Table of Contents
- The main sources of fructose in the diet are the disaccharide sucrose (cane sugar) and high fructose corn syrups (HFCS) found in processed foods and drinks.
- It is also found in free form in honey and many fruits.
- Insulin does not control how much fructose gets into the cells in the body. Glucose, on the other hand, is controlled in how it gets into most of the body’s tissues.
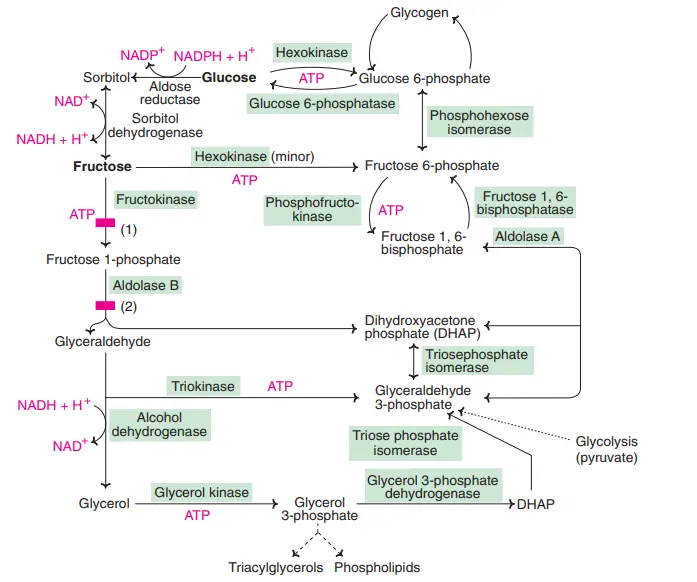
- Fructose is mostly phosphorylated by fructokinase to fructose 1-phosphate. Fructokinase has been found in the intestine, liver, and kidney.
- Hexokinase can act on fructose to make fructose 6-phosphate. It does this by phosphorylating different monosaccharides.
- But hexokinase doesn’t like fructose very much (it has a high Km), so this is a minor pathway.
- Aldolase B breaks down fructose 1-phosphate into glyceraldehyde and dihydroxyacetone phosphate (DHAP). On the other hand, aldolase A changes fructose 6-phosphate into fructose 1, 6-bisphosphate and splits it.
- The enzyme triokinase changes glyceraldehyde into glyceraldehyde 3-phosphate, which enters glycolysis or gluconeogenesis along with DHAP.
- The liver uses glycolysis to break down fructose faster than it does glucose. This is because phosphofructokinase, the enzyme that slows down the rate-limiting step in glycolysis, is skipped over.
- When you eat more fructose, your body makes a lot more acetyl CoA and starts to make fat (fatty acid, triacylglycerol and very low density lipoprotein synthesis).
- When you eat a lot of fructose or sucrose, it can cause a lot of health problems.
Location of Fructolysis
- Fructolysis mainly occurs in liver cells.
Why does fructose metabolism or fructolysis occur in liver cells?
- Even though glucose and fructose are both broken down by many of the same molecules and chemical reactions, they are processed in very different ways. The biggest difference is that glucose can take part in glycolysis but fructose can’t.
- Most of the cells in our bodies start the process of glycolysis by phosphorylating glucose. This is done with the help of an enzyme called hexokinase. But hexokinase only works on sugars with a 6-sided ring structure, like glucose.
- Because fructose is made up of a 5-sided ring, hexokinase can’t phosphorylate it. This means that most of our body’s cells can’t break down fructose. Because of this, fructose is almost always broken down in the liver through a process called fructolysis.
- Hepatocytes in the liver can use an enzyme that most other cells don’t have to break down fructose. The name of this enzyme is fructokinase.
- Fructokinase first phosphorylates fructose. This is the first step in the process of fructolysis. After a few more steps, fructolysis makes the same intermediate molecules (called GAP and DHAP) that glycolysis makes from glucose.
- But GAP and DHAP can’t leave the liver cell because they both have phosphate groups attached to them.
- Like other cells, the liver cell can burn some of them for energy, but it can only use so many.
- Since the rest of the body can’t process fructose, a high-fructose diet makes the liver cells work too hard.
- The liver cell stores the extra fructose as glycogen as the first thing it does with it. This is the best solution, since glycogen can be broken down into glucose and sent to other cells in the body to be used.
- But each liver cell can only hold so much glycogen. If you eat a lot of fructose, your liver will run out of room.
- When the liver has stored all the glycogen it can, it uses the extra fructose to make triglycerides, which can be moved out of the liver and stored as fat for the long term.
- If a person’s diet is always high in fructose, the extra fat doesn’t get used and keeps building up.
- Even though too much glucose can also be stored as fat, the fatty acids made from fructose have their own health risks. Fat produced in the liver isn’t regulated or stored in the same way as the fat from glucose.
Properties and Sources of Fructose
- Fructose tastes more like fruit and is sweeter than sucrose.
- Compared to the standard, sucrose, which is rated at 100%, fructose is between 130% and 180% as sweet. This depends in part on how hot or cold the serving is.
- Both sucrose and fructose are used a lot in foods to make them sweet, give them texture, and make them taste good. These sugars also make the food look better, keep it fresh longer, and give it more energy.
- Fruits, fruit juices, and some vegetables are natural sources of the sugar fructose. In these foods, fructose is found as the monosaccharide and also as a component of the disaccharide sucrose.
- However, the primary source of fructose in Western diets is sugars added to baked goods, candies, soft drinks, and other beverages sweetened with sucrose and highfructose corn syrup (HFCS) (HFCS).
- HFCS is made by using a-amylase and glucoamylase to turn the starch in corn into glucose. The next step is to use glucose isomerase, which makes a mix of glucose and fructose.
- Most of the time, the process makes an HFCS with 42% fructose, 50% glucose, and 8% other sugars (HFCS-42). By “fractionation,” a 90% fructose concentrated fructose syrup can be made (HFCS-90).
- The mixture of HFCS-42 and HFCS-90 makes HFCS-55, which is 55% fructose, 41% glucose, and 4% other sugars.
- The soft drink industry prefers to use HFCS-55 as a sweetener, but HFCS-42 is also often used as a sweetener in many processed foods.
- Apple juice and white grape juice are two examples of concentrated fruit juices that can be used to sweeten drinks. Fruit juices have different amounts of fructose and different ratios of fructose to glucose, but it is clear that concentrated apple juice has more fructose to glucose than either sucrose or HFCS-55.
- Nevertheless, considering the variety of sweeteners commonly available, it is likely that fructose constitutes close to 50% of energy from added sweeteners.
- As a result of the addition of sweeteners and sugars to so many food products, the consumption of fructose has increased from the mid-1970s to the mid-2000s.
- Sugars added to the diet are hard to measure accurately, but based on data from food intake surveys, adults get about 8–12% of their energy from fructose, or 40–60 g day1 if they eat 2,000 kcal a day.
- People who drink a lot of soft drinks, like teenage boys, usually get more than twice the average amount of fructose from added sweeteners, or more than 100 g a day1.
- Considering the US population as whole and using both food disappearance data and food survey data, total fructose consumption has increased by approximately 25% over the course of three decades.
Absorption of Fructose
- Fructose is a type of sugar found in many common foods, both as a standalone monosaccharide and as a component of the disaccharide sucrose.
- At the intestinal brush border, sucrase hydrolyzes sucrose into glucose and fructose.
- Glucose is transported to the liver via the portal vein at a high rate thanks to a sodium-coupled cotransporter.
- Fructose absorption occurs by facilitated diffusion, enabled by a fructose-specific hexose transporter, GLUT-5. The brush border membranes of the jejunum are home to this transporter.
- Fructose enters the portal circulation from the enterocytes using the basolateral transporter, GLUT-2, which also transports glucose and galactose.
- Hours after consuming a fructose-rich diet, GLUT-5 expression rises, suggesting that the transporter is under the control of light.
- However, consumption of a considerable amount of pure fructose can surpass the capacity of intestinal fructose absorption, resulting in diarrhoea.
- More than half of healthy adults experience malabsorption after consuming a single dose of 50 g of fructose, and in some studies, malabsorption is also seen with a dose of 25 g. Diarrhea, gas, and bloating in the abdomen characterise fructose malabsorption.
- However, the intestinal absorptive capacity for fructose rises when glucose is ingested along with fructose.
- Thus, coingesting glucose to nearly balance fructose, as occurs when most fruits or sucrose are taken, greatly alleviates problems of fructose malabsorption.
- Additionally, adaptation to increased fructose intake is suggested by an increase in fructose absorption during prolonged fructose consumption.
From Fructose to Fat/Fructolysis/fructose metabolism pathway
- With the help of fructokinase, liver cells can break down fructose in the same way that most other cells in the body break down glucose.
- The liver cells can use the fructose to satisfy their immediate energy needs or convert it into glycogen for short-term storage.
- The third option is to store the extra fructose as fat for a long time. This flowchart shows what happens when liver cells use this third way to turn the energy in fructose into the fatty acids of triglycerides.
- When the liver has stored all the glycogen it can, this metabolic pathway is used by liver cells.
- The molecules that are created at the end of each step are shown in blue.
- The enzymes that facilitate each step are shown in red. Path A makes acyl-CoA, which is then used in Path B to make triaglycerides.

Steps of fructose metabolism pathway
1. Step 1
- Hepatocytes in the liver use the fructokinase enzyme to change fructose into fructose 1-phosphate. This starts the process of fructolysis.
- Fructokinase takes ATP and adds a phosphate group to fructose. This makes fructose 1-phosphate.
2. Step 2
- Fructose 1-phosphate aldolase breaks fructose 1-phosphate into two molecules: glyceraldehyde and dihydroxyacetone (DHAP).
- Since these two molecules react in different ways, the flowchart is split in two after step two. We’ll start with Path A and then move on to Path B. Remember that the things that come out of Path A end up going back into Path B.
3. Step 3A
- Triokinase moves a phosphate group from ATP to glyceraldehyde, making a molecule of glyceraldehyde 3-phosphate (G3P).
Metabolic Pathway Notes: At this point, the liver cell can use the same steps (6–10) for breaking down G3P as it does for breaking down glucose. It can’t send any extra G3P to other cells, though, because phosphorylated molecules like G3P can’t pass through the cell membrane. Since the liver has to process all of the fructose itself, it gets a lot more than it can use for its own energy needs. So instead of using it as energy, the liver stores it as glycogen. When the body has enough glycogen, it will use it to make triglycerides. This flowchart shows the steps that are taken to make triglycerides. See the flowchart for glycolysis to see how glyceraldehyde 3-phosphate is used to make ATP, which can be used right away for energy.
4. Step 4A
- Glyceraldehyde 3-phosphate dehydrogenase uses the energy stored in NAD+ to add a second phosphate group to glyceraldehyde-3 phosphate, making 1,3 bisphosphoglycerate.
- During the process, NAD+ changed into NADH. Since an inorganic phosphate molecule that wasn’t attached was used, ATP wasn’t needed.
5. Step 5A
- Phosphoglycerate kinase removes a phosphate group from carbon-1 on 1,3 bisphosphoglycerate, changing it into 3-phosphoglycerate.
- During the process, a molecule of ATP is made.
6. Step 6A
- Phosphoglycerate mutase changes 3-phosphoglycerate into 2-phosphoglycerate by moving the phosphate group from carbon-3 to carbon-2.
7. Step 7A
- Enolase changes 2-phosphoglycerate into phosphoenolpyruvate through a process called dehydration, which gets rid of one water molecule.
8. Step 8A
- Pyruvate kinase removes the phosphate group from phosphoenolpyruvate. This makes ATP and pyruvate.
9. Step 9A
- Pyruvate dehydrogenase takes a CO2 molecule from pyruvate and changes it into an acetyl group with two carbons.
- The acetyl group is then added to CoA by the same enzyme, making acetyl-CoA.
10. Step 10A
- Acetyl-CoA carboxylase uses energy from ATP to combine bicarbonate and acetyl-CoA to make malonyl-CoA. Both acetyl-CoA and malonyl-CoA are made up of two carbon acetyl groups.
- The chains of fatty acids will be made up of these groups.
11. Step 11A
- Fatty acid synthase uses a 4-step process to attach the two-carbon acetyl groups from seven malonyl-CoA molecules to the two-carbon acetyl group at the end of a single acetyl-CoA molecule.
- This is done again and again until the acetyl group on acetyl-CoA has 16 carbons. At this point, the 16-carbon long string separates from the newly made acyl-CoA to become palmitate, a free fatty acid. There is also one molecule of CoA left, which can be used again.
12. Step 12A
- Acyl-CoA synthetase brings together palmitate and CoA to make acyl-CoA.
- This is the same as the acyl-CoA molecule we had near the end of step 11A, before palmitate separated from CoA.
- They could have skipped step 11A and gone straight to step 13A as acyl-CoA instead of separating. Step 12A is only needed if you have palmitate and CoA that are not attached to anything.
13. Step 13A
- In our metabolic pathway, this step feeds into steps 4B and 6B of Path B. Acyl transferase moves two 16-carbon palmitate chains from two acyl-CoA molecules to one glycerol 3-phosphate molecule. This gives the glycerol 3-phosphate molecule two of the three chains it needs to be a triglyceride.
- In step 6B, after the molecule has been changed into 1,2 diacylglyceral, the same enzyme adds a third strand of palmitate to the same molecule.
- See the Path B steps below for more information about how all of these parts come together to make a triglyceride.
Note: Let me explain what’s going on with all of these CoA molecules. In Step 11A, we started with a molecule of malonyl-CoA, and in Step 12A, we finished with a molecule of acyl-CoA. CoA (coenzyme A) is used in these steps as a transporter for two-carbon groups that can take different shapes. Acetyl-CoA is CoA with a single acetyl group with two carbons added to it. Acyl-CoA is acetyl-CoA that has eight groups of two carbons added to it (The one it had originally as acetyl-CoA, and seven donated by seven different malonyl-CoA molecules). Malonyl-CoA is made by adding bicarbonate to acetyl-CoA. When it gets seven more acetyl groups, acetyl-CoA changes into acyl-CoA. When this string of eight acetyl groups with two carbon atoms breaks away from CoA, it changes into palmitate.
14. Step 3B
- Glycerol 3-phosphate dehydrogenase turns DHAP into glycerol 3-phosphate by using H- from a NADH molecule and a free proton H+.
- It does this by changing the C=O double bond on the centre carbon of the 3-carbon molecule into a C-O single bond.
- One hydrogen atom is attached to the carbon, and the other is attached to the oxygen.
- Now, the oxygen atoms on carbon-1 and carbon-2 of the glycerol 3-phosphate molecule have an O-H single bond that can be used to attach a palmitate strand.
15. Step 4B
- Acyl transferase moves palmitate from acyl-CoA to the two oxygens on glycerol 3-phosphate that are connected to carbons 1 and 2. Carbon 3 is inaccessible because it is attached to a phosphate group.
- The O- ion at the end of each palmitate combines with each of the two OH groups on glycerol 3-phosphate, getting rid of an OH- hydroxyl group in the process. It makes one molecule of phosphatidic acid.
16. Step 5B
- Two of the three carbon atoms in glycerol 3-phosphate are now linked to palmitate, while the third carbon is linked to a phosphate group.
- Glycerol 3-phosphate is changed into 1,2-diacylglycerol when phosphatidic acid phosphatase dephosphorylates it.
17. Step 6B
- With the phosphate group on the third carbon of 1,2 diacylglycerol now changed to an OH group, the same acyl transferase enzyme that moved palmitate strands from acyl-CoA to carbons 1 and 2 in step 4B can now move a third strand to carbon-3. This is the last step in making a triacylglycerol molecule (a triglyceride).
- Fructose is now a triglyceride, which means that it has been turned from a carbohydrate into fat. And because there are no phosphate groups attached to the triglyceride, it can be moved out of the liver cell and stored somewhere else for a long time.
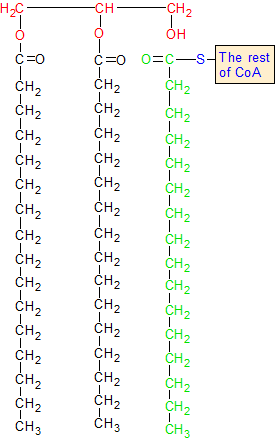
On 1,2 diacylglycerol, acyl transferase adds a third fatty acid chain to the glycerol backbone. This happens when the green carbon breaks away from the blue sulphur atom and forms a bond with the oxygen in the red OH group. The red hydrogen bonds with the sulphur, and the CoA group is thrown away.
The CoA group is shown in blue, and the glycerol backbone is shown in red. The glycerol backbone is the backbone of the 1,2 diacyglycerol molecule. The two black fatty acid chains are attached to it. Acyl-CoA is the blue group of CoA that has the green chain of fatty acids attached to it.
What is Sorbitol / Polyol pathway?
- Sorbitol is a polyhydroxy sugar, which is how the polyol pathway got its name. Sorbitol is used to change glucose into fructose.
- The liver doesn’t have this path.
- Sorbitol pathway is directly linked to glucose concentration and is higher in diabetes that is not under control.
- In the presence of NADPH, the enzyme aldose reductase changes glucose into sorbitol (also called “glucitol”).
- Then, sorbitol dehydrogenase and NAD+ turn the sorbitol into fructose. Aldose reductase is not found in the liver, but it is in many other tissues, such as the lens and retina of the eye, kidney, placenta, Schwann cells of peripheral nerves, erythrocytes, and seminal vesicles.
- The seminal vesicle, the spleen, and the ovaries all have sorbitol dehydrogenase.
- Because sperm cells have a sorbitol pathway, they prefer to use fructose as a source of energy.
Sorbitol pathway in diabetes mellitus
- In diabetes that isn’t under control (hyperglycemia), the cells take in a lot of glucose even though they don’t need it.
- Importantly, the cells in diabetes that have high levels of glucose inside of them (such as the lens, retina, nerve cells, kidney, etc.) have a lot of aldose reductase and enough NADPH.
- This makes it easy and quick for glucose to turn into sorbitol.
- But the enzyme sorbitol dehydrogenase is either not active or not present in these cells. Because of this, sorbitol is not changed into fructose.
- Sorbitol builds up in the cells where it is made because it can’t pass through the cell membrane.
- Because sorbitol is hydrophilic, it has strong osmotic effects that cause cells to swell.
- As was said above, the buildup of sorbitol is thought to cause some of the health problems that come with diabetes, such as cataracts, peripheral neuropathy, nephropathy, etc.
- It is clear that diabetic animals have more sorbitol in their lens, nerves, and glomerulus. This hurts the body’s tissues.
- As a result of the polyol pathway, it seems that most of the problems that can happen with diabetes have the same cause.
- Some inhibitors of aldose reductase can stop the buildup of sorbitol and the problems that come with it. But this approach is still in the testing phase.
Effect of hyperglycemia on sorbitol metabolism
- Because glucose can get into the cells listed in the last sentence without insulin, a lot of glucose can get into these cells when blood sugar is too high (for example, in uncontrolled diabetes).
- Aldose reductase makes a lot more sorbitol when the amount of glucose inside the cell is high and there is enough reduced nicotinamide adenine dinucleotide phosphate (NADPH). Sorbitol can’t easily pass through cell membranes, so it stays inside the cell.
- This is made worse when there is little or no sorbitol dehydrogenase (for example, in retina, lens, kidney, and nerve cells).
- Sorbitol builds up in these cells, which has strong osmotic effects and causes cells to swell because they hold onto water.
- Some of the pathological changes that come with diabetes, like cataracts, peripheral neuropathy, and microvascular problems that lead to nephropathy and retinopathy, can be partly blamed on this. When NADPH is used in the aldose reductase reaction, less of the important antioxidant reduced glutathione is made. This may be linked to complications in diabetics.
Defects in fructose metabolism
Essential fructosuria
- Because the liver doesn’t have enough fructokinase, fructose can’t be turned into fructose 1-phosphate.
- This is a symptomless condition in which fructose is passed out of the body in the urine.
- The treatment is to limit the amount of fructose in the diet.
Hereditary fructose intolerance
- This is because there isn’t enough aldolase B. Hereditary fructose intolerance makes intracellular fructose 1-phosphate build up, which leads to severe low blood sugar, vomiting, liver failure, and jaundice.
- Allosterically, fructose 1-phosphate stops liver phosphorylase from working and stops glycogenolysis, which causes low blood sugar.
- Fructose intolerance can be fixed by finding it early and eating a diet free of fructose and sucrose.
Consumption of high fructose
- Fructokinase works quickly to change fructose into fructose 1-phosphate. The enzyme aldolase B doesn’t work as well as it should, so fructose 1-phosphate builds up in the cell.
- This lowers the amount of inorganic phosphate (Pi) inside the cell.
- Sequestering of phosphate is the process by which Pi binds to organic molecules (like fructose) and makes it less available for important metabolic functions.
- When a person eats too much fructose, there is less Pi available. This makes the liver’s metabolism work less well. This includes the fact that ADP and Pi are used less to make ATP.
- When people eat a lot of fructose over a long period of time, their blood levels of uric acid rise, which can lead to gout. This is because ADP and AMP are broken down too quickly into uric acid when there isn’t enough Pi.
High fructose consumption and the risk of atheroslerosis
Atherosclerosis is a disease in which fat builds up in the arteries, making them thicker. Fructose gets into tissues quickly and speeds up glycolysis, which leads to lipogenesis in the end. It is clear that fructose makes the liver make more fatty acids and triacylglycerol and make more VLDL. All of these metabolic processes lead to higher levels of triacylglycerol and LDL-cholesterol in the blood, which makes atherosclerosis more likely.
Regulation
Regulation of fructose metabolism in the liver
- The liver doesn’t use hormones or allosteric mechanisms to control how fructose is broken down. Instead, fructose goes around the rate-limiting step of glycolysis, which is catalysed by phosphofructokinase 1. (PFK 1).
- Because of this, the metabolism of fructose is much faster and less tightly controlled than the metabolism of glucose.
- In glycolysis, aldolase-B does a lysis step that is different from aldolase. This step makes dihydroxyacetone phosphate and glyceraldehyde.
- In contrast to the products of aldolase, these ones need to be phosphorylated to work.
Regulation at fructose uptake and phosphorylation
- Fructose 1-phosphate changes the way glucokinase works by being a positive allosteric modulator. The attachment of an inhibitory glucokinase-regulatory protein inside the nucleus, which decreases the affinity of glucokinase for fructose, is the cause of glucokinase’s possible allosteric activity and high Km.
- When the glucokinase-regulatory protein complex moves to the nucleus, it stops the glucokinase from doing its job.
- Fructose-1-phosphate binds to the glucokinase-regulatory protein and stabilises the dissociated protein to stop this inhibition. Insulin and glucose after a meal cause glucokinase-regulatory protein to break apart and move out of the nucleus.
- Fructose phosphorylation is mostly dependent on the amount of fructose in tissues like the liver, and when the amount of fructose goes up, the amount of ATP in the cell goes down.
- Metabolites of fructose enter the pool of triose phosphate farther away from PFK 1 than the step that controls PFK 1.
- Because hepatic fructolysis is not limited, fructose loads can cause rapid and large increases in the triose and hexose phosphate pools. This could give more substrate to all central carbon metabolic pathways, like glycolysis, gluconeogenesis, glycogenesis, oxidative phosphorylation, and lipogenesis.
Importance
- Fructose makes it easier for the liver to take in and store glucose.
- Fructose also speeds up the process of burning off carbs after a meal.
- Most of the energy that spermatozoa need to move around comes from fructose. Fructose is made by small sacs called seminal vesicles. But some people have azoospermia because the duct of the seminal vesicles is blocked. Semen from these people is tested to see how much fructose is in it. Depending on whether fructose is present or not, the block will be above or below the seminal vesicular duct.
- Fructose may be an important part of preadipocytes becoming mature, which lets them store more fat.
- People who work out hard may benefit from fructose because it keeps hepatic gluconeogenesis going and gives skeletal muscles more energy in the form of lactate to contract.
References
- Lippincott Illustrated Reviews: Biochemistry
- Tappy, Luc & Lê, Kim-Anne. (2010). Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiological reviews. 90. 23-46. 10.1152/physrev.00019.2009.
- Lee, Ho-Jae & Cha, Ji-Young. (2018). Recent insights into the role of ChREBP in intestinal fructose absorption and metabolism. BMB reports. 51. 10.5483/BMBRep.2018.51.9.197.
- Dholariya SJ, Orrick JA. Biochemistry, Fructose Metabolism. [Updated 2021 Oct 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK576428/
- Keim, N. L., & Havel, P. J. (2013). Fructose: Absorption and Metabolism. Encyclopedia of Human Nutrition, 361–365. doi:10.1016/b978-0-12-375083-9.00128-8
- https://dm5migu4zj3pb.cloudfront.net/manuscripts/96000/96702/cache/96702.1-20201218131313-covered-e0fd13ba177f913fd3156f593ead4cfd.pdf
- https://www.pharmacy180.com/article/fructose-metabolism-1890/
- https://en.wikipedia.org/wiki/Fructolysis
- https://almerja.com/reading.php?idm=158078
- https://microbenotes.com/fructose-metabolism/
- http://gardenandplate.com/fructolysis.html
- http://biocheminfo.com/2020/04/09/metabolism-of-fructose-fructolysis/

Много полезна страница!
Благодаря ви много! Радвам се, че намерихте страницата полезна. Ако имате още въпроси или нуждаете се от допълнителна информация, не се колебайте да питате. Винаги съм тук, за да помогна.