Table of Contents
Gerhardt’s test, often known as Gerhardt’s reaction, is a chemical test for detecting the presence of specific organic compounds in a sample. It was created by the German scientist Charles Gerhardt in the middle of the 19th century and is still widely used today in numerous industries, including pharmaceuticals, food, and environmental testing.
Midway through the 19th century, Charles Gerhardt was a notable chemist who made substantial advances to the science of organic chemistry. In 1848, he reported for the first time the reaction that currently bears his name, which involves heating an organic molecule treated with nitric acid. If the chemical contains specific functional groups, such as primary and secondary alcohols or aldehydes, it will generate a yellow-orange colour when combined with nitric acid.
The significance of Gerhardt’s test lies in its capacity to detect chemical molecules rapidly and easily. For instance, it can be used to identify the presence of ethanol in alcoholic beverages, such as wine and beer, and aldehydes in food goods. In the pharmaceutical business, it is often used to test for contaminants in pharmaceuticals and to assure the quality of chemical reagents.
Overall, Gerhardt’s test is a useful instrument in the field of chemistry and is still employed to identify the presence of specific organic compounds in a variety of substances.
Gerhardt’s test Definition
- Gerhardt’s test is a type of laboratory test which is performed for the qualitative detection of ketone bodies in urine.
- During the “ketosis” three ketones bodies or acetone bodies are found in the urine which are the products of fat metabolism, known as acetone (2%), acetoacetic acid (20%) and beta-hydroxybutyrate (78%).
- During starvation and uncontrolled diabetes, the Ketone bodies are synthesized within the liver to re-utilized energy. These are types of acid that can results in metabolic acidosis in uncontrolled diabetes.
- If the rate of ketone bodies production exceeds, the excess amounts of ketone bodies will be eliminated through the urine and the condition is termed as ketonuria. Acetone is volatile and also excreted in breath.
- Ketosis may be correlated with diabetes mellitus termed Diabetic ketoacidosis, or it may be due to starvation, persistent vomiting and high fat and low carbohydrate diet.
- There are present different methods for the detection of ketones in urine such as Rothera’s test, Gerhardt’s test, Lang’s test, Lindeman’s test, Han’s test, and Tablet test. All the tests used for the detection of ketonuria are based on the principle of Rothera’s nitroprusside test.
- This method only can detect about 25 to 50 mg/dl of acetoacetic acid, that’s why it’s not considered a very sensitive test.
Objective
- To detect the presence of ketone bodies within the supplied urine sample.
Gerhardt’s test Principle
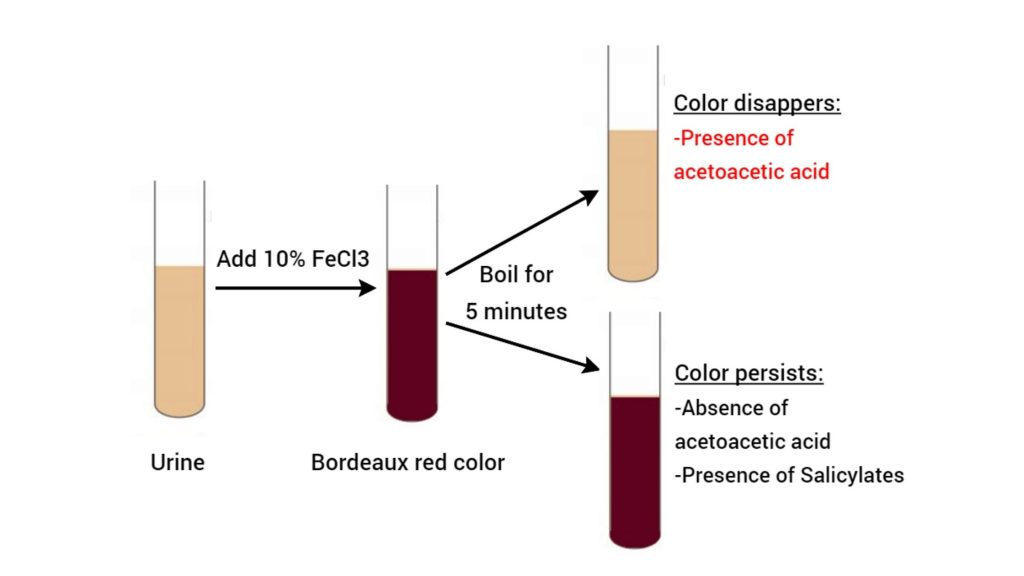
In Gerhardt’s test, the ferric chloride reacts with the acetoacetic acid and forms a port wine or Bordeaux red color. This test only identifies acetoacetic acid, Acetone and beta-hydroxybutyrate can’t be detected by this method. It also detects salicylates in urine.
During this test, if the color disappears, it means the acetoacetic acid is present within the sample. On boiling acetoacetic acid loses carbon dioxide and transforms into acetone. Acetone doesn’t react with ferric chloride. If the color persists, acetoacetic acid is absent. The previous color is due to salicylates.
Requirements of Gerhardt’s test
- Specimen: Urine
- Glassware: Test tubes
- Chemical:10% Ferric chloride (10ml of ferric chloride in 100 ml of distilled water).
Gerhardt’s test Procedure
- Take a clean test tube and add about 3-5 ml of urine to it.
- Then add 5ml of ferric chloride to the test tube. If phosphates are present, they get precipitated as ferric phosphates.
- If the diacetic acid is present, a Bordeaux red color will develop in addition to ferric chloride.
- After that boil the test solution for 5 minutes to confirm the presence of acetoacetic acid within the supplied urine sample.
- Now observe the test tubes for color change.
Observations and Results
- Positive Test: If the color disappears, the Acetoacetic acid is present within the urine.
- Negative Test: If the color persists, acetoacetic acid is absent within the urine sample.
Precautions
- Wash the apparatus before and then after the experiment.
- Carefully manage all the chemicals within the laboratory.
- Don’t touch the urine sample during the experiment.
- Holds the test tubes by using test tube holders.
- Use a clear and clean test tube, make sure the test tubes are free of any dirt and chemicals because this will give a false result.
- Place all the apparatus in their respective place after the completion of the experiment.
FAQ
What is Gerhardt’s test?
Gerhardt’s test is a laboratory test used to qualitatively detect the presence of ketone bodies in urine.
What are ketone bodies?
Ketone bodies are a byproduct of fat metabolism and include acetone, acetoacetic acid, and beta-hydroxybutyrate.
What can cause ketonuria?
Ketonuria, the presence of excess ketone bodies in urine, can be caused by conditions such as starvation, uncontrolled diabetes, persistent vomiting, and a high-fat, low-carbohydrate diet.
How does Gerhardt’s test work?
Gerhardt’s test is a type of nitroprusside test that detects acetoacetic acid in urine. It is not considered very sensitive, as it can only detect levels of acetoacetic acid between 25 to 50 mg/dl.
What is the relationship between ketosis and diabetic ketoacidosis?
Ketosis is the presence of ketone bodies in the body, while diabetic ketoacidosis is a condition where the body produces excess ketone bodies due to uncontrolled diabetes. Ketosis can also occur due to starvation, a high-fat, low-carbohydrate diet, or persistent vomiting.
What is the color formed when ferric chloride reacts with acetoacetic acid in Gerhardt’s test?
When ferric chloride reacts with acetoacetic acid in Gerhardt’s test, a port wine or Bordeaux red color is formed.
Can acetone be detected using Gerhardt’s test?
No, acetone cannot be detected using Gerhardt’s test as it does not react with ferric chloride.
What happens to acetoacetic acid when it is boiled during Gerhardt’s test?
When acetoacetic acid is boiled during Gerhardt’s test, it loses carbon dioxide and transforms into acetone.
What does the disappearance of the color during Gerhardt’s test indicate?
The disappearance of the color during Gerhardt’s test indicates that acetoacetic acid is present within the sample being tested.
What is the significance of the color disappearance in a urine test?
The disappearance of color in a urine test indicates the presence of Acetoacetic acid in the urine sample.
What does a persistent color mean in a urine test?
The persistence of color in a urine test indicates the absence of Acetoacetic acid in the urine sample.
Can Acetoacetic acid be found in a negative urine test result?
No, Acetoacetic acid cannot be found in a negative urine test result, as the persistent color indicates its absence.
Is the disappearance of color the only indicator of Acetoacetic acid in a urine test?
Yes, the disappearance of color is the only indicator of Acetoacetic acid in a urine test, as per the given text.
What are the possible reasons for a false negative result in a urine test?
The possible reasons for a false negative result in a urine test could be an error in the testing process, such as not following the instructions correctly, or a low concentration of Acetoacetic acid in the urine sample.

Thanks ,the content has been helpful to writing my report and being on top