Table of Contents
What is Gram staining?
- Gram staining, a pivotal technique in microbiology, was pioneered by the Danish bacteriologist Hans Christian Gram in 1884. The primary objective of this method is to differentiate bacterial species into two principal groups based on the chemical and physical properties of their cell walls: gram-positive bacteria and gram-negative bacteria.
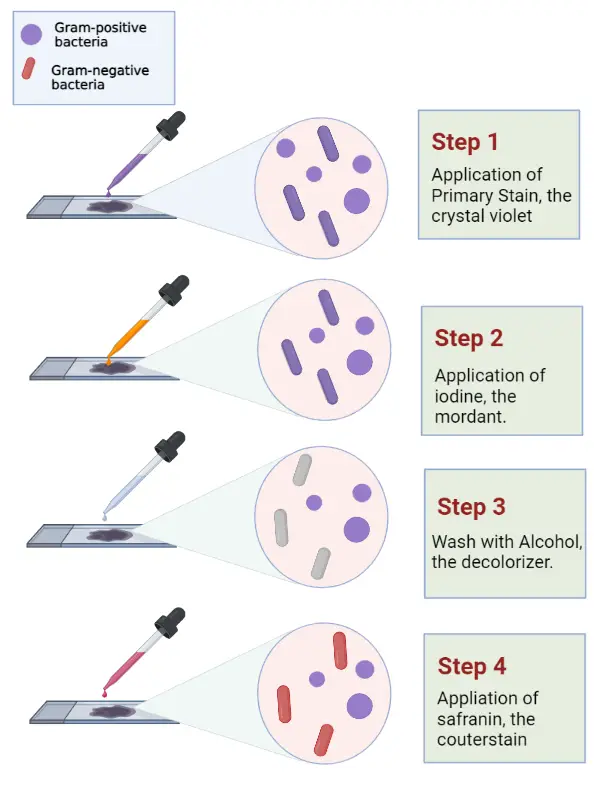
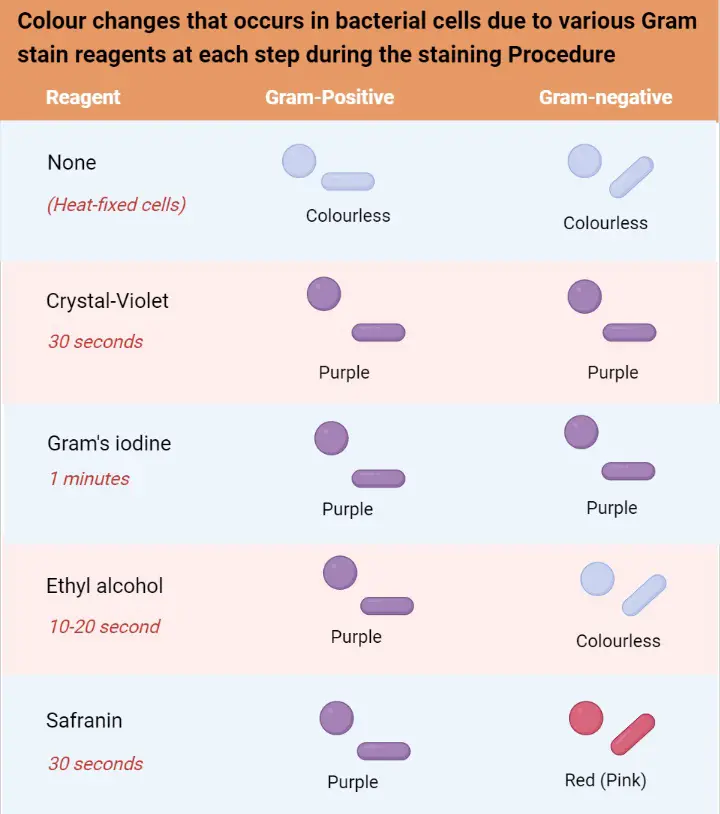
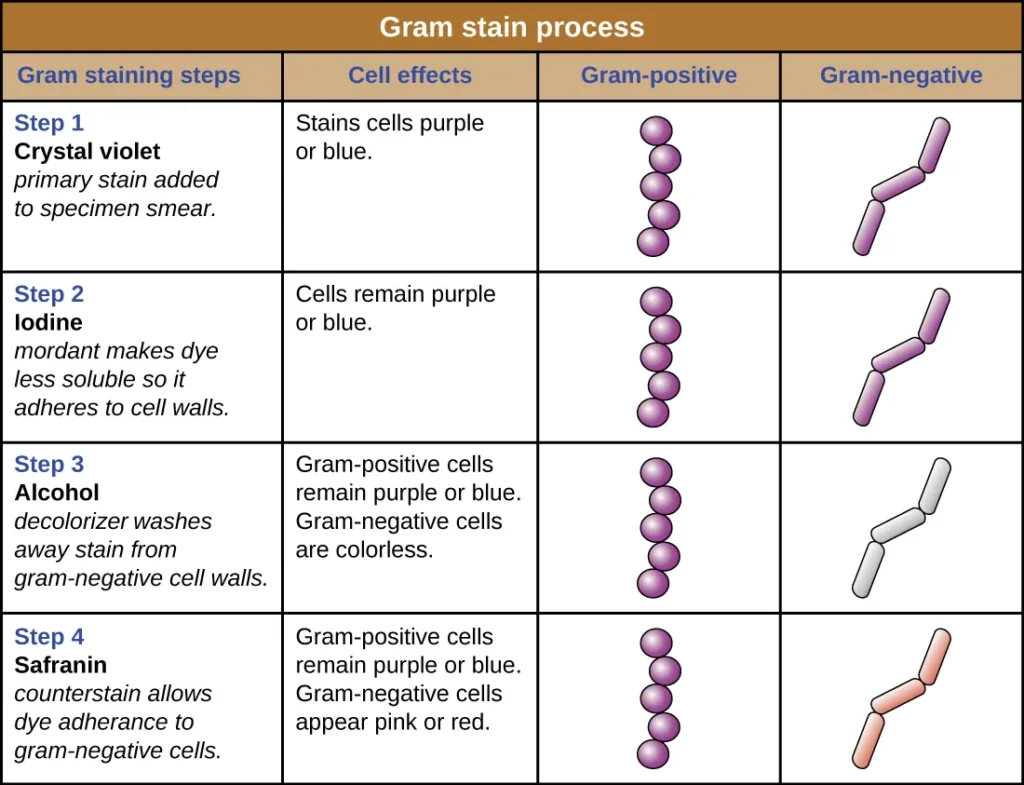
- The procedure begins with the application of a primary stain, crystal violet, to the bacterial sample. Following this, Lugol’s iodine solution, which acts as a mordant, is added to strengthen the bond between the stain and the bacterial cell membrane. Then, the sample is treated with ethanol, a decolorizer. The ethanol’s role is crucial as it determines the final coloration of the bacteria. Gram-positive bacteria, characterized by a thick layer of peptidoglycan in their cell walls, retain the crystal violet stain, resulting in a blue/violet appearance. In contrast, gram-negative bacteria possess a thinner peptidoglycan layer, causing the crystal violet to be washed out during the ethanol treatment. Therefore, a counterstain, typically safranin or fuchsine, is applied, rendering the gram-negative bacteria pink or red.
- Besides its role in distinguishing between gram-positive and gram-negative bacteria, the Gram stain provides valuable insights into the bacterial cell wall’s composition. For instance, the thick peptidoglycan layer in gram-positive bacteria is responsible for retaining the primary stain. On the other hand, the thinner peptidoglycan layer in gram-negative bacteria allows the primary stain to be easily decolorized, necessitating the use of a counterstain.
- Moreover, Gram staining plays a crucial role in both clinical and research settings, often serving as the initial step in bacterial identification. However, it’s essential to note that while the Gram stain is an indispensable tool, not all bacteria can be definitively classified using this method alone. Some bacteria exhibit properties of both groups, leading to classifications such as gram-variable and gram-indeterminate.
- In conclusion, Gram staining is a fundamental, differential staining technique in bacteriology that offers a clear and concise method to categorize bacteria based on their cell wall properties. By understanding the functions and characteristics of different bacterial groups, researchers and clinicians can make informed decisions regarding treatment and further investigations.
Definition of Gram Staining
Gram staining is a microbiological technique used to differentiate bacterial species into two groups, gram-positive and gram-negative, based on the chemical properties of their cell walls.
Purpose of Gram Staining
- The primary objective of Gram staining is to categorize bacteria into two distinct groups: Gram-positive and Gram-negative. This differentiation is achieved by observing the coloration of the bacterial cells post-staining. Gram-positive bacteria, characterized by a thick peptidoglycan layer in their cell walls, retain the primary stain, resulting in a blue to purple appearance. On the other hand, Gram-negative bacteria, possessing a thinner peptidoglycan layer, do not retain the primary stain and, therefore, take up the counterstain, leading to a red to pink hue.
- Besides this primary classification, another objective of Gram staining is to study the morphological structure of bacteria. By understanding the thickness and composition of the peptidoglycan layer, researchers can gain insights into the bacterial cell wall’s structural intricacies. This knowledge, in turn, aids in understanding the bacteria’s physiological properties, potential pathogenicity, and possible mechanisms of resistance.
Therefore, Gram staining is not merely a method of differentiation but also a tool that provides valuable insights into the structural and functional aspects of bacteria. By emphasizing the functions and characteristics of the bacterial cell wall, this technique offers a clear and concise understanding of bacterial taxonomy and physiology.
Gram Staining Principle
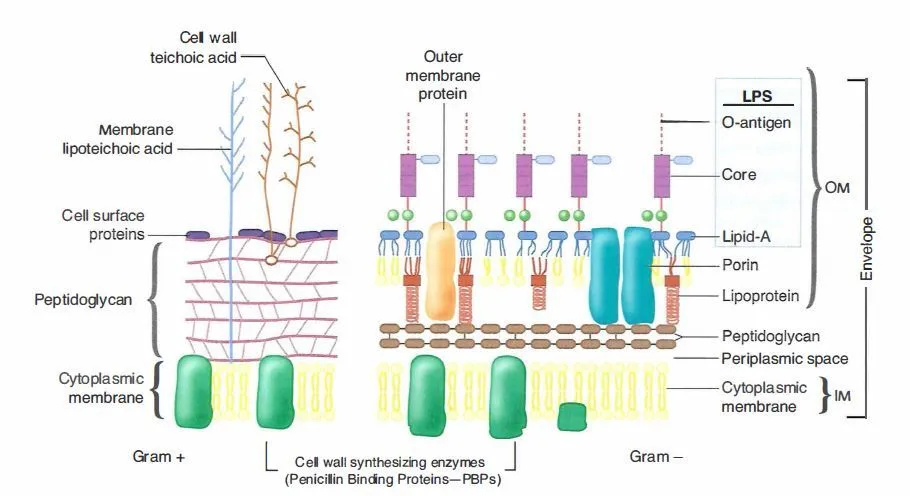
The principle of Gram staining, a foundational technique in bacteriology, revolves around the differentiation of bacteria based on the structural and compositional differences in their cell walls. This differentiation is achieved through a series of meticulously sequenced staining and decolorization steps, which categorize bacteria into two primary groups: Gram-positive and Gram-negative.
Initially, an aqueous solution of crystal violet is applied to the bacterial sample. In this solution, crystal violet dissociates into CV+ and Cl– ions. Both Gram-positive and Gram-negative bacterial cell walls readily allow these ions to penetrate. Within the bacterial cells, the CV+ ions interact with the negatively charged components, staining the cells a purple hue. Following this, Gram’s iodine, a mordant, is added. The iodine, either in the form of I- or I3-, binds with the CV+ ions, forming large crystal violet-iodine (CV-I) complexes within the bacterial cytoplasm and outer layers.
The subsequent step involves the application of a decolorizing agent, typically a solution of ethanol or a mixture of ethanol and acetone. This agent interacts with the lipids present in the cell walls of both Gram-positive and Gram-negative bacteria. For Gram-negative bacteria, their outer membrane is dissolved by the decolorizing solution, exposing the thin peptidoglycan layer. Due to its thinness and lesser cross-linkage, this layer becomes permeable, allowing the CV-I complexes to be washed away. Conversely, Gram-positive bacteria possess a thick peptidoglycan layer with extensive cross-linkage. The decolorizing solution dehydrates this layer, effectively trapping the CV-I complexes within, ensuring the bacteria retain the purple coloration of the crystal violet.
The final step involves counterstaining with safranin, a positively charged dye. In Gram-negative bacteria, which have lost the CV-I complexes, the safranin binds to the negatively charged components, staining them pink or red. However, in Gram-positive bacteria, the dehydrated peptidoglycan layer, already saturated with the CV-I complex, does not take up the safranin, and they remain purple or violet.
Therefore, the essence of the Gram staining principle lies in the differential response of bacterial cell walls to the staining process. By emphasizing the functions and interactions of the staining agents with the bacterial cell wall components, this technique provides a clear, concise, and objective means to categorize bacteria based on their cell wall composition and structure.
Requirements for Gram Staining
Gram staining, a fundamental technique in bacteriology, necessitates specific tools and reagents to ensure its accurate execution. The process, which aims to differentiate bacteria based on their cell wall composition, requires meticulous preparation and the right equipment. Here is a detailed and sequential explanation of the requirements for Gram staining:
- Sample bacterial colonies or suspension: The starting point of the Gram staining procedure is the bacterial sample. This can be in the form of isolated bacterial colonies grown on a culture medium or a suspension of bacteria in a liquid medium.
- Gram Staining Kit (Reagents): A standard Gram staining kit contains all the essential reagents required for the procedure. This typically includes the primary stain (crystal violet), the mordant (Gram’s iodine), the decolorizing agent (usually an alcohol or alcohol-acetone solution), and the counterstain (safranin).
- Glass slide: A clean, grease-free glass slide serves as the platform where the bacterial smear is prepared. It’s crucial that the slide is free from contaminants to ensure accurate staining results.
- Inoculating loop: This tool, often made of metal or plastic, is used to transfer the bacterial sample from the culture medium to the glass slide. It ensures a uniform smear is prepared for staining.
- Bunsen burner: The Bunsen burner is employed to heat-fix the bacterial smear onto the glass slide. Heat-fixing ensures the bacteria adhere to the slide and also kills the bacteria, making them safe to handle.
- Staining rack: This apparatus holds the glass slides during the staining process. It ensures that the slides remain horizontal and stable, allowing for even application of the staining reagents.
- Wash bottle (or Tap water): A wash bottle filled with distilled water or tap water is used to gently rinse off excess stains between the staining steps. Proper washing ensures that each stain is applied and removed accurately, leading to clear differentiation between Gram-positive and Gram-negative bacteria.
- Microscope with 100X objective lens (compound microscope): After the staining procedure is complete, the stained bacterial smear is examined under a compound microscope. The 100X objective lens, often oil immersion, provides the necessary magnification to clearly visualize the stained bacteria and determine their classification based on color.
Therefore, to achieve accurate and clear results in Gram staining, each of these components plays a pivotal role. From the preparation of the bacterial smear to the final observation under the microscope, every step is purposeful, emphasizing the functions and importance of each requirement in the process.
Reagents Required for Gram Staining – Reagent Preparation for Gram Staining
Gram staining, a pivotal technique in bacteriology, requires a series of specific reagents to differentiate bacterial species based on their cell wall composition. The reagents play distinct roles in the staining process, and their preparation is crucial for the accuracy of the results. Here’s a detailed and sequential explanation of the reagents required for Gram staining and their preparation:
- Primary Stain: Crystal Violet
- Function: Crystal violet, an intensely purple-colored organic compound, serves as the primary stain in the Gram staining procedure. It provides a violet color to Gram-Positive bacteria.
- Preparation:
- Solution A: Combine 2g of crystal violet (with a certified 90% dye content) with 20 ml of 95% ethanol.
- Solution B: Dissolve 0.8g of ammonium oxalate in 80 ml of distilled water.
- Mix Solutions A and B to obtain the crystal violet staining reagent. Allow it to sit for 24 hours and then filter through paper before use.
- Mordant: Gram’s Iodine
- Function: Gram’s iodine acts as a mordant, forming a complex with the crystal violet, which gets trapped in the Gram-Positive cell wall’s dehydrated peptidoglycan layer.
- Preparation: Grind 1.0g of iodine and 2.0g of potassium iodide in a mortar. Slowly add water while continuously grinding until the iodine dissolves. The resulting solution should be stored in amber bottles.
- Decolorizing Solution
- Function: The decolorizing agent, typically ethanol or a mixture of ethanol and acetone, dissolves the lipid content in bacterial cell walls, affecting their permeability.
- Preparation:
- Standard Decolorizing Agent: Use 95% ethanol.
- Alternate Decolorizing Agent: Some professionals prefer a mixture of acetone and ethanol. To prepare, combine 50 ml of acetone with 50 ml of 95% ethanol. Commercial variations might include small quantities of isopropyl alcohol or methanol.
- Counterstain: Safranin
- Function: Safranin serves as a counterstain, staining decolorized Gram-Negative cells pink or red. It interacts with the negatively charged components of the bacterial cell wall and membrane.
- Preparation:
- Stock Solution: Dissolve 2.5g of Safranin O in 100 ml of 95% ethanol.
- Working Solution: Mix 10 ml of the stock solution with 90 ml of distilled water.
| Reagent | Function | Preparation |
|---|---|---|
| Primary Stain: Crystal Violet | Serves as the primary stain, providing a violet color to Gram-Positive bacteria. | Solution A: Combine 2g of crystal violet (with a certified 90% dye content) with 20 ml of 95% ethanol. Solution B: Dissolve 0.8g of ammonium oxalate in 80 ml of distilled water. Mix Solutions A and B to obtain the crystal violet staining reagent. Allow it to sit for 24 hours and then filter through paper before use. |
| Mordant: Gram’s Iodine | Acts as a mordant, forming a complex with the crystal violet, which gets trapped in the Gram-Positive cell wall’s dehydrated peptidoglycan layer. | Grind 1.0g of iodine and 2.0g of potassium iodide in a mortar. Slowly add water while continuously grinding until the iodine dissolves. The resulting solution should be stored in amber bottles. |
| Decolorizing Solution | Dissolves the lipid content in bacterial cell walls, affecting their permeability. | Standard Decolorizing Agent: Use 95% ethanol. Alternate Decolorizing Agent: Combine 50 ml of acetone with 50 ml of 95% ethanol. Some variations might include small quantities of isopropyl alcohol or methanol. |
| Counterstain: Safranin | Serves as a counterstain, staining decolorized Gram-Negative cells pink or red. It interacts with the negatively charged components of the bacterial cell wall and membrane. | Stock Solution: Dissolve 2.5g of Safranin O in 100 ml of 95% ethanol. Working Solution: Mix 10 ml of the stock solution with 90 ml of distilled water. |
Gram Staining Procedure
1. Preparation of a slide smear for Gram Staining
The preparation of a slide smear is a crucial step in the Gram staining procedure. It ensures that bacterial cells are adequately spread and fixed on the slide for effective staining and microscopic examination. The following steps provide a detailed and sequential explanation of the process:
- Slide Selection and Preparation: Begin by selecting a clean, clear, and grease-free glass slide. It’s essential to ensure that the slide is free from any contaminants or residues that might interfere with the staining process.
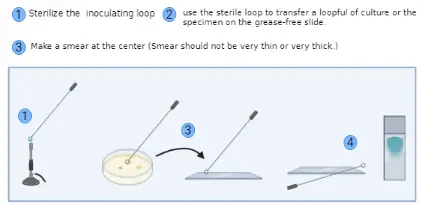
- Sterilization of Inoculating Loop: Using a Bunsen burner or a similar flame source, sterilize the inoculating loop. This step ensures that the loop is free from any unwanted microorganisms.
- Transfer of Bacterial Culture:
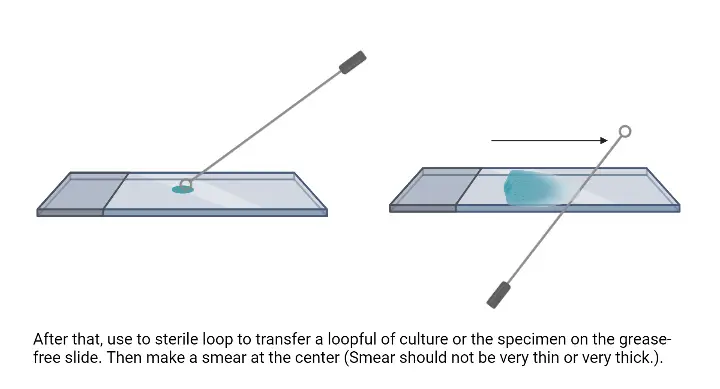
- If working with a bacterial culture suspension, transfer a loop full of the suspension to the center of the glass slide using the sterilized inoculating loop.
- However, if the culture is sourced from a Petri dish or a slant, place a small drop of water in the middle of the slide. Using the sterile loop, pick a small amount of the bacterial colony and mix it with the water drop on the slide.
- Spreading the Suspension: With the sterile inoculating loop, spread the bacterial suspension to form a thin smear on the slide. It’s vital to ensure that the smear is neither too thin nor too thick. An evenly spread smear facilitates uniform staining and clear microscopic observation.
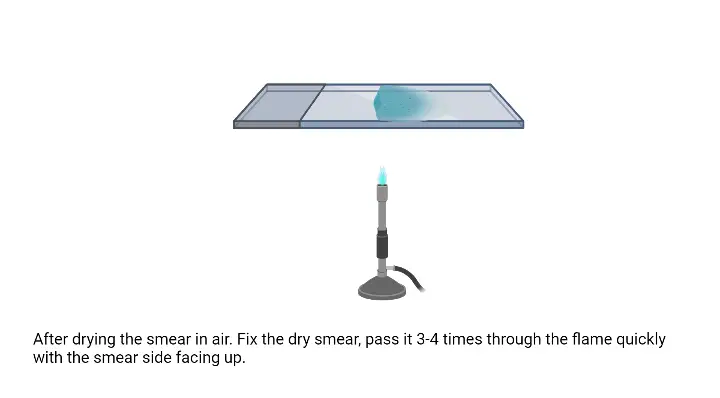
- Drying and Fixation: Allow the smear to air dry completely. Once dried, the smear needs to be fixed to the slide. This is achieved by passing the slide over a gentle flame. The slide should be moved in an up-and-down or circular motion over the flame to prevent overheating. This fixation step is crucial as it adheres the bacterial cells to the slide, ensuring they remain intact during the subsequent staining process.
2. Gram Staining Protocol
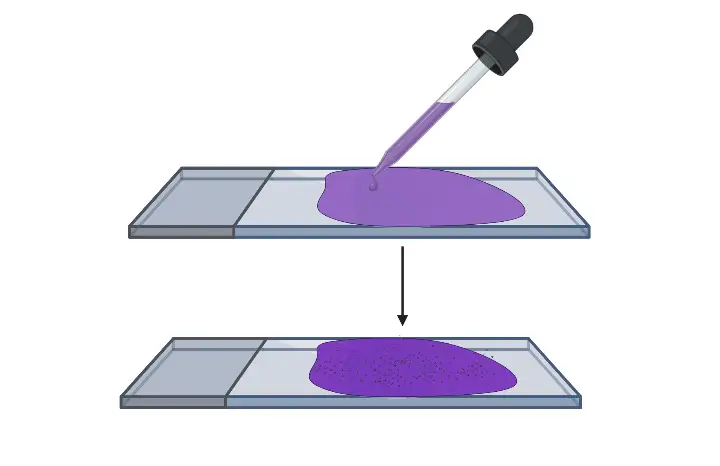
- Application of Primary Stain: Begin by flooding the fixed smear with the crystal violet solution. This primary stain will bind to all bacterial cells present on the slide. Allow the stain to remain on the smear for approximately 30 to 60 seconds.
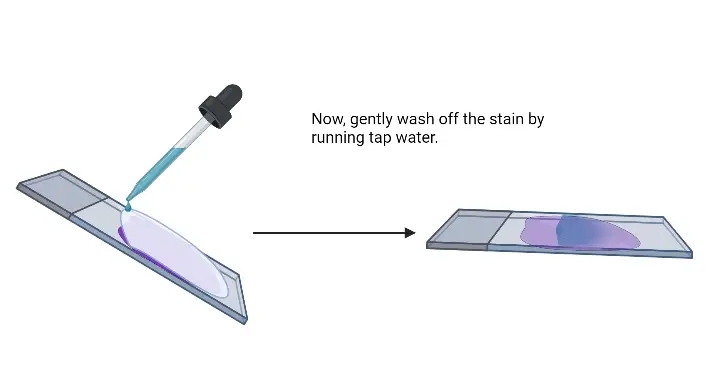
- Rinsing: After the stipulated time, pour off the crystal violet solution and gently rinse the slide with running water. This step ensures the removal of any unbound stain.
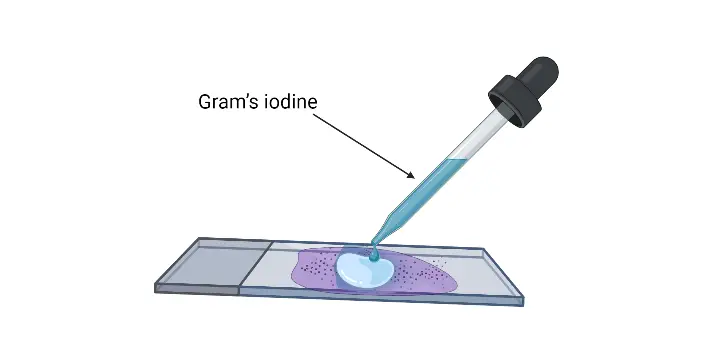
- Mordant Application: Flood the smear with Gram’s iodine solution. This acts as a mordant, forming a complex with the crystal violet, thereby fixing the dye onto the bacterial cells. Leave the iodine solution on the smear for another 30 to 60 seconds.
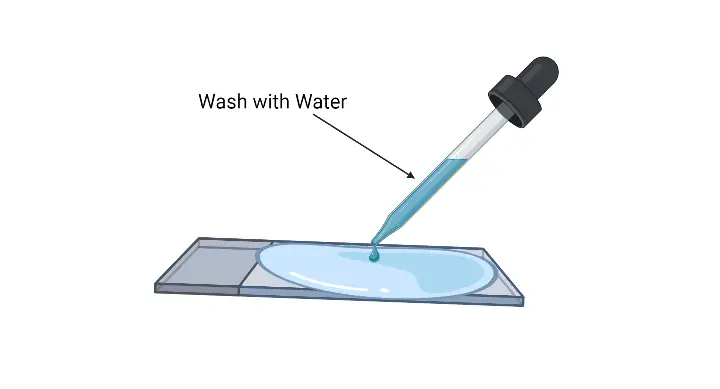
- Rinsing Again: Pour off the excess iodine solution and rinse the slide with gentle running water. Shake off any excess water from the slide.
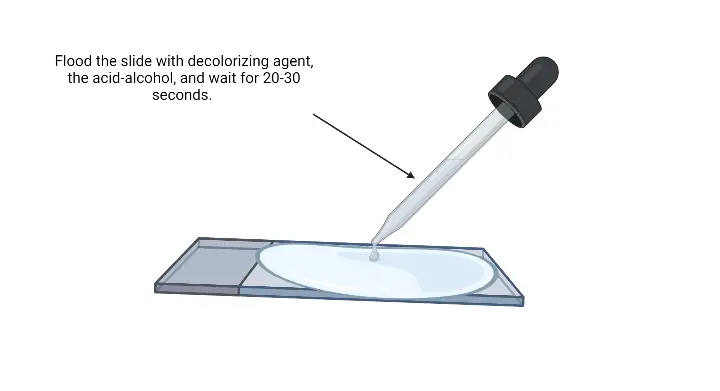
- Decolorization: Decolorize the smear by adding the decolorizing solution. This step differentiates the bacterial cells based on their cell wall composition. Continue adding the decolorizing solution until it runs clear. Alternatively, a few drops of the solution can be added, followed by a gentle shake, and then rinsed with distilled water after about 5 seconds.
- Counterstaining: After rinsing off the decolorizer and shaking off excess water, pour the counterstain (typically safranin or basic fuchsin) over the smear. Allow the counterstain to remain on the smear for 30 to 60 seconds. This stain will color the Gram-negative bacteria, which would have been decolorized in the previous step.
- Final Rinsing and Drying: Wash the slide with gentle running water to remove any excess counterstain. Following this, the slide can be air-dried or blow-dried for microscopic examination.


3. Procedure of Microscopic Observation of Gram Stain
Microscopic observation of a Gram-stained slide is a crucial step in the Gram staining protocol. It allows for the differentiation and identification of bacterial cells based on their staining characteristics. The following steps provide a detailed and sequential explanation of the microscopic observation procedure:
- Slide Placement: Begin by positioning the slide with the Gram-stained, air-dried smear on the stage of a compound microscope. Secure the slide in place using the stage clips to ensure stability during observation.
- Light Focusing: Adjust the stage to focus the light source directly over the smear. Proper illumination is essential for clear visualization of bacterial cells.
- Initial Magnification: Align the 10X objective lens over the smear. Using the coarse adjustment knob, bring the smear into focus. This initial magnification provides a broad view of the smear distribution.
- Intermediate Magnification: Switch to the 40X objective lens. Refine the focus using the fine adjustment knob. This magnification offers a closer look at the bacterial cells and their arrangement.
- Preparation for High Magnification: Rotate the nosepiece of the microscope so that the smear is positioned between the 40X and 100X objective lenses. This step prepares the slide for oil immersion observation.
- Oil Immersion: Carefully place a drop of immersion oil directly over the smear. The oil bridges the gap between the slide and the 100X objective lens, enhancing the resolution of the image.
- High Magnification Observation: Rotate the nosepiece to align the 100X oil immersion objective lens over the smear. Using the fine adjustment knob, bring the bacterial cells into sharp focus. This high magnification allows for detailed examination of individual bacterial cells and their staining characteristics.
- Detailed Examination: All areas of the slide should be examined, with special attention given to areas where the smear is only one cell thick. These regions provide the most accurate staining results. It’s essential to note that thick areas on the slide can yield variable and potentially incorrect results.
Procedure for Change Magnification in Microscope
Changing the magnification in a microscope is a fundamental skill for anyone working in a laboratory setting. It allows for detailed examination of specimens at varying levels of magnification. The following is a detailed and sequential explanation of the procedure to change the magnification in a microscope:
- Positioning: Begin by positioning yourself comfortably in front of the microscope. Ensure that your eyes are level with the eyepieces and that you have a clear view of the specimen.
- Initial Focus: Look through the eyepieces and use the coarse and fine focusing knobs to bring the specimen into clear focus at the current magnification level, which in this case is 10x.
- Locating the Objective Lenses: Direct your attention to the objective lenses located on the rotating nosepiece beneath the stage of the microscope. These lenses are responsible for magnifying the specimen. Each lens is labeled with its magnification power, such as 10x, 40x, or 100x.
- Selecting the Desired Magnification: Gently turn the nosepiece either to the right or left until the 100x objective lens clicks into place directly above the specimen. Ensure that the lens is aligned correctly to avoid any obstructions in the viewing field.
- Refocusing: After changing the objective lens, the specimen’s focus may be slightly off. Therefore, look through the eyepieces once again and use the fine adjustment knob to bring the specimen into sharp focus at the new 100x magnification level.
- Observation: With the specimen now in focus at the desired magnification, you can proceed with your observations, taking note of any details or structures that were not visible at the lower magnification.
Oil Immersion steps in microscope for 100x magnification
The oil immersion technique is a pivotal method in microscopy, especially when one aims to achieve the highest resolution with 100x magnification. This technique employs immersion oil to bridge the gap between the microscope slide and the objective lens. The following is a systematic guide to the oil immersion steps for 100x magnification:
- Preparation: Begin by ensuring that the microscope is positioned on a stable surface. Switch on the microscope’s light source to ensure adequate illumination.
- Slide Placement: Place the prepared microscope slide on the stage, ensuring that the specimen is facing upwards. Adjust the slide so that the area of interest is directly beneath the objective lens.
- Initial Magnification: Before applying the oil, it’s essential to first use a lower magnification objective, such as the 40x, to locate the area of interest on the slide.
- Applying Immersion Oil: Once the area of interest is located, rotate the nosepiece away from the slide and place a drop of immersion oil directly onto the specimen. The oil serves to reduce light refraction, thereby enhancing image clarity at high magnifications.
- Engaging the 100x Objective: Carefully rotate the nosepiece to align the 100x oil immersion objective lens with the specimen. The objective lens should immerse into the oil drop placed on the slide.
- Focusing: With the 100x objective lens in place, look through the eyepieces. Utilize the fine adjustment knob to bring the specimen into sharp focus. The immersion oil will ensure that more light rays are captured, providing a clearer and more detailed image.
- Observation: At this magnification, cellular structures and minute details become more evident. Therefore, take your time to study the specimen, noting any intricate details that were not discernible at lower magnifications.
- Post-Observation Cleanup: After completing the observations, rotate the nosepiece to disengage the 100x objective. Carefully clean the objective lens using lens tissue or a soft, lint-free cloth to remove any residual immersion oil. Similarly, clean the slide to remove the oil before storing it.
Gram Staining Result and Interpretation
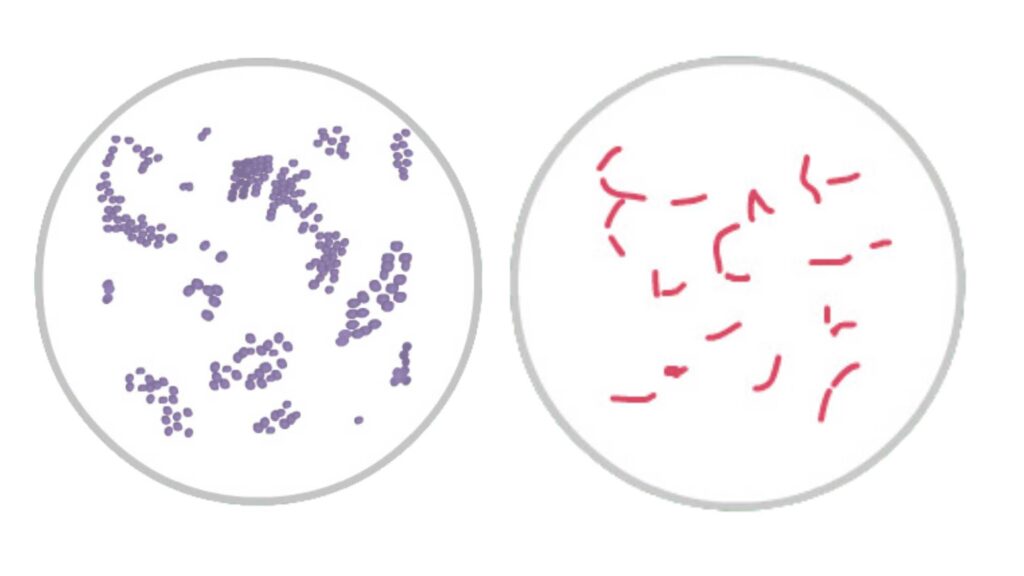
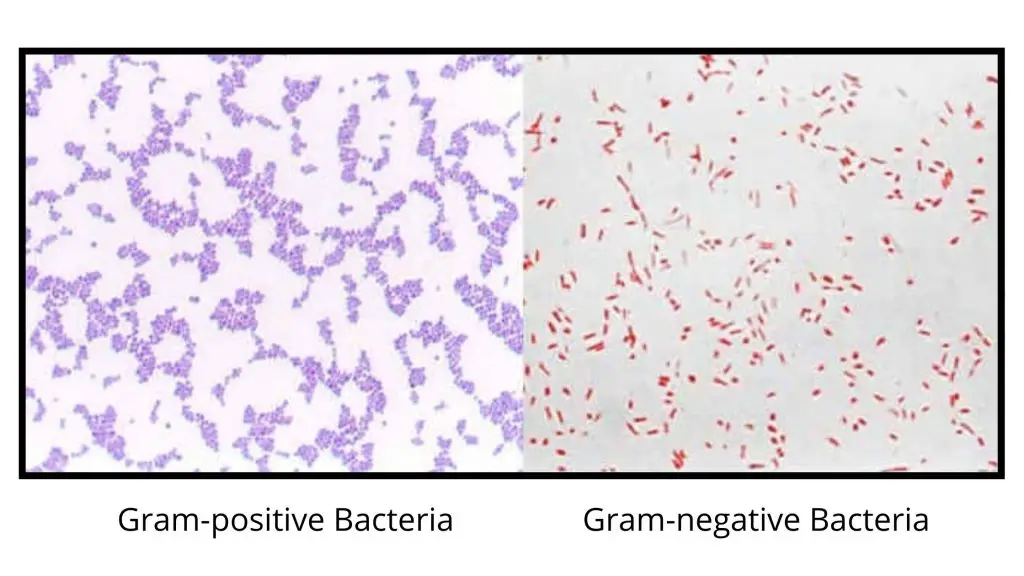
- Gram-Positive Bacteria:
- Appearance: These bacteria retain the crystal violet stain and appear violet or purple under the microscope.
- Cell Wall Composition: The cell wall of Gram-positive bacteria is characterized by a thick layer of peptidoglycan.
- Examples:
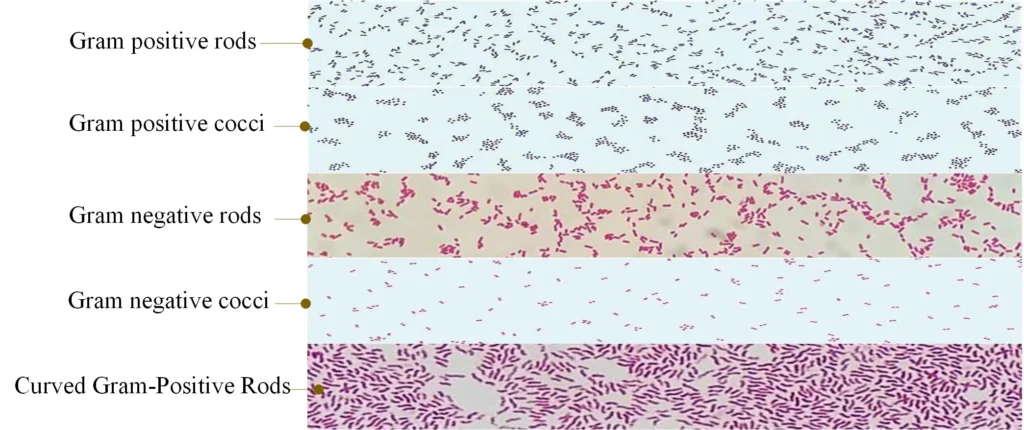
- Gram-positive cocci: This group includes species like Staphylococcus spp., Streptococcus spp., and Enterococcus spp.
- Gram-positive bacilli: Examples in this category are Bacillus spp., Clostridium spp., Lactobacillus spp., Streptomyces spp. and other Actinobacteria, Listeria spp., and Corynebacterium spp.
- Gram-Negative Bacteria:
- Appearance: These bacteria do not retain the crystal violet stain but take up the counterstain, safranin, and therefore appear pink or red under the microscope.
- Cell Wall Composition: Gram-negative bacteria have a thinner peptidoglycan layer and an additional outer membrane containing lipopolysaccharides.
- Examples:
- Gram-negative cocci: This group encompasses species like Neisseria spp., Moraxella spp., and Acinetobacter spp.
- Gram-negative bacilli: Bacteria in this category include E. coli, Klebsiella spp., Salmonella spp., Shigella spp., Pseudomonas spp., and Proteus spp.
Therefore, the Gram staining technique is not just about color differentiation; it provides valuable insights into the structural differences in bacterial cell walls. Besides aiding in bacterial identification, this information is crucial for determining the susceptibility of bacteria to certain antibiotics and for understanding their pathogenic mechanisms. In essence, the Gram stain serves as a foundational tool in both clinical and research settings, enabling a deeper understanding of bacterial diversity and behavior.
| Gram-Positive Bacteria | Gram-Negative Bacteria |
|---|---|
| Staphylococcus aureus | Escherichia coli |
| Streptococcus pneumoniae | Salmonella spp. |
| Bacillus anthracis | Klebsiella pneumoniae |
| Clostridium tetani | Proteus mirabilis |
| Listeria monocytogenes | Pseudomonas aeruginosa |
| Corynebacterium diphtheriae | Haemophilus influenzae |
| Streptococcus pyogenes | Neisseria gonorrhoeae |
| Enterococcus faecalis | Shigella spp. |
| Clostridium perfringens | Vibrio cholerae |
| Mycobacterium tuberculosis | Francisella tularensis |
Risk of errors
Gram staining is a crucial technique in microbiology, but like all procedures, it is susceptible to errors. These errors can lead to false positive or false negative results, which can impact the correct identification and treatment of bacterial infections. Therefore, understanding these potential pitfalls is essential for accurate interpretation.
False Positive Errors:
- Spread Issues: If the bacterial smear is spread out excessively, it can lead to poor decoloration of bacteria in depth, resulting in a false positive.
- Dye Deposit: The presence of dye deposits in the gentian violet bottle can cause false positives. Filtering the dye can rectify this issue.
- Iodine Solution: An iodine solution that doesn’t drain well can lead to false positive results.
- Bleach Duration: If bleach doesn’t have enough contact time, it can result in a false positive.
- Use of Fuchsin: Certain bacteria, especially Neisseria and Acinetobacter, have a high affinity for fuchsin. They concentrate it, leading to a dark red color that can be mistaken for violet, causing a false positive.
False Negative Errors:
- Presence of Dead Bacteria: Within every group of Gram-positive bacteria, there are some Gram-negative bacteria. These are often dead Gram-positive bacteria, which can lead to false negatives.
- Dye Quality: Using low-quality or overly diluted dyes can result in false negatives.
- Lugol’s Iodine: If Lugol’s iodine is left on for an extended period, it can lead to false negatives.
- Bleach Issues: Leaving bleach on for too long or not rinsing it adequately can result in false negatives.
In conclusion, while Gram staining is a valuable tool for differentiating bacterial types, it is essential to be aware of the potential errors and take steps to minimize them. Proper technique, quality reagents, and careful interpretation are crucial for accurate results.
Complications of Gram Staining
- Smear Thickness: The interpretation of slides can become challenging if the microscopic smear is too thick or clumped. Thicker smears necessitate a longer decolorizing time, which can affect the outcome.
- Decolorization: The time taken for decolorization requires meticulous monitoring. Both under-decolorization and over-decolorization can lead to inaccurate results.
- Age of Cultures: It’s imperative to evaluate cultures when they are fresh. Older cultures might lose their peptidoglycan cell walls, causing gram-positive cells to appear as gram-negative or gram variable.
- Limitations of the Technique: Gram staining is not effective for organisms without a cell wall, such as Mycoplasma species. Additionally, smaller bacteria like Chlamydia and Rickettsia species are not accurately detected using this method.
- Potential for False Negatives: Several scenarios can lead to a Gram stain not revealing organisms accurately:
- Prior use of antibiotics before specimen collection.
- Evaluating cultures that are too young or too old.
- Fixing the smear before it has dried completely.
- Excessively thick smears.
- Using a low concentration of crystal violet.
- Overheating during fixation.
- Washing excessively between steps.
- Insufficient exposure to iodine.
- Extended decolorization.
- Overdoing counterstaining.
- Lack of experience in both slide preparation and review.
- Mismatched Results: There are instances where the results of the Gram stain may not align with the final culture results. This discrepancy can potentially lead to the inappropriate use of antibiotics.
Therefore, while the Gram staining technique is invaluable in microbiology, it’s crucial to be aware of its potential complications. Proper training, adherence to protocols, and understanding the intricacies of the method can help mitigate these challenges and ensure accurate results.
Potential Diagnosis Using Gram Staining
Gram staining is a pivotal technique in microbiology, offering a rapid method to differentiate bacterial types and subsequently aid in the diagnosis of various diseases or pathological conditions. By distinguishing bacteria based on their cell wall composition, medical professionals can identify the potential causative agents of infections and tailor treatment accordingly.
- Gram-Positive Organisms:
- Cocci:
- Staphylococcus species: These are often responsible for skin infections, food poisoning, and sometimes more severe conditions like pneumonia or bloodstream infections.
- Streptococcus species: These can cause a range of diseases, from throat infections (streptococcal pharyngitis) to severe conditions like rheumatic fever.
- Bacilli:
- Corynebacterium species: One notable member is Corynebacterium diphtheriae, the causative agent of diphtheria.
- Clostridium species: These can cause conditions like tetanus or botulism.
- Listeria species: Listeria monocytogenes, for instance, can cause a severe foodborne illness known as listeriosis.
- Cocci:
- Gram-Negative Organisms:
- Cocci:
- Neisseria gonorrhoeae: The causative agent of gonorrhea, a sexually transmitted infection.
- Neisseria meningitidis: Responsible for meningococcal meningitis.
- Moraxella species: Some species can cause infections in the respiratory system.
- Bacilli:
- Escherichia coli (E. coli): While many strains are harmless, some can cause food poisoning, urinary tract infections, and other illnesses.
- Pseudomonas species: These can lead to skin rashes, ear infections, and more severe conditions in immunocompromised individuals.
- Proteus species: Often associated with urinary tract infections.
- Klebsiella species: Can cause conditions ranging from pneumonia to bloodstream infections.
- Cocci:
- Gram-Variable Organisms:
- Actinomyces species: These bacteria are typically found in the human mouth and can cause actinomycosis, a rare kind of slowly progressing infection.
Therefore, by identifying the type of bacteria present in a sample, Gram staining provides invaluable information that can guide medical professionals in their diagnostic process and subsequent treatment decisions.
Normal and Critical Findings Using Gram Staining
Gram staining is a fundamental technique in microbiology that differentiates bacteria based on their cell wall composition. This method provides crucial insights into the potential causative agents of infections, guiding subsequent diagnostic and treatment decisions.
Normal Findings: In sterile body fluids, the expected normal finding is the absence of any pathogenic organisms. When observing under the microscope, the organisms, if present, are identified based on their color and shape. Gram-positive organisms will appear purple or blue, while gram-negative organisms will manifest as pink or red. The shape further classifies them: bacilli are rod-shaped, and cocci are spherical.
Critical Findings: Certain morphological characteristics on a Gram-stained smear can suggest specific bacterial infections:
- Gram-Positive Organisms:
- Cocci in clusters: Typically indicative of Staphylococcus species, such as S. aureus.
- Cocci in chains: Characteristic of Streptococcus species like S. pneumoniae and B group streptococci.
- Cocci in tetrads: Often associated with Micrococcus spp.
- Thick bacilli: Commonly seen in Clostridium spp., including C. perfringes and C. septicum.
- Thin bacilli: Indicative of Listeria spp.
- Bacilli with branches: Characteristic of Actinomyces and Nocardia.
- Gram-Negative Organisms:
- Diplococci: Often seen in Neisseria spp., such as N. meningitidis. It’s worth noting that Moraxella spp. and Acinetobacter spp. can also exhibit a diplococcal morphology. Acinetobacter might sometimes appear as Gram-positive cocci and can be pleomorphic.
- Coccobacilli: Typically associated with Acinetobacter spp. They can vary in their staining, appearing as gram-positive, gram-negative, or gram-variable.
- Thin bacilli: Commonly associated with Enterobacteriaceae, such as E. coli.
- Coccobacilli: Indicative of Hemophilus spp., like H. influenzae.
- Curved rods: Characteristic of Vibrio spp. and Campylobacter spp., including V. cholerae and C. jejuni.
- Thin, needle-shaped rods: Often seen in Fusobacterium spp.
- Gram-Variable Organisms: These organisms do not distinctly fall into either the gram-positive or gram-negative categories, making them unique in their staining properties.
Therefore, understanding the morphology and staining characteristics of bacteria is pivotal in the diagnostic process, allowing clinicians to make informed decisions about patient care.
Interfering Factors in Gram Staining
Gram staining is a pivotal technique in microbiology, allowing for the differentiation of bacterial species based on their cell wall properties. However, several factors can interfere with the accuracy and reliability of this method. Understanding these interfering factors is crucial to ensure the validity of the results.
- Specimen Collection Issues:
- Contamination: If the specimen collection is not carried out under sterile conditions, it can lead to contamination by multiple organisms, thereby affecting the accuracy of the results.
- Improper Collection: The manner in which the specimen is collected can influence the outcome. For instance, prior use of antibiotics can hinder the growth of organisms, leading to false-negative results.
- Interpretation of the Gram Stain: As outlined by the World Health Organization in 2003, several steps should be meticulously followed during the interpretation:
- Low Power Magnification (10X): At this magnification, the general nature of the smear should be analyzed. The background of the slide should predominantly be gram-negative or clear. White blood cells, if present, should also stain gram-negative. It’s essential to ensure that thin crystal violet or gentian violet precipitates are not misinterpreted as gram-positive bacillus bacteria. Ideally, the smear should be one cell thick without any overlapping of cells.
- Observations under Low Power Magnification: This magnification level is used to observe:
- The relative numbers of polymorphonuclear neutrophils (PMNs), mononuclear cells, and red blood cells (RBCs).
- The relative numbers of squamous epithelial cells and normal microbiota bacteria.
- The location, arrangement, and shape of the organisms.
- Oil Immersion Examination: A thorough examination under oil immersion across multiple fields is vital to note:
- Micro-organisms: If identified, their numbers and morphology should be noted.
- Shapes: These can range from coccus, bacillus, coccobacillus, to filaments and yeast-like forms.
- End Appearances: These can be rounded, tapered, concave, clubbed, or flattened.
- Side Appearances: These can be parallel, ovoid, irregular, or concave.
- Axis of the Organism: This can be straight, curved, or spiral.
- Pleomorphism: This refers to the variation in shape.
- Branching or Cellular Extensions: These are unique features that some bacteria might exhibit.
Therefore, while Gram staining is a valuable diagnostic tool, it’s imperative to be aware of the potential interfering factors and ensure meticulous adherence to the procedure to achieve accurate results.
Variations in Gram Reaction
Gram staining is a fundamental technique in microbiology, pivotal for distinguishing between different bacterial groups based on their cell wall properties. However, various factors can influence the results, leading to unexpected outcomes.
- Gram-Positive Bacteria Variations: Gram-positive bacteria, typically retaining the crystal violet stain and appearing purple, might sometimes stain as Gram-negative due to several reasons:
- Damage to the bacterial cell wall, which can be a result of antibiotic therapy or excessive heat fixation of the smear.
- Over-decolorization of the smear.
- Utilization of an old iodine solution that has turned yellow instead of its original brown color. It’s crucial to store iodine in brown glass or other light-opaque containers to maintain its efficacy.
- Preparing smears from old cultures.
- The thickness of the smear also plays a role. A thick smear might require more decolorization than a thin one. If not fully decolorized, Gram-negative bacteria might appear as Gram-positive.
- Pitfalls in Interpretation: Certain organisms can present variations in their staining, leading to potential misinterpretations:
- Streptococcus pneumoniae: Typically seen as Gram-positive, lancet-shaped diplococci, they can sometimes appear as elongated cocci resembling short bacilli. Over-decolorized cells might be mistaken for gram-negative coccobacilli.
- Acinetobacter spp.: Generally observed as Gram-negative coccobacilli, they can sometimes appear as Gram-negative cocci or even demonstrate gram-variable staining. They might be mistaken for Neisseria spp. and reported as gram-negative cocci. It’s essential to search the smear for elongated forms, which Neisseria doesn’t exhibit.
- Clostridium perfringens: Known for their boxcar-shaped gram-positive bacilli appearance, they can sometimes appear as Gram-positive cocci or even Gram-variable or Gram-negative bacilli. The unique boxcar shape is a clue that the organism is gram-positive. Other Clostridia and Bacillus spp. might also appear similar.
- Yeast, especially Cryptococcus neoformans: Typically appearing as Gram-positive round or oval cells with budding, they might sometimes demonstrate gram-variable staining. Their size and shape distinguish them from bacteria, ensuring they aren’t mistaken for artifacts.
Therefore, while Gram staining remains an invaluable tool in microbiology, it’s imperative to be aware of these variations and potential pitfalls to ensure accurate interpretation and diagnosis.
Gram-positive bacteria and Gram-negative bacteria
Gram positive cocci
| Description of the Morphotype | Most Common Organisms |
| Pairs | Staphylococcus, Streptococcus, Enterococcus spp. |
| tetrads | Micrococcus, Staphylococcus, Peptostreptococcus spp |
| Groups | Staphylococcus, Peptostreptococcus, Stomatococcus spp. |
| Chains | Streptococcus, Peptostreptococcus spp. |
| Clusters, intracellular | Streptococcus spp. Microaerophilic, viridans streptococci, Staphylococcus spp. |
| Encapsulated | Streptococcus pneumoniae, Streptococcus pyogenes (rarely), Stomatococcus mucilaginosus |
| In the form of an ancestor | Streptococcus pneumoniae |
| Neisseria spp., Moraxella catarrhalis. |
Gram-positive bacilli
| Description of the Morphotype | Most Common Organisms |
| Little | Listeria monocytogenes, Corynebacterium spp. |
|---|---|
| Medium | Lactobacillus, anaerobic bacilli |
| Big | Clostridium, Bacillus spp. |
| Diphtheroid | Corynebacterium, Propionibacterium, Rothia spp. |
| Pleomorphic, Gram variables | Gardnerella vaginalis |
| Pearl | Mycobacteria, lactobacilli affected by antibiotics and corynebacteria |
| Filamentous | Anaerobic morphotypes, cells affected by antibiotics |
| Filamentous, beaded, branched | Actinomycetes, Nocardia, Nocardiopsis, Streptomyces, Rothia spp. |
| Bifid or V shapes | Bifidobacterium spp., brevibacteria |
| Gram-negative coccobacilli | Bordetella, Haemophilus spp. (pleomorph) |
| Masses | Veillonella spp. |
| Chains | Prevotella, Veillonella spp. |
Gram-negative bacilli
| Description of the Morphotype | Most Common Organisms |
| Little | Haemophilus, Legionella (thin with filaments), Actinobacillus, Bordetella, Brucella, Francisella, Pasteurella, Capnocytophaga, Prevotella, Eikenella spp. |
|---|---|
| Bipolar | Klebsiella pneumoniae, Pasteurella spp., Bacteroides spp. |
| Medium | Enterics, pseudomonads |
| Big | Clostridia or devitalized bacilli |
| Curved | Vibro, Campylobacter spp. |
| Spiral | Campylobacter, Helicobacter, Gastrobacillum, Borrelia, Leptospira, Treponema spp |
| Fusiform | Fusobacterium nucleatum |
| Filaments | Fusobacterium necrophorum (pleomorph) |
Applications of Gram Staining
- Bacterial Classification in Research: Gram staining is employed extensively in research settings to classify bacteria into two primary groups: Gram-positive and Gram-negative. This classification aids researchers in understanding the structural and functional differences between these groups.
- Diagnostic Identification: In diagnostic laboratories, Gram staining is a crucial step for the preliminary identification of pathogens. By observing the staining pattern, microbiologists can infer the nature of the bacterial invader and its potential implications.
- Hospital Applications: In clinical settings, especially hospitals, Gram staining plays a vital role in guiding the initial choice of antibiotics for treatment, even before the complete identification of the bacterial species. This rapid intervention can be life-saving, especially in cases of severe infections.
- Studying Bacterial Morphology: Besides classification, Gram staining is also used to study the morphology or shape and structure of bacteria, providing insights into their biology and behavior.
- Identification of Bacterial Species: As a preliminary step, Gram staining aids in narrowing down the potential bacterial species in a sample. By discerning whether a bacterium is Gram-positive or Gram-negative, further specific tests can be conducted to confirm the bacterium’s identity.
- Detection of Bacterial Infections: Clinical samples, such as sputum, urine, and wound swabs, often undergo Gram staining to detect bacterial infections. Identifying the type of bacteria and its Gram nature helps healthcare providers determine the most effective treatment strategy.
- Antibiotic Susceptibility Testing: The Gram nature of bacteria can hint at their susceptibility or resistance to certain antibiotics. For instance, Gram-negative bacteria often exhibit resistance to specific antibiotics, necessitating alternative treatment strategies.
- Research and Discovery: Beyond clinical applications, Gram staining is invaluable in research. By analyzing bacterial cell structures and their staining patterns, scientists can delve deeper into bacterial biology, understanding their interactions with various environments and other organisms.
Advantages of Gram Staining
Gram staining is a widely used laboratory technique that is used to differentiate bacterial species based on the structure and composition of their cell walls. It has several advantages over other methods of bacterial identification, including:
- Widely available and relatively easy to perform: Gram staining is a simple and inexpensive procedure that can be performed in most laboratories. It requires only basic equipment, such as a microscope, a heat source, and a few chemical reagents.
- High degree of accuracy: Gram staining has a high degree of accuracy, especially when combined with other identification techniques. This makes it a reliable method for identifying bacterial species in clinical samples.
- Can be performed on a variety of samples: Gram staining can be performed on a wide range of samples, including liquid cultures, solid media, and clinical specimens. This makes it a versatile technique for identifying bacteria in different types of samples.
- Provides information about the structure of bacterial cells: In addition to identifying bacterial species, Gram staining can also provide information about the structure of bacterial cells. By examining the appearance of the cells under the microscope, microbiologists can learn more about the characteristics and behavior of different bacterial species.
Overall, Gram staining is an important tool for microbiologists and healthcare providers for the identification and characterization of bacterial species, as well as for the detection and treatment of infections.
Limitation of Gram Staining
- Inability to Stain Specific Bacteria: Gram staining cannot effectively stain Acid Fast Bacilli, notably Mycobacterium species. Additionally, bacteria without a cell wall, such as Mycoplasma species, remain unstained.
- Ineffectiveness for Minute Bacteria: The technique is unsuitable for staining minute bacteria like Rickettsia species and Chlamydia species.
- Requirement of Multiple Reagents: The procedure necessitates the use of multiple reagents, complicating the process.
- Risk of Over-decolorization: Over-decolorizing the smear can lead to false gram-negative results, while under-decolorizing can produce false gram-positive outcomes.
- Issues with Smear Thickness: Thick or viscous smears might retain excessive primary stain, complicating the differentiation between Gram-positive and Gram-negative organisms.
- Age of Cultures: Cultures that have aged beyond 16 to 18 hours consist of both living and dead cells. The deteriorating dead cells might not retain the stain appropriately.
- Stain Precipitation: With time, the stain can form a precipitate. Filtering the stain through gauze can help remove these excess crystals.
- Impact of Antibiotics: Gram stains from patients undergoing antibiotic or antimicrobial treatment might display altered Gram stain reactivity due to the medication’s effectiveness.
- Growth Limitations: Occasionally, pneumococci identified in the lower respiratory tract might not grow in culture, as some strains are obligate anaerobes.
- Toxin-producing Organisms: Organisms that produce toxins, such as Clostridia, staphylococci, and streptococci, might destroy white blood cells in a purulent specimen.
- Faintly Staining Organisms: Some Gram-negative organisms, like Campylobacter and Brucella, may stain faintly. Using an alternative counterstain, such as basic fuchsin, can help visualize these organisms.
What is the correct order of staining reagents in gram-staining?
The correct order of staining reagents in gram staining is as follows:
- Crystal violet
- Iodine
- Alcohol or acetone
- Safranin (counterstain)
Here is a brief description of each step:
- Crystal violet: The sample is first treated with crystal violet, a violet-colored dye, which stains the bacterial cells.
- Iodine: The sample is then rinsed and treated with an iodine solution, which serves as a mordant to help the crystal violet dye bind to the bacterial cell walls.
- Alcohol or acetone: The sample is then rinsed again and treated with a decolorizing solution, which typically contains alcohol or acetone. This step helps to remove the excess crystal violet dye from the sample, resulting in the decolorization of the sample.
- Safranin: Finally, the sample is treated with a counterstain called safranin, which stains the bacteria that were not retained by the crystal violet-iodine staining. This step allows for the differentiation between Gram-positive and Gram-negative bacteria based on their different responses to the crystal violet-iodine staining.
Comments And Tips
- Smear Thickness: The thickness of the smear directly influences the outcome of the Gram stain. A thin smear with no clumping or inconsistency is recommended for optimal results.
- Decolorizing Step: This is the most critical step in the staining process. Over-decolorizing can cause gram-positive cells to appear as gram-negative, while under-decolorizing can produce the opposite effect. The decolorizing time varies based on the smear’s thickness. Some experts suggest flooding the slide for 15 seconds or less, while others recommend a drop-wise approach for 5-15 seconds.
- Use of Young Cultures: For accurate Gram staining, young, actively growing cultures are preferred. Older cultures might have compromised cell walls, leading to gram-variable results.
- Gram Stain Control: Including a known gram stain reaction sample on the same slide as the test culture can serve as a control, ensuring the technique’s success.
- Microscopy: After staining, bacteria should be viewed under a brightfield microscope at 1000X magnification using oil immersion. Proper brightness adjustment is crucial to discern the specimen’s color.
- Fresh Staining Reagents: It’s recommended to use freshly made staining reagents. If using older reagents, filtering before use is advised.
- Positive Charge Confusion: The Gram stain technique uses two positively charged dyes: crystal violet and safranin. The term “gram-positive” should not be confused with staining cells with a simple stain carrying a positive charge.
- KOH String Test: This test can confirm the Gram Stain results. The formation of a string (DNA) in 3% KOH indicates a gram-negative organism.
- Decolorizing Agents: Various formulations can be used, including acetone, acetone/ethanol, and ethanol. Acetone is the fastest decolorizer, followed by acetone/ethanol and then ethanol. For student use, ethanol is recommended to prevent over-decolorization.
- Counterstain Duration: Overexposure to counterstain can affect results. The mordant’s presence slows down the counterstaining process, so it’s essential to monitor the exposure time.
Animation of gram staining
Gram Staining Images
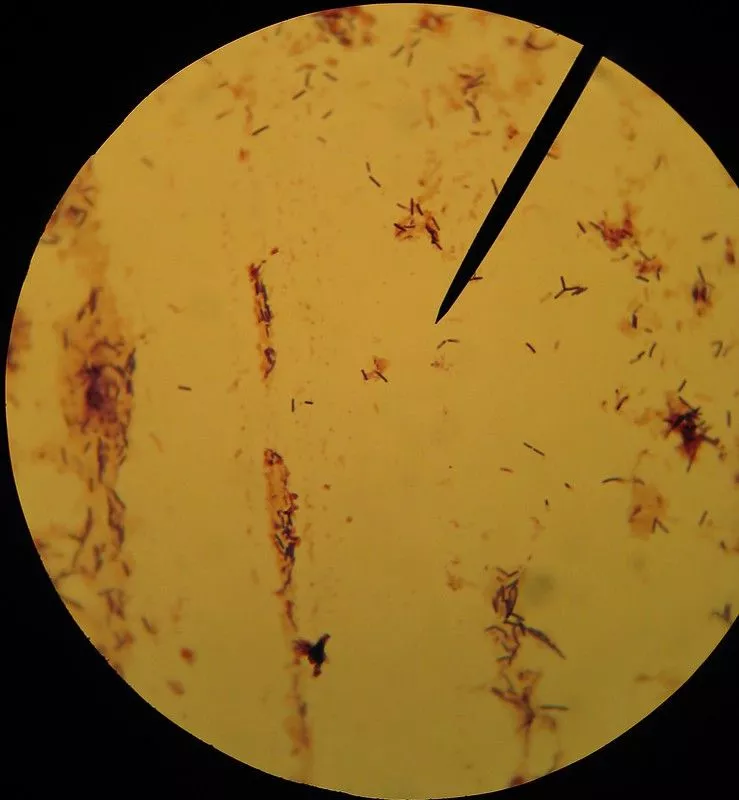
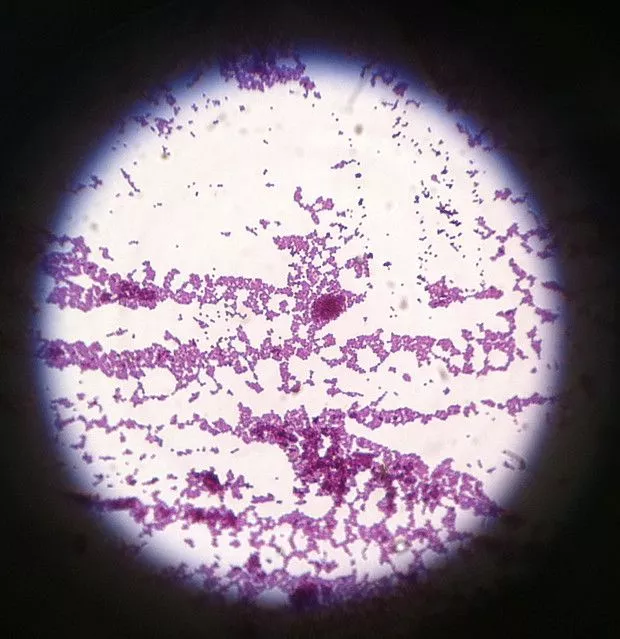
MCQ on Gram Staining
FAQ
What is gram staining?
Gram staining is a laboratory technique used to differentiate bacterial species based on the structure and composition of their cell walls. It was developed by Danish bacteriologist Hans Christian Gram in 1884 and is still widely used today.
In the Gram staining procedure, a bacterial sample is first treated with a crystal violet stain, which colors the cells purple. The sample is then washed with a decolorizing solution, which removes the stain from most bacterial cells. However, the cells of Gram-positive bacteria are able to retain the crystal violet stain due to the thick peptidoglycan layer in their cell walls. The sample is then treated with a counterstain, typically safranin, which colors the cells red.
When observed under the microscope, Gram-positive bacteria will appear purple due to the retained crystal violet stain, while Gram-negative bacteria will appear red due to the counterstain. This allows microbiologists to differentiate between the two groups based on the appearance of the cells.
Gram staining is a widely used technique for the identification and characterization of bacterial species, as well as for the detection and treatment of infections. It is a simple and inexpensive procedure that can be performed in most laboratories, and it provides rapid and accurate results.
Who discovered gram staining?
Gram staining is a laboratory technique that was developed by Danish bacteriologist Hans Christian Gram in 1884. It is used to differentiate bacterial species based on the differences in their cell wall composition. Gram staining is still widely used today in microbiology laboratories to identify and classify bacteria.
What is the role of alcohol during gram staining?
Alcohol is used during the gram staining procedure to decolorize the sample after it has been dyed with crystal violet. After the sample has been dyed with crystal violet, it is rinsed with water and then treated with an iodine solution, which serves as a mordant to help the crystal violet dye bind to the bacterial cell walls. The sample is then rinsed again and treated with a decolorizing solution, which typically contains alcohol. The alcohol in the decolorizing solution helps to remove the excess crystal violet dye from the sample, resulting in the decolorization of the sample. This step is important because it allows for the differentiation between Gram-positive and Gram-negative bacteria based on their different responses to the crystal violet-iodine staining.
Gram stain is example of which staining?
Gram staining is an example of a differential stain. Differential stains are used to distinguish between different types of cells or microorganisms based on their physical or chemical properties. In the case of gram staining, the technique is used to differentiate between two major groups of bacteria: Gram-positive and Gram-negative. Gram-positive bacteria have a thick peptidoglycan layer in their cell walls, which retains the crystal violet dye during the gram staining procedure and appears purple or blue under the microscope. Gram-negative bacteria have a thinner peptidoglycan layer and an outer membrane made of lipopolysaccharides, which does not retain the crystal violet dye and appears pink or red when counterstained with safranin.
Which dye is used for gram staining?
The dye used for gram staining is crystal violet. Crystal violet is a violet-colored dye that is used to stain bacterial cells during the gram staining procedure. After the bacterial cells have been treated with crystal violet, they are rinsed and treated with an iodine solution, which serves as a mordant to help the crystal violet dye bind to the bacterial cell walls. The sample is then rinsed again and treated with a decolorizing solution, typically containing alcohol or acetone, which removes the excess crystal violet dye from the sample. Finally, the sample is treated with a counterstain called safranin, which stains the bacteria that were not retained by the crystal violet-iodine staining. This allows for the differentiation between Gram-positive and Gram-negative bacteria based on their different responses to the crystal violet-iodine staining.
Which primary stain is used during gram staining?
The primary stain used during gram staining is crystal violet. Crystal violet is a violet-colored dye that is used to stain bacterial cells during the gram staining procedure. After the bacterial cells have been treated with crystal violet, they are rinsed and treated with an iodine solution, which serves as a mordant to help the crystal violet dye bind to the bacterial cell walls. The sample is then rinsed again and treated with a decolorizing solution, typically containing alcohol or acetone, which removes the excess crystal violet dye from the sample. Finally, the sample is treated with a counterstain called safranin, which stains the bacteria that were not retained by the crystal violet-iodine staining. This allows for the differentiation between Gram-positive and Gram-negative bacteria based on their different responses to the crystal violet-iodine staining.
Which bacteria appears purple violet colour after gram staining?
Gram-positive bacteria appear purple or blue after gram staining. Gram-positive bacteria have a thick peptidoglycan layer in their cell walls, which retains the crystal violet dye during the gram staining procedure. The retained dye gives the bacterial cells a purple or blue color when viewed under the microscope. Gram-negative bacteria, on the other hand, have a thinner peptidoglycan layer and an outer membrane made of lipopolysaccharides, which does not retain the crystal violet dye. These bacteria appear pink or red when counterstained with safranin, a counterstain used in the gram staining procedure.
What is mordant in gram staining?
In gram staining, a mordant is a substance that is used to fix the crystal violet dye to the cells being stained. The most commonly used mordant in gram staining is crystal violet, which is used to color the cells purple. Other mordants that can be used in gram staining include iodine and safranin. The choice of mordant will depend on the specific protocol being used and the characteristics of the cells being stained.
