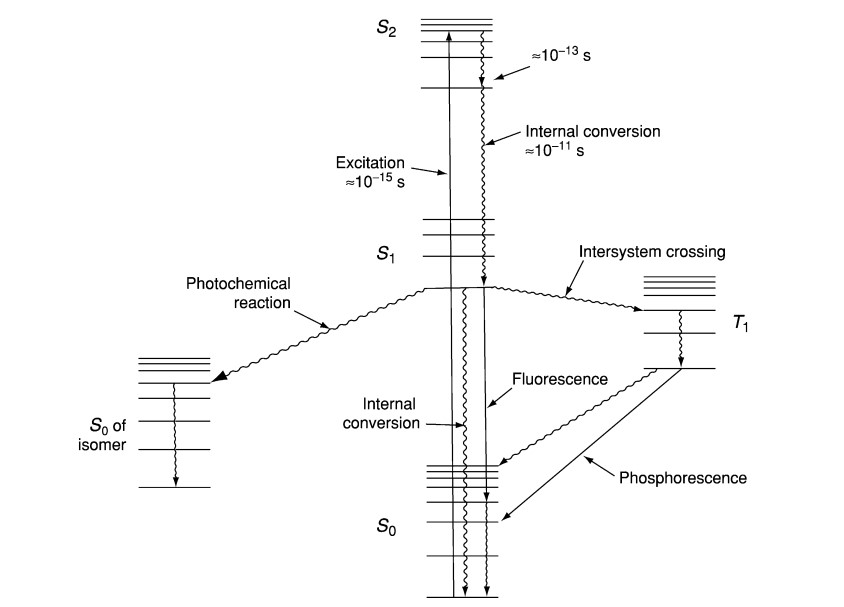
- The electronic excited state is a fundamental concept in the realm of fluorescence and phosphorescence processes. These processes are fundamentally associated with the relaxation of molecules from their excited electronic states to their ground states, involving the emission of photons. This phenomenon is notably pertinent to polyatomic fluorescent compounds, often referred to as fluorophores, which undergo transitions between electronic and vibrational states.
- To elucidate the structural aspects of the excited state and the associated transitions, the Jablonski diagram serves as a valuable graphical representation. In this diagram, the energy differences between electronic states are typically in the range of 10,000 to 1 cm. Each electronic state further consists of sublevels that correspond to the vibrational modes of the molecule, with an energy separation between vibrational levels of approximately 100 to 1 cm.
- Crucially, to induce an electronic transition, photons with energy falling within the ultraviolet to blue-green portion of the electromagnetic spectrum are requisite. This is due to the substantial energy gap between the excited and ground electronic states, which far exceeds thermal energy levels. Consequently, thermodynamic considerations dictate that molecules predominantly exist in their electronic ground state.
- The electronic excited states of polyatomic molecules can be further classified based on their multiplicity. Electrons’ indistinguishability and the Pauli exclusion principle dictate that electronic wave functions exhibit symmetric or asymmetric spin states. Symmetric wave functions, known as triplet states, possess a multiplicity of three, while the antisymmetric wave function, known as the singlet state, has a singular multiplicity.
- Optical transitions typically couple states with the same multiplicity to the first order. This optical transition propels molecules from the lowest vibrational level of the electronic ground state to a vibrational level attainable in the electronic excited state. Since both the ground and excited electronic states are singlets, rapid vibrational relaxation ensues following excitation, occurring on a femtosecond to picosecond timescale. This process gives rise to the Stoke shift, which describes the observation that fluorescence photons exhibit longer wavelengths than the excitation light.
- Fluorescence lifetime, on the order of nanoseconds, denotes the duration during which the fluorophore retains the lowest vibrational level of the excited electronic state. Fluorescence emission transpires when the singlet electronic excited states of the fluorophore decay to a permissible vibrational level in the electronic ground state.
- The absorption and emission spectra of fluorescence provide insights into the vibrational level structures of the ground and excited electronic states, respectively. The Frank–Condon principle posits that electronic transitions minimally alter vibrational levels, leading to a resemblance between the vibrational level structures of the ground and excited electronic states. Consequently, absorption and emission spectra often exhibit mirrored characteristics.
- Furthermore, the excited state of an electron possesses specific polarization features. When light polarization aligns with a particular chemical axis, fluorophores are preferentially activated, a phenomenon referred to as the excitation dipole. Subsequently, fluorescence photons released by the molecule exhibit polarization aligned with another molecular axis, known as the emission dipole. These excitation and emission dipoles typically do not overlap, contributing to the unique characteristics of fluorescence phenomena.
Operating Procedure of Fluorescence spectroscopy
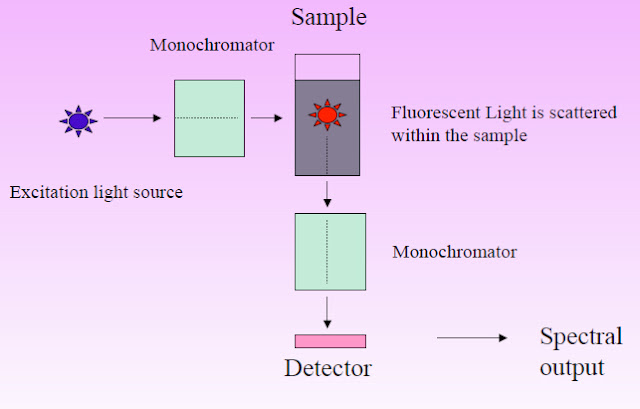
Fluorescence spectroscopy is executed through a well-defined operating procedure involving several critical components to ensure precise measurement. The instrument employed for this purpose consists of essential elements, including a light source, two monochromators, a sample holder, and a detector. The following steps elucidate the standard operational protocol:
- Light Source: The instrument is equipped with a light source capable of emitting radiation spanning the ultraviolet, visible, and near-infrared spectra. This source serves as the excitation beam for the sample.
- Monochromators: Two monochromators are employed in the setup. The first monochromator is responsible for selecting the specific wavelength for excitation, while the second monochromator is utilized for the precise analysis of emitted light.
- Detector Orientation: The detector, which typically comprises a photomultiplier tube, is positioned at a right angle to the excitation beam. This orientation allows for the efficient collection of fluorescence emitted from the sample in all directions.
- Excitation of Sample: The sample to be analyzed is placed within a specialized sample compartment, often in a cuvette with translucent quartz or glass sides. When the sample is exposed to the excitation light, the molecules within the solution become excited, leading to the emission of fluorescence.
- Excitation Monochromator: The excitation light from the lamp source is directed through an optical system to the excitation monochromator. This monochromator enables precise selection or scanning of a specific wavelength range for excitation.
- Fluorescence Emission: The selected excitation wavelength is then directed into the sample compartment containing the fluorescent cuvette. When the excitation light interacts with the sample, molecules within it become excited, and a portion of them emit light in response.
- Emission Monochromator: The emitted fluorescence, which radiates in all directions, is examined by the emission monochromator. This monochromator is positioned perpendicular to the incoming excitation beam and is responsible for wavelength analysis.
- Wavelength Analysis: The emission monochromator facilitates the measurement of fluorescence intensity at a predetermined wavelength, which is crucial for obtaining spectral information about the sample.
- Detector and Measurement: The selected wavelength of emitted light is directed by the analyzer monochromator to the detector, which is typically a photomultiplier tube. The photomultiplier tube measures the intensity of the emitted light, converting it into an electrical current.
- Data Display: The output current from the photomultiplier tube is sent to a measuring device that displays the level of fluorescence. This device provides a quantitative measure of the fluorescence intensity, allowing for the characterization and analysis of the sample.
In summary, fluorescence spectroscopy involves the precise manipulation and analysis of excitation and emitted light using monochromators, a sample compartment with a cuvette, and a detector. This well-defined procedure allows scientists to gather valuable information about the fluorescent properties of molecules and substances, making fluorescence spectroscopy an indispensable tool in various scientific disciplines.
Types of luminescence
Luminescence is a fascinating natural phenomenon that encompasses several mechanisms and applications. It involves the emission of light by a substance without the need for high temperatures, as in incandescence. Luminescence can be categorized based on various mechanisms and excitation sources, each with its unique properties and applications:
By Mechanism
- Fluorescence:
- Mechanism: Fluorescence is a luminescent phenomenon where a substance absorbs light at a specific excitation wavelength and then promptly re-emits light at a longer wavelength, known as the emission wavelength.
- Process: When a photon of light excites an electron in a molecule from its ground state to a higher energy state, the electron rapidly relaxes back to the ground state, releasing energy as emitted light.
- Applications: Fluorescence is prevalent in organic and inorganic compounds, including dyes, pigments, and biomolecules. It is utilized in fluorescence spectroscopy to study molecular properties and detect specific substances in samples.
- Phosphorescence:
- Mechanism: Phosphorescence shares similarities with fluorescence but involves a longer-lived excited state. In phosphorescence, a substance absorbs light at an excitation wavelength and emits light at a longer wavelength, even after the excitation source is removed.
- Process: Phosphorescence arises from a transition from a triplet state to the ground state, with slower relaxation compared to fluorescence.
- Applications: Phosphorescence is observed in certain organic and inorganic molecules, and it can be used for studying molecular properties and substance detection, although it is less commonly utilized than fluorescence.
By Excitation Source
- Chemiluminescence:
- Mechanism: Chemiluminescence is the emission of light resulting from a chemical reaction rather than excitation by an external light source.
- Process: Chemical reactions between reactants generate light-emitting species, such as excited atoms, ions, or molecules, leading to light emission.
- Applications: Chemiluminescence is used in various fields, including forensics, medical diagnostics, and environmental monitoring. Notable examples include bioluminescent reactions in fireflies and chemiluminescent assays for detecting substances in samples.
- Cathodoluminescence:
- Mechanism: Cathodoluminescence involves a substance emitting light when exposed to a high-energy electron beam.
- Process: A material is stimulated by high-energy electrons, leading to light emission, which is employed to investigate properties of semiconductors, minerals, and other materials.
- Applications: Cathodoluminescence is used in diverse applications, such as semiconductor and material characterization, geology, and defect analysis in materials.
- Electroluminescence:
- Mechanism: Electroluminescence occurs when a material emits light as a result of the passage of an electric current through it.
- Process: Semiconductor materials are commonly used in electroluminescent devices, like light-emitting diodes (LEDs), where electrical current excites the material to emit light.
- Applications: LEDs are prevalent in electronics, displays, lighting, and indicators due to their efficiency and versatility in producing various colors and brightness levels.
- Photoluminescence:
- Mechanism: Photoluminescence encompasses fluorescence and phosphorescence and occurs when a material emits light upon exposure to light.
- Process: A substance absorbs photons of light, exciting electrons to higher energy states, and subsequently emits light as the electrons return to lower energy states.
- Applications: Photoluminescence is utilized to study molecular characteristics, detect specific substances, and investigate material properties in various fields, including chemistry, biology, and materials science.
In summary, luminescence encompasses a diverse array of mechanisms and excitation sources, each with its own characteristics and applications. Understanding these mechanisms and their applications is fundamental to various scientific and technological disciplines.
What is a Fluorescence Spectrum?
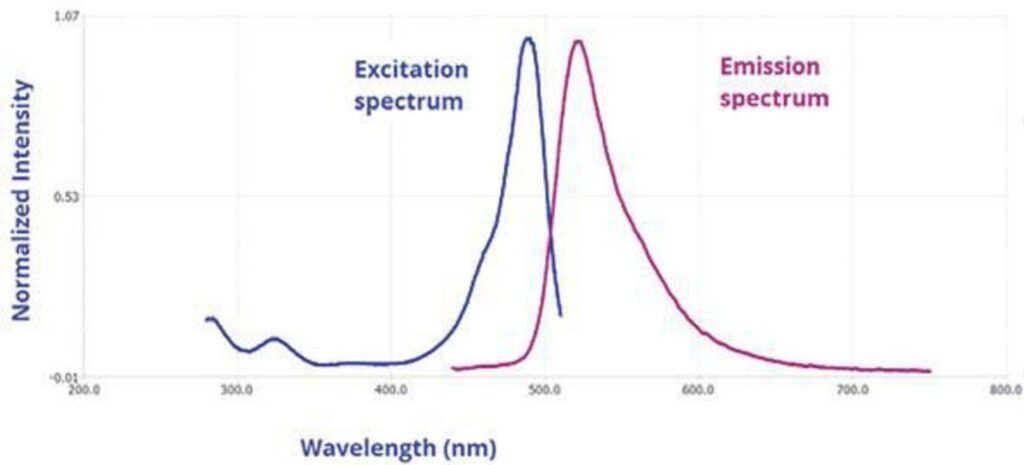
Fluorescence spectrum, a fundamental concept in fluorescence spectroscopy, provides valuable insights into the behavior of molecules when exposed to light. This scientific technique involves the measurement of emitted photons or intensity as a function of wavelength. It offers critical information about the excitation and emission properties of fluorophores, aiding in various applications, including molecular analysis, environmental monitoring, and material characterization.
Fluorescence Emission Spectrum:
A fluorescence emission spectrum is generated by keeping the excitation wavelength constant and scanning the emission wavelength. This process results in a plot of intensity against emission wavelength. Essentially, it reveals how a sample responds when exposed to a specific excitation wavelength, showing the wavelengths at which it emits fluorescence.
Key characteristics of a fluorescence emission spectrum include:
- Peak Emission Wavelength: This is the wavelength at which the intensity of emitted fluorescence is highest. It is a unique identifier for each fluorophore.
- Spectral Shape: The shape of the emission spectrum provides information about the fluorophore’s environment and any interactions it may have with neighboring molecules or conditions.
- Stokes Shift: The difference between the excitation and emission wavelengths is known as the Stokes shift. It reflects the energy lost during the relaxation process from the excited state to the ground state and is a fundamental property of the fluorophore.
Fluorescence Excitation Spectrum:
In contrast, a fluorescence excitation spectrum is obtained by holding the emission wavelength constant and scanning the excitation wavelength. This approach helps identify the wavelengths at which a sample absorbs light to emit fluorescence at a specific emission wavelength.
Crucial aspects of a fluorescence excitation spectrum include:
- Absorption Peaks: These correspond to the wavelengths at which the sample efficiently absorbs light and transitions from the ground state to the excited state. Absorption peaks are often used to excite fluorophores for subsequent fluorescence measurements.
- Relationship with Absorption Spectrum: In many cases, the excitation spectrum closely resembles the absorption spectrum, especially when the sample’s fluorescence is independent of the excitation wavelength. This similarity reinforces the connection between absorption and excitation processes.
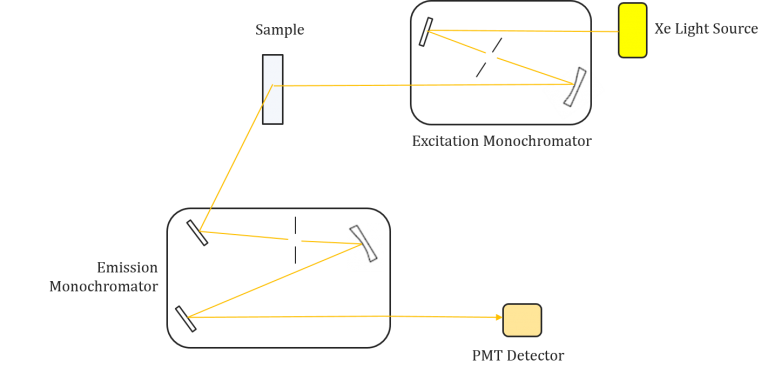
Significance:
Fluorescence spectra are powerful tools for understanding molecular properties and behavior. Researchers can utilize these spectra to investigate how various factors, such as temperature, concentration, or interactions with surrounding molecules, influence a sample’s fluorescence. Additionally, some fluorophores exhibit sensitivity to environmental factors like pH, polarity, and ion concentrations, which can be monitored using fluorescence spectroscopy.
Moreover, fluorescence spectra aid in the identification and quantification of specific substances in samples. By comparing the emitted fluorescence to known spectra of reference compounds, researchers can determine the presence and concentration of target molecules.
In summary, fluorescence spectra provide critical information about how molecules respond to light excitation, making them invaluable in diverse scientific and technological applications, from probing the properties of biomolecules to detecting environmental pollutants. These spectra offer valuable insights into the behavior of matter at the molecular level and play a central role in modern scientific research.
Instrumentation of fluorescence spectroscopy
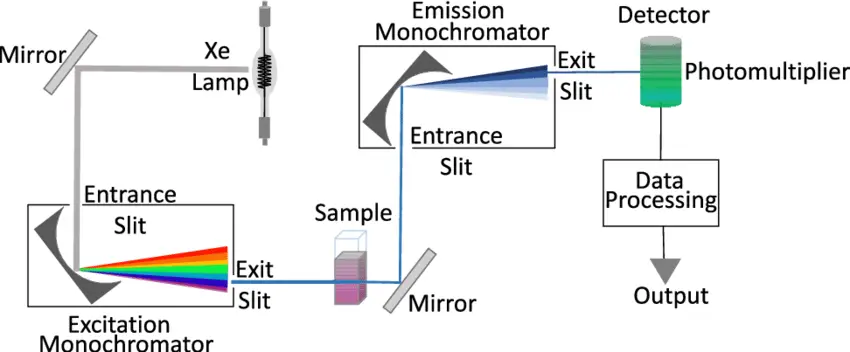
Fluorescence spectroscopy is a powerful analytical technique used to study the properties of molecules and detect specific substances in various applications. To perform fluorescence spectroscopy effectively, specialized instrumentation is required. This section discusses the key components and considerations involved in fluorescence spectroscopy instrumentation.
Components of Fluorescence Spectroscopy Instrumentation
- Light Sources:
- Fluorescence instruments require a suitable light source capable of generating excitation light. Various light sources can be employed, including gas discharge lamps (e.g., mercury vapor lamps), light-emitting diodes (LEDs), and lasers.
- Lasers are particularly powerful as they can provide narrow-band excitation at a wide range of wavelengths, including the ultraviolet region.
- The choice of light source depends on the specific application and the spectral range of interest.
- Photodetectors:
- Photodetectors are essential for capturing the emitted fluorescence from the sample.
- Common photodetectors include photodiodes and photomultiplier tubes (PMTs). PMTs are often preferred for their high sensitivity and fast response times, making them suitable for fluorescence lifetime measurements.
- The selection of photodetectors depends on the desired sensitivity and the nature of the fluorescence signal.
- Wavelength Selection:
- Fluorescence spectroscopy requires precise wavelength selection to isolate specific excitation and emission wavelengths.
- In basic filter fluorimeters, filters are used to select the desired wavelengths. More advanced instruments employ monochromators to provide flexibility in wavelength selection.
- Monochromators allow for the measurement of both excitation and emission spectra, enhancing the analytical capabilities of the instrument.
- Read-Out Devices:
- The output from the photodetector is typically amplified and displayed on a readout device.
- Digital displays are common for their clarity and precision. Microprocessor-based electronics can further enhance data processing and compatibility with external devices such as printers and computers.
- Continuous sensitivity adjustment in the amplifier allows for concentration measurements across a wide range.
- Sample Holders:
- Most fluorescence experiments are conducted in solution, and samples are held in cuvettes or flow cells.
- Cuvettes come in various shapes (circular, square, rectangular) and are made of materials that transmit both excitation and emission light.
- The choice of cuvette shape depends on factors such as pathlength and optical considerations.
- Different cuvette orientations (e.g., right-angled, front surface) can be used depending on the nature of the sample and the desired measurements.
- For highly concentrated samples, proper dilution is essential to prevent quenching of fluorescence.
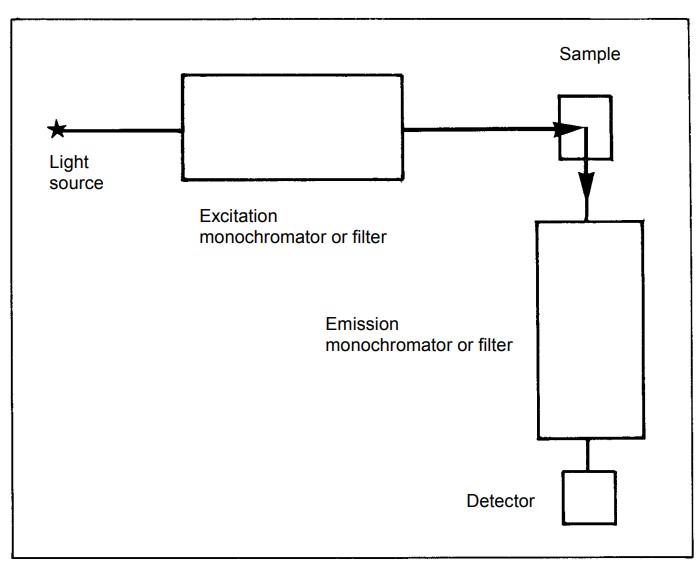
Considerations in Fluorescence Spectroscopy Instrumentation:
- Sensitivity: The sensitivity of the instrument, including the choice of light source and photodetector, should match the requirements of the application. High sensitivity is crucial for detecting low-concentration analytes.
- Resolution: The instrument’s resolution, determined by factors such as the slit width of monochromators, affects its ability to distinguish closely spaced spectral features. High resolution may be required for certain applications.
- Light Source Stability: The stability of the light source is critical for obtaining reliable and reproducible results. Light source output fluctuations can be monitored and corrected using additional photodetectors.
- Sample Preparation: Proper sample preparation, including dilution when necessary, is essential to ensure accurate measurements and prevent sample-related artifacts.
- Data Processing: Advanced instrumentation may include features for data processing, such as integration techniques, to improve precision and reduce noise in fluorescence measurements.
In summary, fluorescence spectroscopy instrumentation comprises several key components that work together to excite and detect fluorescence emissions from samples. The selection of appropriate components and careful consideration of factors like sensitivity and resolution are crucial for the success of fluorescence experiments in various scientific and analytical applications.
Factors that affect fluorescence spectroscopy
- Molecular rigidity: For fluorescence spectroscopy, rigid fluorophores are chosen since they have less vibrations and a lower probability of changing to triplet state. Fluorescein and eosin have hard structures and are very luminous, but phenolphthalein has a flexible structure and is not fluorescent.
- Solvent polarity: The degree of fluorescence can also be determined by the polarity of the solution. In the presence of heavy atoms in the solvent, the fluorescence of the structures can diminish.
- Dissolved oxygen: Oxygen dissolved in the solvent can also reduce the intensity of fluorescence emission. Fluorophore undergoes photochemical oxidation to accomplish this. Oxygen’s paramagnetic characteristics can also result in fluorescence quenching.
- pH: pH can impact the fluorescence of a substance. An illustration of this is aniline, which is a cation at low pH and an anion at high pH. In both situations, fluorescence is lost.
- Quenching: Quenching refers to a decrease in the intensity of fluorescence. This may be the result of fluorescence being absorbed by the solution or the fluorescent substance itself being absorbed. This effect is known as self-quenching.
- Conjugation: Molecules must be unsaturated, i.e., they must have π electrons, in order to absorb UV/vis radiation. If there is no absorption of radiation, fluorescence will not occur.
- Rigidity of structures: Rigid structures will emit more fluorescence, whereas flexible structures would emit less.
- Nature of substituent groups: Electron-donating groups, such as amino and hydroxyl, boost fluorescence activity. Electron-withdrawing groups, such as Nitro and carboxyl, decrease fluorescence. Fluorescence intensity is unaffected by groups such as SO3H and NH4+.
- Effect of temperature: Increase in temperature causes an increase in molecular collisions and a fall in fluorescence intensity, whereas a decrease in temperature causes a decrease in collisions and an increase in fluorescence intensity.
- Viscosity: Increased viscosity decreases molecular collisions, which increases fluorescence intensity, whereas decreasing viscosity increases molecular collisions, which decreases fluorescence intensity.
Applications of fluorescence spectroscopy
Fluorescence spectroscopy is a versatile and indispensable analytical technique with a wide range of applications across various industries. Its ability to provide detailed information about the properties of molecules and their interactions makes it a valuable tool in scientific research and practical applications. The following are selected examples of the diverse applications of fluorescence spectroscopy in different fields:
- Bioscience:
- Nucleic Acid Analysis: Fluorescence spectroscopy is widely used for the precise quantification of DNA and RNA samples. Extrinsically labeled fluorophores, such as ethidium bromide, are often employed to measure DNA concentration accurately.
- DNA Sequencing: Single-molecule real-time (SMRT) DNA sequencing utilizes fluorescence to achieve high-precision sequencing of long DNA molecules, revolutionizing genetic diagnostics.
- Industrial:
- Pollution Monitoring: Fluorescence spectroscopy serves as a rapid and non-invasive method for assessing pollution in industrial settings. For instance, it can detect organic contaminants in groundwater following hydraulic fracturing activities in the gas exploration industry.
- Chemical:
- Nanoparticle Characterization: Fluorescence spectroscopy plays a crucial role in characterizing nanoparticles used in applications such as drug delivery. It helps study interactions between nanoparticles and biomolecules, particularly the protein corona formed when nanoparticles interact with biological fluids.
- Environmental:
- Water Remediation: Fluorescence spectroscopy is applied in environmental monitoring, including the remediation of landfill leachates. It helps analyze dissolved organic matter in water samples and optimize treatment techniques for landfill leachate.
- Pharmaceutical:
- Quality Control: Fluorescence spectroscopy is used in the pharmaceutical industry for quality control of pharmaceuticals. For example, synchronous fluorescence spectroscopy is employed to test co-formulated pills, ensuring their quality and efficacy.
- Agricultural:
- Crop Identification: Spectroscopic techniques, such as laser-induced fluorescence emission technique (LIFS), are used in agriculture to identify different crop varieties. This can aid in crop management and quality control.
- Tea Quality Assessment: Total luminescence spectroscopy is used by tea companies as an objective and cost-effective method to distinguish between similar types of tea.
- Other Applications:
- Biochemistry and Molecular Biology: Fluorescence spectroscopy is essential for studying the structure, function, and interactions of biomolecules like proteins, nucleic acids, and lipids.
- Medical Diagnostics: It is employed to detect disease markers in biological samples and for imaging purposes in various medical diagnostics.
- Environmental Monitoring: Used to monitor pollutants and contaminants in water, air, and soil.
- Food Safety and Quality Control: Helps detect contaminants and ensure food product quality.
- Industrial Applications: Used for quality control, process monitoring, and impurity detection in industrial processes.
- Drug Discovery and Development: Employed to study drug-biomolecule interactions and assess drug efficacy.
- Nanotechnology: Used to investigate nanomaterial properties and behavior in biological systems.
- Forensic Science: Applied to detect and identify trace evidence in forensic investigations.
Fluorescence spectroscopy’s versatility and precision make it an indispensable tool in scientific research and various industries, enabling scientists and professionals to gain valuable insights into molecular structures, interactions, and environmental conditions. Researchers and practitioners across these fields rely on fluorescence spectroscopy to address complex challenges and advance knowledge and technology.
Advantages of fluorescence spectroscopy
Fluorescence spectroscopy has several advantages over other spectroscopic techniques, some of them include:
- High sensitivity: Fluorescence spectroscopy is able to detect very low concentrations of molecules with high sensitivity, making it an ideal tool for detecting trace amounts of biological molecules, pollutants, and other contaminants.
- Specificity: Fluorescence spectroscopy can be used to selectively detect specific molecules or groups of molecules by using appropriate excitation and emission wavelengths.
- Low background noise: Fluorescence spectroscopy generally has a low level of background noise, which makes it easy to detect weak fluorescence signals.
- Multiplexing: Fluorescence spectroscopy can be used to detect multiple molecules simultaneously by using different fluorescent labels.
- Non-destructive: Fluorescence spectroscopy can be used to study samples without altering or destroying them.
- In-vivo imaging: Fluorescence spectroscopy can be used in-vivo with the help of fluorescent labels which can be targeted to specific cells or tissues and can be used to track the movement of biomolecules in living organisms.
- Cost-effective: Fluorescence spectroscopy equipment is relatively inexpensive and easy to use, making it accessible to many researchers and scientists.
- Versatility: Fluorescence spectroscopy can be used in a wide variety of applications, including chemistry, biology, medicine, environmental monitoring, and industrial analysis.
Overall, fluorescence spectroscopy is a powerful and versatile tool that can be used to study a wide range of samples and provide valuable information in a wide range of applications.
Disadvantages of fluorescence spectroscopy
While fluorescence spectroscopy has many advantages, it also has some limitations and disadvantages. Some of the main disadvantages include:
- Photobleaching: Fluorescence spectroscopy can cause the fluorescence signal to decrease over time due to photobleaching, which occurs when the fluorescent molecules are exposed to light and lose their fluorescence.
- Quenching: Some molecules can quench the fluorescence of other molecules, leading to a decrease in the fluorescence signal.
- Interference from other sources: Fluorescence spectroscopy can be affected by interference from other sources such as scatter, absorption, and autofluorescence, which can make it difficult to interpret the results.
- Limited penetration depth: Fluorescence spectroscopy is limited by the penetration depth of the excitation light, which means that it is not suitable for studying deep tissue samples or samples that are not transparent to the excitation light.
- Fluorophore dependent: The fluorescence signal is dependent on the presence of fluorescent molecules, so it is not suitable for samples that do not contain fluorescent molecules.
- Limited to certain molecular species: Fluorescence spectroscopy can only be used to detect molecules that can fluoresce, which means that it is not suitable for detecting all types of molecules.
- Limited dynamic range: Fluorescence spectroscopy has a limited dynamic range, meaning it can only detect a certain range of concentration and not very high or very low.
- High-cost of reagents: Some applications of fluorescence spectroscopy require the use of expensive reagents, such as fluorescent labels.
- Others
- As fluorescence intensity may be highly reliant on buffering, it is vital to buffer with care.
- Excitation with ultraviolet light may result in photochemical changes or the destruction of the fluorescent molecule.
- Increased photochemical damage may result from the presence of dissolved oxygen.
- Trace amounts of iodide and nitrogen oxides are effective quenchers, and as such, they interfere.
- The approach is unsuitable for determining a sample’s principal constituents due to its low accuracy for big volumes.
- Because not every element or compound may glow, the scope of this technique’s applicability is limited.
Overall, while fluorescence spectroscopy is a powerful and versatile tool, it also has limitations and disadvantages that should be considered when planning experiments and interpreting results.
Precautions
Fluorescence analysis is a powerful technique for trace chemical detection, but it requires careful handling and precautions to ensure accurate and reliable results. Here are some important precautions to consider when conducting fluorescence analysis:
- Contamination Prevention:
- Stoppers and Corks: Avoid the use of rubber or cork stoppers in sample containers, as these materials can contain fluorescent pigments that may leach into the solvent and contaminate the sample. Use inert materials like Teflon or glass for stoppers.
- Filter Paper: Be cautious when using filter paper, as it may contain fluorescent impurities. Ensure that the filter paper used is of high purity or pre-treated to minimize contamination.
- Grease: Grease from stopcocks or other sources can introduce fluorescence contaminants. Use grease-free or specially designed stopcocks and equipment to minimize this issue.
- Glass Contaminants: All glassware can release extractable elements like aluminum (Al), calcium (Ca), and silicon dioxide (SiO2) into the solution. Choose high-quality glassware and thoroughly rinse with appropriate solvents before use to minimize contamination.
- Reagent Concentration:
- Express the concentration of reagents in micromoles (µmol) per unit volume rather than in other units to ensure consistency and facilitate the determination of the reagent-to-metal ratio. This standardization is crucial for accurate quantitative analysis.
- Temperature Control:
- Avoid significant temperature differences between the unknown sample and the standard solutions. Temperature variations can affect the fluorescence properties of the samples and lead to inaccurate results. Use temperature control devices to maintain a constant and uniform temperature throughout the analysis.
- UV Radiation Exposure:
- Prolonged exposure of the sample solution to ultraviolet (UV) radiation should be minimized. UV light can induce photochemical reactions and alter the fluorescence properties of the sample. Shield samples from direct UV exposure when not actively performing measurements.
These precautions are essential to minimize sources of contamination and ensure the accuracy and reliability of fluorescence analysis. By following these guidelines and using proper laboratory techniques, researchers can obtain precise and meaningful results in fluorescence-based experiments.
Quiz
Question 1: What is the fundamental principle of fluorescence spectroscopy?
A) Absorption of photons by a sample
B) Emission of photons by a sample
C) Scattering of photons by a sample
D) Reflection of photons by a sample
Question 2: In fluorescence spectroscopy, which of the following processes occurs when a molecule returns to its ground state after being excited?
A) Absorption
B) Emission
C) Scattering
D) Reflection
Question 3: What is the name of the wavelength of light that is used to excite a molecule in fluorescence spectroscopy?
A) Excitation wavelength
B) Absorption wavelength
C) Emission wavelength
D) Scattering wavelength
Question 4: Which component of a fluorescence instrument is responsible for selecting the wavelength of stimulation in fluorescence spectroscopy?
A) Detector
B) Sample holder
C) Excitation monochromator
D) Emission monochromator
Question 5: What is the term for the phenomenon in fluorescence spectroscopy where emitted light has a longer wavelength than the excitation light?
A) Fluorescence shift
B) Wavelength inversion
C) Absorption reversal
D) Stokes shift
Question 6: Which type of luminescence is similar to fluorescence but has a longer-lived excited state?
A) Absorption
B) Phosphorescence
C) Reflection
D) Scattering
Question 7: In fluorescence spectroscopy, what is the name of the device used to measure the intensity of emitted light?
A) Excitation monochromator
B) Sample holder
C) Detector
D) Emission monochromator
Question 8: What is the primary purpose of using a monochromator in fluorescence spectroscopy?
A) To select the sample holder
B) To select the emission wavelength
C) To select the excitation wavelength
D) To measure the intensity of emitted light
Question 9: Which of the following industries commonly uses fluorescence spectroscopy for applications such as DNA measurement?
A) Aerospace
B) Automotive
C) Bioscience
D) Construction
Question 10: What precaution should be taken to minimize contamination in fluorescence analysis when using stoppers or cork?
A) Use rubber or cork stoppers
B) Rinse with water before use
C) Avoid using stoppers altogether
D) Use inert materials like Teflon or glass
FAQ
What is Fluorescence Spectrophotometry?
Fluorescence Spectrophotometry is a technique used to measure the intensity of light emitted by a substance when it is excited by a specific wavelength of light.
What is the principle of Fluorescence Spectrophotometry?
The principle of Fluorescence Spectrophotometry is based on the ability of certain molecules to absorb light at one wavelength and re-emit it at a longer wavelength.
What are the key parts of a Fluorescence Spectrophotometer?
The key parts of a Fluorescence Spectrophotometer include a light source, a monochromator, a sample holder, a detector, and a readout device.
What are the advantages of Fluorescence Spectrophotometry?
Some of the advantages of Fluorescence Spectrophotometry include high sensitivity, high selectivity, and the ability to measure very small amounts of a substance.
What are some common uses of Fluorescence Spectrophotometry?
Common uses of Fluorescence Spectrophotometry include studying the structure of proteins and nucleic acids, monitoring chemical reactions, and measuring the concentration of analytes in a sample.
What is the difference between Fluorescence Spectrophotometry and Absorption Spectrophotometry?
Fluorescence Spectrophotometry measures the light emitted by a substance after it has been excited by a specific wavelength of light, while Absorption Spectrophotometry measures the amount of light absorbed by a substance.
Is Fluorescence Spectrophotometry harmful to the environment?
Fluorescence Spectrophotometry is not harmful to the environment as it uses small amounts of energy and does not produce any harmful by-products.
How is Fluorescence Spectrophotometry used in medical research?
Fluorescence Spectrophotometry is used in medical research to study the structure and function of proteins and nucleic acids, and to monitor the effectiveness of drugs and other treatments.
What are the most common Fluorescence Spectrophotometry instruments?
The most common Fluorescence Spectrophotometry instruments include Fluorometers, Fluorimeters, and Fluorescence Microscopes.
Can Fluorescence Spectrophotometry be used to measure the concentration of analytes in real-time?
Yes, Fluorescence Spectrophotometry can be used to measure the concentration of analytes in real-time, making it a useful tool for monitoring chemical reactions and other processes.


