Table of Contents
What is Gluconeogenesis?
Gluconeogenesis, often abbreviated as GNG, is a vital metabolic pathway responsible for the synthesis of glucose from non-carbohydrate carbon substrates. This process is not limited to a specific group of organisms; rather, it is universally observed in plants, animals, fungi, bacteria, and various microorganisms. In vertebrates, the liver predominantly undertakes this task, with the kidney’s cortex also playing a role, albeit to a lesser degree. Therefore, gluconeogenesis, along with glycogen degradation (known as glycogenolysis), serves as the primary mechanisms that many animals, including humans, rely on to regulate blood sugar levels and prevent hypoglycemia. Besides, in ruminants, gluconeogenesis is a constant process due to the metabolism of dietary carbohydrates by rumen organisms.
Then, considering the human body, the substrates essential for gluconeogenesis are derived from non-carbohydrate sources that can be transformed into pyruvate or intermediates of glycolysis. When proteins are broken down, glucogenic amino acids serve as substrates. However, ketogenic amino acids do not. Additionally, the breakdown of lipids, such as triglycerides, yields glycerol and odd-chain fatty acids as substrates. It’s important to note that even-chain fatty acids are not used in this process. Furthermore, lactate from the Cori cycle and, under extended fasting conditions, acetone from ketone bodies can also be utilized as substrates. This latter pathway offers a bridge from fatty acids to glucose. While the liver is the primary site for gluconeogenesis, it’s worth noting that the kidney’s contribution becomes more significant in situations like diabetes and prolonged fasting.
The process of gluconeogenesis is inherently endergonic. However, when coupled with ATP or GTP hydrolysis, it becomes exergonic. For instance, the conversion of pyruvate to glucose-6-phosphate demands 4 ATP and 2 GTP molecules to proceed spontaneously. These ATP molecules are primarily sourced from the catabolism of fatty acids through beta oxidation. In conclusion, gluconeogenesis is a complex yet essential pathway that ensures the body’s energy needs are met, especially in the absence of dietary carbohydrates.
Gluconeogenesis Definition
Gluconeogenesis is a metabolic pathway that synthesizes glucose from non-carbohydrate carbon substrates, primarily occurring in the liver and kidneys to maintain blood sugar levels.
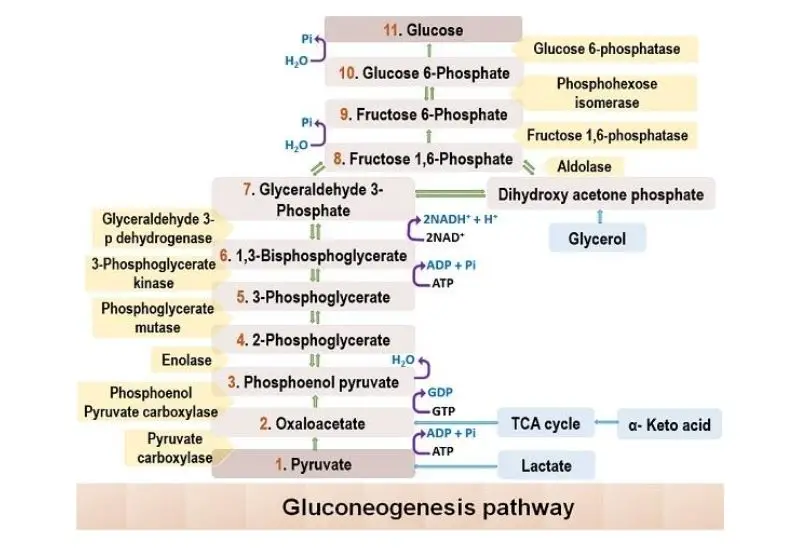
Location of Gluconeogenesis – Where does Gluconeogenesis Occur?
Gluconeogenesis is a crucial metabolic pathway responsible for the synthesis of glucose from non-carbohydrate precursors. The location where this process occurs is vital for understanding its regulation and integration with other metabolic pathways.
Primary Sites of Gluconeogenesis: Gluconeogenesis predominantly takes place in specific organs, ensuring a steady supply of glucose to the body. The primary sites include:
- Liver: The liver is the primary site for gluconeogenesis in mammals. It preferentially utilizes lactate, glycerol, and glucogenic amino acids, especially alanine, as substrates. After meals, the liver focuses on glycogen synthesis, but during fasting or extended periods without food, it shifts towards gluconeogenesis to maintain blood glucose levels.
- Kidney: The kidney is another significant site for gluconeogenesis. It primarily uses lactate, glutamine, and glycerol as substrates. Unlike the liver, which can use both glycogenolysis and gluconeogenesis, the kidney relies solely on gluconeogenesis to produce glucose.
- Intestine: The intestine, though not the primary site, also contributes to gluconeogenesis, mainly using glutamine and glycerol as substrates.
- Muscle: Muscles have the capability for gluconeogenesis, but it’s not their primary function.
- Astrocytes in the Brain: Recent studies indicate the presence of gluconeogenesis in astrocytes within the brain, highlighting the brain’s potential role in glucose synthesis.
Ruminants and Gluconeogenesis: In ruminants, the liver’s gluconeogenesis process differs slightly. Propionate serves as the principal substrate for gluconeogenesis in the ruminant liver. When glucose demand rises, the ruminant liver might also increase its use of gluconeogenic amino acids, such as alanine. Interestingly, the capacity of liver cells in ruminants like calves and lambs to use lactate for gluconeogenesis decreases as they mature.
Cellular Location: On a cellular level, the formation of oxaloacetate from pyruvate and TCA cycle intermediates occurs exclusively within the mitochondria. The enzymes converting Phosphoenolpyruvic acid (PEP) to glucose-6-phosphate are located in the cytosol. The enzyme PEP carboxykinase (PEPCK), which bridges these two segments of gluconeogenesis by converting oxaloacetate to PEP, varies in location across species. In humans, it’s dispersed evenly between the mitochondria and the cytosol.
Transport Mechanisms: PEP is transported across the mitochondrial membrane through specific transport proteins. However, no such proteins exist for oxaloacetate. In species lacking intra-mitochondrial PEPCK, oxaloacetate is converted into malate or aspartate, exported from the mitochondrion, and then reverted back to oxaloacetate to continue gluconeogenesis.
In summary, gluconeogenesis is a tightly regulated process occurring in specific organs and cellular locations, ensuring that glucose production meets the body’s demands, especially during periods when dietary glucose is unavailable.
Gluconeogenesis Pathway Steps
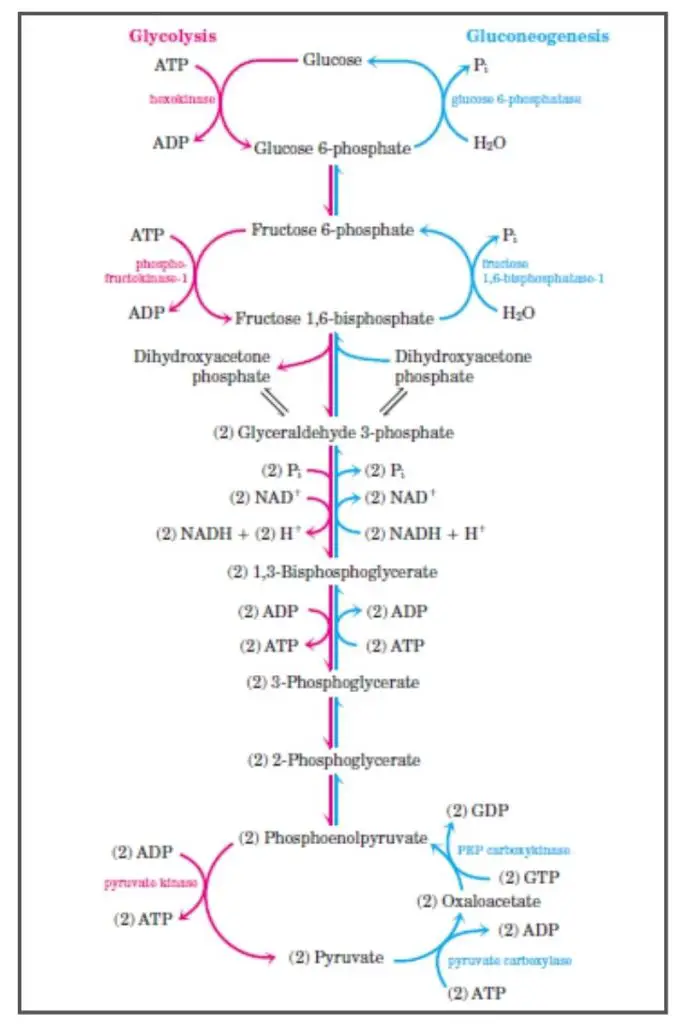
Gluconeogenesis is a vital metabolic pathway that synthesizes glucose from non-carbohydrate precursors. This process ensures that the body maintains adequate glucose levels, especially during periods of fasting or intense physical activity. Here’s a detailed breakdown of the steps involved in gluconeogenesis:
- Conversion of Pyruvate to Oxaloacetate: In the mitochondria, pyruvate undergoes carboxylation to produce oxaloacetate. This reaction is facilitated by the enzyme pyruvate carboxylase, which requires ATP for activation and biotin as a coenzyme. This step is exclusive to gluconeogenesis and serves to circumvent the irreversible glycolytic reaction driven by pyruvate kinase.
- Formation of Phosphoenolpyruvate from Oxaloacetate: Once in the cytosol, oxaloacetate is decarboxylated and rearranged to yield phosphoenolpyruvate (PEP) through the action of PEP carboxykinase. This enzyme utilizes GTP and requires magnesium ions. This step is another unique aspect of gluconeogenesis, bypassing the irreversible action of pyruvate kinase in glycolysis.
- Conversion of PEP to 2-Phosphoglycerate: The enzyme enolase mediates the hydration of PEP, resulting in 2-phosphoglycerate.
- Isomerization to 3-Phosphoglycerate: Through the action of phosphoglycerate mutase, 2-phosphoglycerate is transformed into 3-phosphoglycerate.
- Formation of 1,3-Bisphosphoglycerate: The enzyme phosphoglycerate kinase phosphorylates 3-phosphoglycerate, producing 1,3-bisphosphoglycerate. This reaction consumes ATP.
- Reduction to Glyceraldehyde 3-Phosphate: The enzyme glyceraldehyde 3-phosphate dehydrogenase facilitates the reduction of 1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate, with NADH donating electrons.
- Isomerization to Dihydroxyacetone Phosphate: Triose phosphate isomerase catalyzes the isomerization of glyceraldehyde 3-phosphate, producing dihydroxyacetone phosphate.
- Combination to Form Fructose 1,6-Bisphosphate: The enzyme aldolase combines glyceraldehyde 3-phosphate and dihydroxyacetone phosphate, yielding fructose 1,6-bisphosphate.
- Dephosphorylation to Fructose 6-Phosphate: Unique to gluconeogenesis, the enzyme fructose 1,6-bisphosphatase (FBPase-1) dephosphorylates fructose 1,6-bisphosphate, generating fructose 6-phosphate. This step bypasses the irreversible glycolytic reaction catalyzed by phosphofructokinase-1.
- Conversion to Glucose 6-Phosphate: Phosphohexose isomerase transforms fructose 6-phosphate into glucose 6-phosphate.
- Formation of Glucose: Lastly, glucose 6-phosphatase dephosphorylates glucose 6-phosphate, producing glucose, which can then be released into the bloodstream. This final step is exclusive to gluconeogenesis, bypassing the irreversible glycolytic reaction driven by hexokinase.
In conclusion, gluconeogenesis is a meticulously regulated process that ensures glucose supply during times when dietary intake is insufficient. The pathway involves several unique enzymes and reactions that differentiate it from glycolysis, even though many of its steps are essentially the reverse of glycolytic reactions.
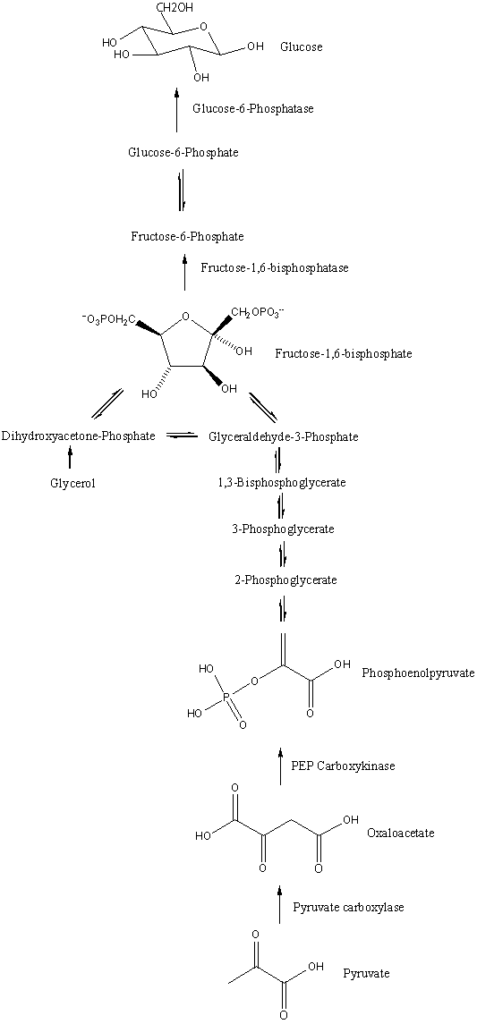
Gluconeogenesis Reactions
Gluconeogenesis is a metabolic pathway that facilitates the synthesis of glucose from non-carbohydrate precursors. This process encompasses a series of enzymatic reactions, each meticulously orchestrated to ensure the accurate conversion of substrates. Here’s a detailed overview of the reactions involved in gluconeogenesis:
- From Pyruvate to Oxaloacetate: The reaction is initiated with pyruvate, which, in the presence of bicarbonate (HCO3-) and ATP, is converted to oxaloacetate. The equation for this reaction is: [Pyruvate]+[HCO3−]+[ATP]→[oxaloacetate]+[ADP]+[Pi]
- Oxaloacetate to Phosphoenolpyruvate: Oxaloacetate undergoes a transformation to produce phosphoenolpyruvate (PEP), releasing carbon dioxide in the process. The reaction is as follows: [Oxaloacetate]+[GTP]⇌[phosphoenolpyruvate]+[CO2]+[GDP]
- Conversion to 2-Phosphoglycerate: PEP is then hydrolyzed to form 2-phosphoglycerate: [Phosphoenolpyruvate]+[H2O]⇌[2−phosphoglycerate]
- Isomerization to 3-Phosphoglycerate: A simple isomerization reaction converts 2-phosphoglycerate to 3-phosphoglycerate.
- Formation of 1,3-Bisphosphoglycerate: 3-Phosphoglycerate reacts with ATP to produce 1,3-bisphosphoglycerate and ADP.
- Conversion to Glyceraldehyde 3-Phosphate: 1,3-Bisphosphoglycerate, in the presence of NADH, is reduced to form glyceraldehyde 3-phosphate.
- Interconversion with Dihydroxyacetone Phosphate: Glyceraldehyde 3-phosphate can interconvert with dihydroxyacetone phosphate.
- Formation of Fructose 1,6-Bisphosphate: The two aforementioned compounds combine to produce fructose 1,6-bisphosphate.
- Conversion to Fructose 6-Phosphate: Fructose 1,6-bisphosphate is then dephosphorylated to yield fructose 6-phosphate.
- Formation of Glucose 6-Phosphate: Fructose 6-phosphate is isomerized to glucose 6-phosphate.
- Final Step to Glucose: The last step involves the hydrolysis of glucose 6-phosphate to release free glucose.
In summary, the final overarching reaction of gluconeogenesis is: 2Pyruvate+4ATP+2GTP+2NADH+[2H+]+4H2O→glucose+4ADP+2GDP+6Pi+[2NAD+]
These reactions, when viewed collectively, highlight the intricate nature of gluconeogenesis and its pivotal role in glucose homeostasis.
Enzymes of Gluconeogenesis
Gluconeogenesis is a metabolic pathway that synthesizes glucose from non-carbohydrate precursors. This process involves several enzymes, some of which are unique to gluconeogenesis, while others are shared with glycolysis but operate in the reverse direction. Here’s a detailed look at these enzymes:
- Pyruvate Carboxylase: This enzyme operates in the mitochondria and is responsible for the conversion of pyruvate to oxaloacetate. It is one of the key enzymes that differentiate gluconeogenesis from glycolysis.
- Malate Dehydrogenase: Oxaloacetate, produced in the mitochondria, cannot directly cross the mitochondrial membrane. Therefore, it is first converted to malate by malate dehydrogenase. Once in the cytoplasm, malate is reconverted to oxaloacetate by another malate dehydrogenase.
- PEP Carboxykinase (PEPCK): This enzyme converts oxaloacetate to phosphoenolpyruvic acid (PEP) in the cytoplasm. It is another enzyme unique to the gluconeogenesis pathway.
- Fructose-1,6-Phosphatase: While glycolysis uses phosphofructokinase to convert fructose-6-P to fructose-1,6-bP, gluconeogenesis does the reverse. The enzyme fructose-1,6-phosphatase converts fructose-1,6-bP back to fructose-6-P.
- Phosphoglucoisomerase: This enzyme facilitates the conversion of fructose-6-P to glucose-6-P, a step shared with glycolysis but operating in the opposite direction.
- Glucose-6-Phosphatase: In the final step of gluconeogenesis, glucose-6-phosphatase converts glucose-6-P to glucose. This enzyme is located in the endoplasmic reticulum and is another distinguishing feature of gluconeogenesis.
Substrate for Gluconeogenesis
Gluconeogenesis is a metabolic pathway that synthesizes glucose from non-carbohydrate precursors. The substrates for this process are derived from various metabolic pathways, ensuring the body’s glucose needs are met, especially during periods of limited carbohydrate intake.
1. Glycerol as a Substrate
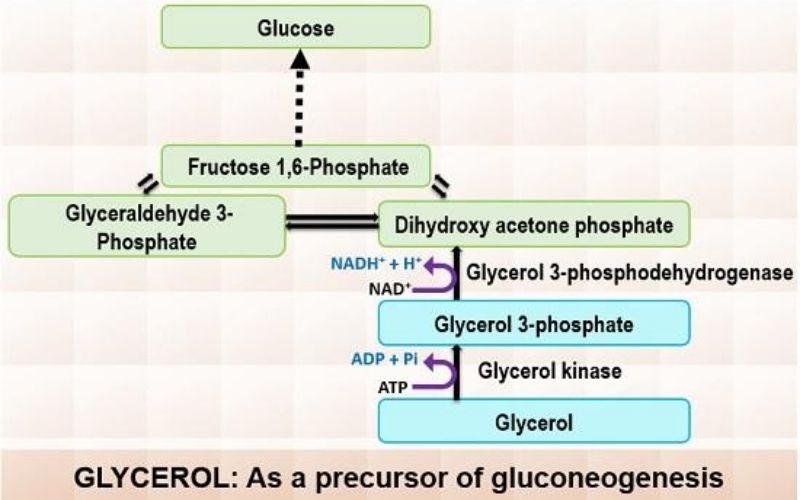
Glycerol is a byproduct of triglyceride hydrolysis in adipose tissue. Once formed, it is transported to the liver through the bloodstream. Within the liver, glycerol undergoes a two-step transformation to enter the gluconeogenesis pathway:
- Phosphorylation: Glycerol is first phosphorylated to produce glycerol 3-phosphate. This reaction consumes one ATP molecule and is catalyzed by the enzyme glycerol kinase.
- Oxidation: Subsequently, glycerol 3-phosphate is oxidized to dihydroxyacetone phosphate. During this step, one NAD molecule is reduced to NADH, with the enzyme glycerol 3-phosphodehydrogenase facilitating the reaction.
2. Lactate as a Substrate
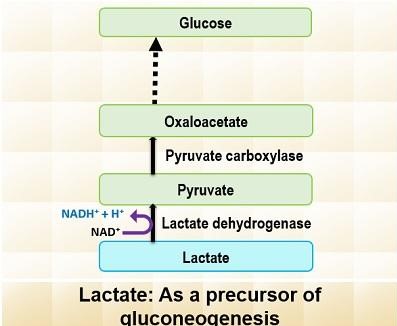
Lactate is primarily produced through anaerobic glycolysis in red blood cells and muscles. It is then transported to the liver via the bloodstream. In the liver, lactate is converted back to pyruvate by the enzyme lactate dehydrogenase. This pyruvate then serves as a substrate for gluconeogenesis, eventually leading to glucose production.
3. Glucogenic Amino Acids
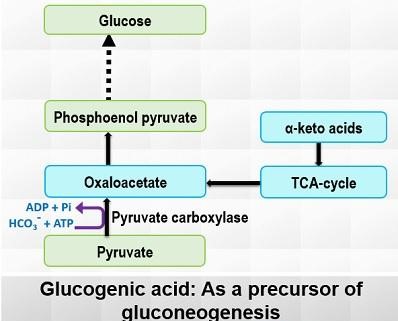
Glucogenic amino acids arise from the hydrolysis of tissue proteins. Examples of these amino acids include succinyl Co-A, α-ketoglutarate, fumarate, oxaloacetate, and fumarate. These amino acids can enter the gluconeogenesis pathway at various points, with pyruvate and oxaloacetate being the primary entry points.
In summary, gluconeogenesis relies on diverse substrates, including glycerol, lactate, and glucogenic amino acids. Whether derived from triglyceride breakdown, anaerobic metabolism, or protein hydrolysis, these substrates collectively ensure the body’s ability to produce glucose when dietary carbohydrates are scarce.
Regulation of Gluconeogenesis
The regulation of this process is intricate, involving various molecules and hormones that either promote or inhibit its progression.
- Acetyl CoA’s Dual Role: Acetyl CoA plays a pivotal role in the regulation of gluconeogenesis. It has both stimulatory and inhibitory effects on the pathway:
- Positive Regulation: Acetyl CoA enhances the activity of the enzyme pyruvate carboxylase, leading to increased production of oxaloacetate, a key intermediate in gluconeogenesis.
- Negative Regulation: Conversely, acetyl CoA inhibits pyruvate dehydrogenase, reducing the conversion rate of pyruvate to acetyl CoA.
- Glucagon’s Influence: Glucagon, a hormone secreted from the α-cells of the pancreas, plays a crucial role in glucose homeostasis. It regulates gluconeogenesis in two primary ways:
- By mediating cyclic AMP, glucagon inactivates pyruvate kinase, decreasing the conversion rate of phosphoenolpyruvate (PEP) to pyruvate.
- It inhibits phosphofructokinase while activating fructose 1,6-bisphosphatase, promoting glucose synthesis.
- Glucogenic Amino Acids: During periods of decreased insulin levels, glucogenic amino acids regulate the conversion rate of glucose 6-phosphate to glucose, further emphasizing their role in glucose production.
- Hormonal Control: Gluconeogenesis is influenced by various hormones, including glucagon, cortisol, and growth hormone. These hormones are released in response to low blood sugar levels, stress, or fasting conditions. They promote the production of essential gluconeogenic enzymes, ensuring glucose synthesis. In contrast, insulin acts as a counter-regulatory hormone, inhibiting gluconeogenesis by reducing the expression of gluconeogenic enzymes and promoting tissue glucose uptake.
- Insulin Resistance and Its Implications: Insulin resistance disrupts the normal regulation of gluconeogenesis. Characterized by reduced cellular responsiveness to insulin, this condition results in excessive glucose production by the liver. This overproduction is due to the liver’s decreased sensitivity to insulin’s inhibitory effects. Such dysregulation contributes to elevated blood glucose levels, commonly observed in conditions like type 2 diabetes. Moreover, insulin resistance not only promotes gluconeogenesis but also inhibits glycolysis, exacerbating hyperglycemia.
In conclusion, the regulation of gluconeogenesis is a complex interplay of molecules and hormones, ensuring glucose homeostasis. Any disruption in this regulation can lead to metabolic disorders, emphasizing the importance of understanding and maintaining this balance.
Clinical Significance of Gluconeogenesis
Gluconeogenesis, a vital metabolic pathway, plays a central role in the synthesis of glucose from non-carbohydrate sources. Its significance becomes even more pronounced when considering various genetic disorders that impact glucose metabolism. Therefore, understanding the clinical relevance of gluconeogenesis and its associated disorders is crucial for medical professionals.
- Von Gierke Disease (Glycogen Storage Disorder Type 1A): One of the primary disorders associated with glucose metabolism is Von Gierke disease. Patients with this condition face a challenge in converting glycogen into glucose. The underlying cause of this disorder is an insufficient amount of the enzyme glucose-6-phosphatase. Clinically, newborns with this condition often present with hepatomegaly and hypoglycemia. These symptoms typically manifest between the ages of three and six months, although the age of presentation can vary. Diagnosis is confirmed through an enzyme test and a liver biopsy. With appropriate nutritional therapy, the long-term complications of this disorder can be mitigated.
- Cori Disease (Glycogen Storage Disorder Type III): Another genetic disorder that emphasizes the importance of gluconeogenesis is Cori disease. This condition arises due to a mutation in the AGL gene, located on chromosome 1p21. This gene codes for the glycogen debranching enzyme (amylo-1,6-glucosidase). Patients with Cori disease often exhibit clinical signs such as hypoglycemia, hyperlipidemia, decreased growth, and hepatomegaly.
- McArdle Disease (Glycogen Storage Disorder Type V): McArdle disease provides another perspective on the clinical relevance of gluconeogenesis. This autosomal recessive inborn error impacts skeletal muscle metabolism. Specifically, the activity of glycogen phosphorylase is affected, preventing the breakdown of glycogen. This disorder underscores the importance of the efficient breakdown and utilization of glycogen for energy.
In conclusion, the pathway of gluconeogenesis and its associated enzymes play a pivotal role in maintaining glucose homeostasis. Genetic disorders like Von Gierke, Cori, and McArdle diseases highlight the clinical implications of disruptions in this pathway. Understanding these disorders and their underlying mechanisms is essential for effective diagnosis and management.
Insulin Resistance
Insulin resistance is not an isolated event. When cells fail to respond adequately to insulin, the pancreas, in an attempt to restore balance, produces extra insulin. Over time, this compensatory mechanism leads to elevated blood sugar levels. Besides, insulin resistance syndrome manifests with a constellation of symptoms, including obesity, high blood pressure, high cholesterol, and type 2 diabetes. It is noteworthy that this syndrome might affect as many as one in every three Americans. In some circles, insulin resistance is also referred to as metabolic syndrome, highlighting its broad metabolic implications.
Reversing Insulin Resistance: Addressing insulin resistance is paramount for metabolic health. The objective is to transition from a state of insulin resistance to insulin sensitivity. In an insulin-sensitive state, cells become more efficient at absorbing blood sugar, reducing the need for excessive insulin production.
- Physical Exercise: Physical activity stands out as a potent tool against insulin resistance. Engaging in regular exercise enhances insulin responsiveness. It’s imperative to understand that one need not wait for a diabetes diagnosis to incorporate exercise into their routine. The earlier one initiates physical activity, the more beneficial the outcomes.
- Lifestyle Modifications: Besides exercise, several other factors play a pivotal role in reversing insulin resistance. Weight loss, for instance, can significantly improve insulin sensitivity. Additionally, maintaining stable blood sugar levels, managing stress, and ensuring adequate sleep are all crucial components of this therapeutic approach. It’s worth noting that physical exercise can also aid in improving sleep quality.
In conclusion, insulin resistance is a multifaceted metabolic disorder with wide-ranging implications. However, with informed interventions like physical activity and lifestyle modifications, it’s possible to mitigate its effects and promote metabolic health.
Gluconeogenesis of Amino acids
Amino acids that can be transformed into glucose are termed gluconeogenic amino acids. These amino acids undergo specific biochemical reactions, primarily deamination or transamination, to enter the citric acid cycle, also known as the tricarboxylic acid (TCA) cycle. Therefore, out of the 20 amino acids, a significant number find their way into the TCA cycle, serving as precursors for glucose synthesis.
- Single Intermediate Production: Some amino acids, upon entering the TCA cycle, yield only one intermediate. For instance, alanine is converted into pyruvate, which subsequently enters the gluconeogenesis pathway.
- Multiple Intermediate Production: Besides those producing a single intermediate, certain amino acids yield two intermediates. A classic example is phenylalanine. This versatility in intermediate production underscores the diverse routes through which amino acids can contribute to glucose synthesis.
Metabolic Pathway of Amino Acids: Once the amino acids are processed into the intermediates of the TCA cycle, they traverse the metabolic pathway to eventually form glucose. Key intermediates in this journey include phosphoenolpyruvic acid and oxaloacetic acid. These intermediates undergo a series of enzymatic reactions, culminating in the production of glucose.
In conclusion, the gluconeogenesis of amino acids is a testament to the adaptability and complexity of human metabolism. Amino acids, beyond their role in protein synthesis, also serve as vital precursors for glucose production, ensuring energy homeostasis in the body. Understanding this intricate process provides valuable insights into the multifaceted roles of amino acids in human physiology.
Importance of Gluconeogenesis
- Central Role in Blood Sugar Regulation: One of the primary functions of gluconeogenesis is to regulate blood sugar levels during periods of deprivation. This ensures that the body’s cells and tissues have a consistent supply of glucose, which is essential for their proper functioning.
- Meeting the Energy Demands of Vital Tissues: Certain cells and tissues are highly dependent on glucose as their primary energy source. These include:
- Red Blood Cells (RBCs): RBCs rely exclusively on glucose for their energy needs, as they lack mitochondria and cannot utilize fatty acids for energy.
- Neurons: The brain and nervous system predominantly use glucose as an energy source. While they can utilize ketone bodies during prolonged fasting, glucose remains their preferred fuel.
- Skeletal Muscle: During intense physical activity, skeletal muscles rely on glucose for rapid energy production.
- Embryonic Tissue: During the early stages of development, embryonic tissues are highly dependent on glucose for growth and differentiation.
- Medulla of the Kidney: This region of the kidney requires glucose to function optimally, especially during the process of urine concentration.
- Testes: Glucose is vital for spermatogenesis, the process by which sperm cells are produced.
- Clearance of Metabolites from Circulation: Gluconeogenesis plays a crucial role in removing specific metabolites from the bloodstream. For instance, lactate, produced by muscles and RBCs during anaerobic metabolism, and glycerol, released during the breakdown of triglycerides in adipose tissue, are utilized as substrates in the gluconeogenesis pathway. This not only ensures that these metabolites do not accumulate to harmful levels but also recycles them to produce glucose, further emphasizing the pathway’s importance.
- Significance During Starvation: During prolonged periods of fasting or starvation, dietary sources of glucose are unavailable. Gluconeogenesis becomes particularly vital during these times, ensuring that blood glucose levels are maintained, thereby preventing hypoglycemia and supporting the energy needs of vital organs.
Difference between Gluconeogenesis and Glycogenolysis – Glycogenolysis vs Gluconeogenesis
Glycogenolysis: Glycogenolysis is the biochemical process that focuses on the breakdown of glycogen, a multi-branched polysaccharide, into glucose-6-phosphate. This process is initiated by the addition of inorganic phosphates, which facilitates the cleavage of the glycogen monomer. Besides being a catabolic process, glycogenolysis is characterized by the following features:
- Site of Occurrence: Contrary to common belief, glycogenolysis does not occur in the gallbladder. Instead, it predominantly takes place in the liver, where glycogen stores are abundant. The liver plays a central role in decomposing glycogen during this process.
- Energy Consumption: Glycogenolysis is an energy-efficient process. Only trace quantities of ATP, the cellular currency of energy, are utilized during this process.
- Purpose: The primary objective of glycogenolysis is to provide glucose-6-phosphate, which can then be converted to glucose for energy production or other metabolic needs.
Gluconeogenesis: On the other hand, gluconeogenesis is a metabolic pathway that synthesizes glucose from non-carbohydrate precursors. This process is essential during periods when glucose intake is low or absent. The characteristics of gluconeogenesis include:
- Substrates Used: Gluconeogenesis employs non-carbohydrate substrates for glucose synthesis. Lactic acid and amino acids are the primary substrates utilized in this process.
- Nature of the Process: Gluconeogenesis is an anabolic process. This means it is involved in building or synthesizing molecules.
- Energy Consumption: Unlike glycogenolysis, gluconeogenesis is energy-intensive. To synthesize one glucose molecule, six ATP molecules are expended.
- Site of Occurrence: Gluconeogenesis primarily occurs in the liver. However, it can also take place in tissues where there is a high demand for glucose.
In conclusion, while both glycogenolysis and gluconeogenesis are integral to glucose metabolism, they differ in their mechanisms, energy consumption, and sites of occurrence. Therefore, understanding these processes is essential for anyone delving into the intricacies of metabolic pathways.
| Criteria | Glycogenolysis | Gluconeogenesis |
|---|---|---|
| Definition | Process of producing glucose-6 phosphate from glycogen. | Metabolic process in which glucose is synthesized from non-carbohydrate substrates. |
| Site of Occurrence | Predominantly in the liver. | Primarily in the liver and tissues with high glucose demand. |
| Nature of the Process | Catabolic (breakdown). | Anabolic (synthesis). |
| Energy Consumption | Only trace quantities of ATP. | Six ATPs for one glucose molecule. |
| Substrates/Inputs | Glycogen. | Lactic acid and amino acids. |
| Primary Objective | Provide glucose-6-phosphate. | Synthesize glucose from non-carbohydrate sources. |
