Table of Contents
What is High-performance liquid chromatography (HPLC)?
- High-performance liquid chromatography (HPLC) is a powerful analytical technique widely used in various scientific fields, including chemistry, pharmaceuticals, biochemistry, and environmental analysis. It enables the separation, identification, and quantification of individual components in a mixture with high precision and accuracy.
- The fundamental principle behind HPLC is derived from column chromatography, where a mixture is passed through a stationary phase, and the different components interact differently with the stationary phase, leading to their separation. However, HPLC takes this concept to a new level by utilizing high pressures, typically ranging from 50 to 400 atmospheres, to force the solvent and sample mixture through the column at a much faster rate.
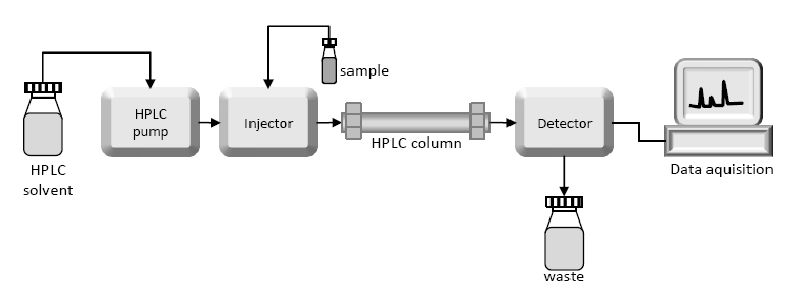
- The HPLC system consists of several key components that work together to achieve efficient separation and analysis. These components include a solvent reservoir, a pump to generate the high-pressure gradient, an injector for introducing the sample into the system, a column packed with a stationary phase, a detector to measure the eluted components, and a data analysis system.
- The separation process in HPLC relies on the differential interactions between the sample components and the stationary phase inside the column. The stationary phase is typically a finely divided solid material, known as the packing material, coated onto a solid support or packed into a tube. This stationary phase can be composed of various materials, such as silica, alumina, or specialty phases designed for specific separation purposes.
- The mobile phase, or the solvent, is another crucial aspect of HPLC. It is carefully chosen based on the sample and the desired separation goals. The mobile phase is typically a mixture of organic solvents, such as methanol or acetonitrile, and an aqueous phase, often buffered for pH control. The composition of the mobile phase is controlled by the pump, which generates a precise and reproducible gradient of solvents over time, enabling optimal separation conditions.
- Once the sample is introduced into the HPLC system via the injector, it is carried by the mobile phase and forced through the column. As the mixture interacts with the stationary phase, the individual components undergo different degrees of retention, resulting in their separation along the column. The eluted components are then detected by a suitable detector, such as ultraviolet (UV) or visible light detectors, refractive index detectors, or mass spectrometers, depending on the nature of the analytes.
- The detector generates a signal proportional to the concentration of each component, which is recorded by the data analysis system. By comparing the retention times and peak areas of the components with those of known standards or reference compounds, the individual components can be identified and quantified accurately.
- HPLC offers numerous advantages over other separation techniques. It provides excellent separation efficiency, high sensitivity, and a wide range of applications. It can handle a broad spectrum of sample types, including small organic molecules, peptides, proteins, nucleic acids, and complex mixtures. HPLC is highly versatile, allowing for different separation modes, such as reversed-phase, normal-phase, ion-exchange, size-exclusion, and affinity chromatography.
- In summary, high-performance liquid chromatography (HPLC) is a sophisticated analytical technique that enables the separation, identification, and quantification of components in a mixture. With its high-pressure operation and precise control over separation conditions, HPLC has become an indispensable tool in scientific research, quality control, and various industries, contributing to advancements in fields ranging from pharmaceutical development to environmental monitoring.
Principle of High-Performance Liquid Chromatography (HPLC)
The principle of High-Performance Liquid Chromatography (HPLC) is based on the separation of components in a mixture using a stationary phase and a mobile phase in a high-pressure system. HPLC offers high resolution and sensitivity, making it a valuable technique for analyzing complex mixtures.
The separation process in HPLC occurs in a separation column, which contains a stationary phase. The stationary phase consists of small porous particles, typically made of a granular material. These particles provide a large surface area for interactions with the sample components.
The mobile phase, often a solvent or solvent mixture, is forced through the separation column at high pressure. This is achieved using a pump that generates the necessary pressure to drive the mobile phase through the system. The mobile phase is responsible for carrying the sample through the column, allowing the components to separate.
To introduce the sample into the mobile phase flow, an injection system is used. This typically involves a valve connected to a sample loop, which is a small tube or capillary made of stainless steel. The sample is injected into the mobile phase flow using a syringe, allowing it to mix with the mobile phase before entering the separation column.
Once inside the column, the individual components of the sample migrate through the column at different rates. This differential migration occurs because each component interacts with the stationary phase to varying degrees. Components that have stronger interactions with the stationary phase will be retained longer and will migrate more slowly through the column, while those with weaker interactions will move through the column more quickly.
After passing through the separation column, the individual substances are detected by a suitable detector. Various types of detectors can be used in HPLC, including UV/Visible light detectors, refractive index detectors, fluorescence detectors, and mass spectrometers. The detector generates a signal proportional to the concentration of the separated components.
The detector signal is then sent to the HPLC software on a computer, where it is processed and analyzed. The software generates a chromatogram, which is a graphical representation of the detector signal as a function of time. The chromatogram displays peaks corresponding to the separated components, allowing for their identification and quantification.
By comparing the retention times of the peaks in the chromatogram with those of known standards or reference compounds, the individual substances in the sample can be identified. The peak areas or heights can be used to quantify the amount of each component present in the mixture.
In conclusion, the principle of High-Performance Liquid Chromatography (HPLC) involves the separation of components in a mixture using a stationary phase and a mobile phase in a high-pressure system. Through interactions with the stationary phase, the components migrate through the separation column at different rates. The separated components are detected and analyzed using suitable detectors, and the resulting chromatogram allows for the identification and quantification of the substances present in the sample.
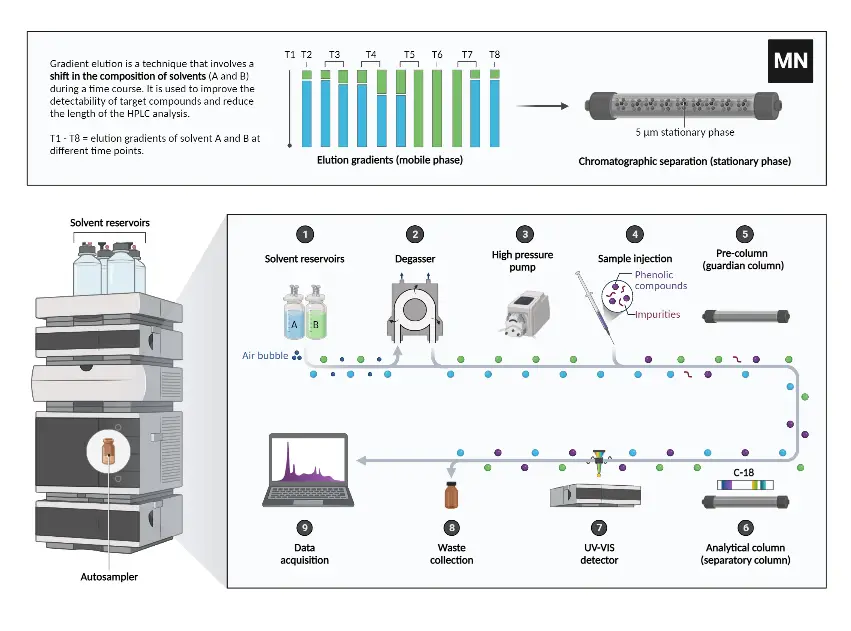
Instrumentation of High-Performance Liquid Chromatography (HPLC)
The Pump
- The pump is a crucial component in High-Performance Liquid Chromatography (HPLC) systems, responsible for generating a consistent and controlled flow of the eluent, which is the mobile phase or solvent mixture used in the separation process.
- With the advancement of HPLC, the development of the pump system became essential to meet the high-pressure requirements of the technique. The pump is typically positioned at the uppermost part of the liquid chromatography system, drawing the eluent from the solvent reservoir and delivering it into the system.
- One of the primary requirements of HPLC pumps is the ability to generate high pressures. The pressures involved in HPLC can range from 50 to 400 atmospheres or more, depending on the specific application and the column used. The pump must be capable of maintaining these high pressures consistently throughout the analysis.
- In addition to generating high pressures, the pump should provide a controllable and reproducible flow rate. The flow rate is an essential parameter in HPLC, as it affects the efficiency of the separation and the time required for analysis. The pump should be capable of delivering a precise and consistent flow rate, allowing for accurate and reproducible results.
- Most pumps used in current HPLC systems employ reciprocating piston mechanisms to generate the flow of the eluent. These pumps rely on the back-and-forth motion of a motor-driven piston to draw in and push out the eluent. This reciprocating motion results in a pulsatile flow, where the eluent is delivered in discrete pulses.
- The pulsatile flow generated by reciprocating pumps can have an impact on the chromatographic performance and the stability of the detector signal. To minimize these effects, various measures are implemented. For example, pulsation dampers or pulse dampeners can be used to reduce the pulsatile flow by smoothing out the pressure variations caused by the reciprocating motion of the pump.
- Modern HPLC pumps often incorporate advanced technologies and design features to enhance performance and minimize pulsations. These may include improved piston designs, optimized flow paths, and sophisticated control systems. Some pumps may utilize alternative pumping mechanisms, such as dual-piston pumps or syringe-driven pumps, which can provide smoother and more continuous flows compared to traditional reciprocating pumps.
- The pump in an HPLC system is typically controlled by the chromatography software, allowing precise control of the flow rate, pressure, and other operating parameters. The software can be used to set and adjust the flow rate based on the specific separation requirements, ensuring optimal conditions for the separation and analysis.
- In summary, the pump is a critical component of an HPLC system, responsible for generating the high-pressure flow of the eluent. It should provide consistent pressures at any condition and deliver a controllable and reproducible flow rate. While reciprocating pumps are commonly used, efforts are made to minimize the pulsatile flow associated with these pumps. The development and improvement of pump technology have played a significant role in the advancement and widespread adoption of HPLC as a powerful analytical technique.
Injector
- The injector is an essential component in High-Performance Liquid Chromatography (HPLC) systems, located adjacent to the pump. Its primary function is to introduce the sample into the flow of eluent, allowing it to mix with the mobile phase and enter the separation column for analysis.
- One of the simplest and most commonly used methods for sample injection is the manual injection using a syringe. In this method, a small volume of the sample is drawn into a syringe, and then it is introduced into the flow of eluent at the appropriate time. Manual injections are often employed when only a few samples need to be analyzed or in situations where precision is not of utmost importance.
- However, for routine and high-throughput analysis, the use of sampling loops in conjunction with an autosampler or auto-injector system is the preferred method. The sampling loop is a small tube or capillary connected to the injector. It acts as a reservoir for the sample and is filled with a precise volume of the sample solution.
- The autosampler, or auto-injector, is a system commonly used in HPLC that allows for automated and repeated injections at set scheduled intervals. It offers enhanced precision, efficiency, and reproducibility compared to manual injections. The autosampler system typically consists of a sample tray or carousel, where multiple sample vials are placed, and a robotic arm or syringe mechanism that retrieves the samples and injects them into the injector.
- During the injection process, the autosampler accurately aspirates the desired volume of the sample from the vial using a syringe or other mechanism. The sample is then introduced into the flow of eluent, either directly or by diverting the eluent flow through the sampling loop. The timing and sequence of injections can be programmed in the HPLC software, allowing for fully automated and unattended operation.
- The use of an autosampler system provides several advantages in HPLC analysis. It reduces manual handling and minimizes the potential for human error, ensuring higher accuracy and reproducibility of injections. It also allows for a higher sample throughput, as multiple samples can be prepared and queued for injection in a continuous and efficient manner.
- In addition to the sampling loop and autosampler system, other injection techniques may be employed in specific applications or specialized HPLC systems. These may include techniques such as on-column injections, where the sample is introduced directly onto the column without the need for an injector, or microfluidic systems that utilize microfluidic channels and valves for precise and controlled sample introduction.
- In summary, the injector in an HPLC system is responsible for introducing the sample into the flow of eluent for analysis. Manual injections using a syringe are suitable for low-throughput or less demanding applications. However, the use of sampling loops with an autosampler system is the preferred method for routine analysis, offering automated and precise injections with enhanced efficiency and reproducibility. The choice of injection technique depends on the specific requirements of the analysis and the desired level of automation.
Column
- The column is a key component in High-Performance Liquid Chromatography (HPLC) systems, where the actual separation of components in a mixture takes place. The column is responsible for the efficient separation of analytes based on their differential interactions with the stationary phase.
- In modern HPLC systems, columns are commonly prepared in a stainless steel housing, offering durability and compatibility with a wide range of solvents. The stainless steel housing provides robustness and resistance to corrosion, allowing the column to withstand the high pressures and diverse solvents used in HPLC.
- The packing material inside the column is crucial for achieving efficient separations. Traditionally, silica-based materials have been widely used as the packing material in HPLC columns. Silica offers a large surface area and a variety of functional groups, providing excellent separation capabilities for a wide range of analytes.
- In addition to silica, polymer gels are also commonly employed as packing materials in HPLC columns. Polymer-based materials offer advantages such as chemical stability, high mechanical strength, and compatibility with a broader range of solvents. They are particularly useful for separating large biomolecules, such as proteins and nucleic acids.
- The choice of the packing material depends on the specific separation requirements and the nature of the analytes. Different packing materials may exhibit different selectivity, efficiency, and stability, allowing for tailored separations in various applications.
- The eluent, or mobile phase, used in HPLC can vary depending on the separation goals and the nature of the analytes. Eluents can range from acidic to basic solvents, allowing for a wide pH range and versatility in separating different types of compounds.
- The selection of the eluent is often based on the solubility, stability, and compatibility of the analytes with the solvent system. The eluent composition is carefully optimized to achieve the desired separation and maximize the resolution of the analytes.
- The column, along with the eluent, plays a crucial role in determining the separation efficiency and selectivity in HPLC. Various factors, such as column dimensions, particle size, and column length, can impact the separation performance. These parameters are carefully chosen to meet the specific separation requirements, balancing factors such as resolution, analysis time, and system pressure.
Detector
The detector is a critical component in High-Performance Liquid Chromatography (HPLC) systems, responsible for observing and measuring the separation of analytes that occurs within the column.
During the chromatographic separation, the eluent or mobile phase flows through the column. When no analyte is present, the composition of the eluent remains consistent. However, as the analytes pass through the column, they interact with the stationary phase and experience differential retention times. These interactions cause changes in the composition of the eluent.
The detector’s primary function is to measure these changes in the eluent composition and convert them into an electronic signal that can be analyzed and interpreted. The detector detects and quantifies the separated analytes based on their unique characteristics, such as absorbance, refractive index, fluorescence, or mass.
There are various types of detectors available for HPLC, each utilizing different principles to detect and measure the analytes. Some of the commonly used detectors include:
- UV/Visible Light Detector: This detector operates based on the principle of absorbance spectroscopy. It measures the absorbance of UV or visible light by the analytes as they pass through the detector cell. The absorbance signal is directly proportional to the concentration of the analyte.
- Refractive Index Detector: This detector measures changes in the refractive index of the eluent caused by the presence of analytes. It is particularly useful for detecting analytes that do not have strong UV absorbance. The refractive index detector provides relatively universal detection, but it may lack sensitivity compared to other types of detectors.
- Fluorescence Detector: This detector measures the fluorescence emitted by analytes that exhibit fluorescence properties. It operates by irradiating the analytes with a specific wavelength of light and detecting the emitted fluorescence at a different wavelength. Fluorescence detectors offer high sensitivity and selectivity for fluorescent compounds.
- Mass Spectrometer: Mass spectrometry (MS) detectors are highly sensitive and specific, capable of providing structural information about the separated analytes. They ionize the analytes and measure the mass-to-charge ratio (m/z) of the resulting ions. Mass spectrometers offer high-resolution detection and can be coupled with HPLC to enable identification and quantification of analytes.
These are just a few examples of the detectors used in HPLC. Other types of detectors, such as electrochemical detectors, evaporative light-scattering detectors (ELSD), and conductivity detectors, are also available and may be suitable for specific applications or analyte classes.
The choice of detector depends on factors such as the analyte’s properties, required sensitivity, selectivity, and the nature of the analysis. Different detectors may be employed individually or in combination to enhance the detection and characterization of analytes in complex samples.
Recorder
- The recorder, also known as a data processor or integrator, is an essential component in High-Performance Liquid Chromatography (HPLC) systems that is responsible for capturing, analyzing, and presenting the electronic signals generated by the detector.
- When the detector detects changes in the eluent composition caused by the presence of analytes, it produces an electronic signal that carries information about the separated components. This signal is not directly visible to the human eye, and thus a recorder or data processor is used to convert it into a format that can be analyzed and interpreted.
- In the past, pen (paper)-chart recorders were commonly used. These recorders featured a moving chart paper and a pen that moved across the paper to create a graphical representation of the detector signal. The chart paper would provide a permanent record of the separation, allowing for visual inspection and analysis.
- However, with advancements in technology, computer-based data processors or integrators have become more prevalent in modern HPLC systems. These data processors offer greater flexibility, accuracy, and advanced data analysis capabilities compared to pen-chart recorders.
- Computer-based data processors can be equipped with various software options designed specifically for LC systems. These software packages provide features beyond simple data acquisition, such as peak-fitting, baseline correction, automatic concentration calculation, molecular weight determination, and more. They allow for sophisticated data analysis, making it easier to extract meaningful information from the detector signals.
- Moreover, data processors often offer data storage capabilities, allowing for the efficient management and retrieval of chromatographic data. The recorded data can be stored electronically, enabling easy access and sharing among researchers and ensuring data integrity and traceability.
- In addition to the advanced software features, data processors can be integrated with other components of the HPLC system, such as pumps and injectors, enabling seamless control and synchronization of the entire chromatographic process.
- The use of a data processor or integrator in HPLC systems provides several advantages. It allows for real-time monitoring of chromatographic separations, accurate quantification of analytes, and enhanced data analysis capabilities. The software-driven features enable more efficient and reliable data interpretation and facilitate the generation of comprehensive reports and results.
- In summary, the recorder or data processor is a crucial component in HPLC systems that captures and analyzes the electronic signals generated by the detector. While pen-chart recorders were previously popular, computer-based data processors have become the norm in modern HPLC systems. These data processors offer advanced software capabilities for data acquisition, analysis, and interpretation, allowing for enhanced efficiency, accuracy, and automation in HPLC analysis. The use of a data processor enables researchers to extract valuable insights from the chromatographic data and facilitates data management and sharing.
Degasser
- The degasser is an important component in High-Performance Liquid Chromatography (HPLC) systems that is used to remove gases from the eluent, ensuring a stable and noise-free baseline during the chromatographic analysis.
- The eluent, which is the mobile phase used in HPLC, can sometimes contain dissolved gases such as oxygen. These gases are not visible to the naked eye but can cause issues during the analysis. When gas is present in the eluent, it can result in the formation of air bubbles, leading to fluctuations in the eluent flow rate and an unstable baseline in the chromatogram.
- To overcome this problem, a degasser is employed in the HPLC system. The degasser utilizes a special polymer membrane tubing that is designed to effectively remove gases from the eluent. This membrane tubing is typically made from materials such as polytetrafluoroethylene (PTFE) or polyetheretherketone (PEEK).
- The membrane tubing of the degasser contains numerous very small pores on its surface. These pores are sized in a way that allows gas molecules, such as oxygen, to pass through while preventing the liquid phase of the eluent from entering the pores. As the eluent flows through the degasser, the dissolved gases diffuse out through the pores of the membrane tubing, effectively removing them from the eluent stream.
- By removing the gases, the degasser helps to eliminate air bubbles and stabilize the eluent flow. This leads to a more consistent eluent composition and a stable baseline in the chromatogram. The absence of gas-related noise allows for accurate detection and quantification of the analytes of interest.
- The degasser can be an integral part of the HPLC system, typically positioned before the pump or injector. Some modern HPLC systems even have integrated degassers, streamlining the setup and operation of the chromatographic analysis.
- In summary, the degasser is a crucial component in HPLC systems used to remove dissolved gases from the eluent. By utilizing a special polymer membrane tubing with small pores, the degasser effectively eliminates gases such as oxygen from the eluent stream. This helps to stabilize the eluent flow, prevent the formation of air bubbles, and ensure a noise-free baseline during chromatographic analysis. The degasser plays a vital role in maintaining the accuracy and reliability of HPLC measurements by providing a stable and consistent eluent composition.
Column Heater
The column heater is a crucial component in High-Performance Liquid Chromatography (HPLC) systems that is used to control and maintain the temperature of the separation column during the chromatographic analysis.
The temperature plays a significant role in the efficiency and selectivity of the chromatographic separation. The LC separation is often influenced by factors such as analyte retention, peak shape, and resolution, which can be greatly affected by the column temperature. Therefore, maintaining consistent and controlled temperature conditions is essential to achieve reproducible and reliable results.
The column heater, also known as a column oven, provides a controlled environment for the separation column. It is designed to enclose and protect the column while ensuring precise temperature regulation. The column heater is typically a thermally insulated compartment that surrounds the column.
There are several reasons why controlling the column temperature is important in HPLC:
- Reproducibility: To obtain consistent and repeatable results, it is crucial to maintain a constant temperature throughout the analysis. Fluctuations in temperature can lead to variations in analyte retention times and peak shapes, affecting the accuracy and precision of the analysis.
- Optimal Resolution: For certain analyses, such as the separation of sugars and organic acids, elevated temperatures (typically between 50 to 80°C) can improve resolution and separation efficiency. The column heater allows the column to be heated to the desired temperature, enhancing the separation of thermally sensitive compounds.
- Efficiency and Selectivity: The temperature can influence the equilibrium between the mobile phase and the stationary phase, affecting analyte retention. By controlling the column temperature, it is possible to optimize the efficiency and selectivity of the separation, leading to improved peak resolution and better separation performance.
The column heater is equipped with temperature control mechanisms that enable precise regulation of the column temperature. This can be achieved through the use of heating elements, such as resistive heating coils or Peltier elements, which provide accurate temperature control within the column oven.
The temperature control system is typically connected to a temperature controller or a programmable logic controller (PLC), allowing the user to set and adjust the desired temperature for the column. The temperature controller monitors and maintains the set temperature, ensuring that the column remains at the specified temperature throughout the analysis.
In summary, the column heater or column oven is a critical component in HPLC systems that provides a controlled environment for the separation column. It enables precise temperature regulation, ensuring consistent and reproducible temperature conditions during chromatographic analysis. By controlling the column temperature, optimal separation efficiency, selectivity, and resolution can be achieved. The column heater plays a vital role in obtaining accurate and reliable HPLC results, especially for analyses that are sensitive to temperature variations or require elevated temperatures for improved resolution.
Types of High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography (HPLC) encompasses various techniques, each designed for specific separation requirements. Here are some of the common types of HPLC:
- Normal Phase HPLC: In normal phase HPLC, the stationary phase is polar, typically packed with materials like silica. The mobile phase, on the other hand, is non-polar. This technique is useful for separating water-sensitive compounds, geometric isomers, cis-trans isomers, and chiral compounds.
- Reverse Phase HPLC: Reverse phase HPLC is widely used and involves a non-polar stationary phase, commonly packed with materials such as C18. The mobile phase is typically a mixture of water and a miscible organic solvent, such as methanol or acetonitrile. Reverse phase HPLC is versatile and can be employed for the separation of polar, non-polar, ionizable, and ionic compounds.
- Ion Exchange HPLC: Ion exchange HPLC utilizes a stationary phase that contains ionic groups. The mobile phase consists of a buffer solution, which allows for the separation of anions and cations based on their charge interactions with the stationary phase. Ion exchange HPLC is useful for the analysis of charged species and is commonly employed in areas such as pharmaceuticals and environmental analysis.
- Size Exclusion HPLC (also known as Gel Filtration or Gel Permeation Chromatography): Size exclusion HPLC separates molecules based on their size. The stationary phase contains a porous medium with different pore sizes. Larger molecules are unable to enter the pores and, therefore, elute first, while smaller molecules can diffuse into the pores and elute later. Size exclusion HPLC is commonly used for determining the molecular weight or size distribution of polymers, proteins, and other macromolecules.
These are just a few examples of the different types of HPLC techniques available. Other variations include affinity chromatography, hydrophilic interaction chromatography (HILIC), and chiral chromatography, each offering unique capabilities for specific separation challenges. The choice of HPLC technique depends on the nature of the analytes, the desired separation mechanism, and the specific objectives of the analysis.
Applications of High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography (HPLC) is a versatile analytical technique that finds applications in a wide range of fields. Here are some of the key areas where HPLC is commonly employed:
- Analysis of Drugs: HPLC is extensively used in pharmaceutical analysis to determine the identity, purity, and concentration of drugs. It plays a crucial role in drug development, quality control, and formulation analysis.
- Analysis of Synthetic Polymers: HPLC is employed for characterizing synthetic polymers, including determining molecular weight distribution, polymer composition, and identifying impurities. This is important in industries such as plastics, coatings, and textiles.
- Analysis of Pollutants in Environmental Analytics: HPLC is utilized for the detection and quantification of pollutants in environmental samples, including water, air, and soil. It enables the monitoring of contaminants such as pesticides, pharmaceuticals, and persistent organic pollutants.
- Determination of Drugs in Biological Matrices: HPLC is used in pharmacokinetic studies to measure drug concentrations in biological samples, such as blood, urine, and tissues. This aids in understanding drug absorption, distribution, metabolism, and elimination.
- Isolation of Valuable Products: HPLC plays a crucial role in the isolation and purification of valuable compounds, such as natural products, pharmaceutical intermediates, and biomolecules. It allows for the separation of target compounds from complex mixtures.
- Product Purity and Quality Control: HPLC is widely employed in industries for assessing the purity and quality of products. It ensures that industrial chemicals, fine chemicals, and consumer products meet specified standards and regulatory requirements.
- Separation and Purification of Biopolymers: HPLC is utilized for the separation and purification of biopolymers, including proteins, enzymes, nucleic acids, and carbohydrates. It enables the isolation of specific biomolecules for further analysis or use in research and biotechnology applications.
- Water Purification: HPLC is employed in water treatment processes for the removal of contaminants and purification of water supplies. It allows for the analysis and quantification of trace elements, organic compounds, and disinfection byproducts.
- Pre-concentration of Trace Components: HPLC can be used for pre-concentration of trace components in complex samples, enabling their detection and analysis at low concentrations. This is particularly important in environmental, forensic, and food analysis.
- Ligand-Exchange Chromatography and Ion-Exchange Chromatography: HPLC techniques such as ligand-exchange chromatography and ion-exchange chromatography are utilized for the separation and analysis of proteins, carbohydrates, and other biomolecules based on their charge properties.
These are just a few examples of the diverse applications of HPLC. The versatility, sensitivity, and reliability of HPLC make it an indispensable tool in various scientific disciplines, including chemistry, biochemistry, pharmacy, environmental science, and many more.
Advantages of High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography (HPLC) offers several advantages that make it a widely used analytical technique in various fields. Here are some key advantages of HPLC:
- Speed: HPLC allows for rapid analysis, making it suitable for high-throughput applications. With advancements in instrument technology and column design, HPLC can provide fast separations, reducing analysis time and increasing laboratory efficiency.
- Efficiency: HPLC provides excellent separation efficiency, allowing for the resolution of complex mixtures with high peak capacity. The use of small particle sizes in modern HPLC columns and optimized operating conditions leads to improved peak shapes and better separation of closely related compounds.
- Accuracy: HPLC offers high accuracy in identifying and quantifying chemical components. It provides precise and reliable measurements, ensuring the accuracy of analytical results. This is crucial in various applications, such as pharmaceutical analysis, environmental monitoring, and quality control of industrial products.
- Versatility: HPLC is a versatile technique that can be applied to a wide range of sample types, including small molecules, large biomolecules, polar compounds, non-polar compounds, and ionizable compounds. It offers flexibility in selecting the appropriate column, stationary phase, and mobile phase to achieve optimal separations for specific analytes.
- Precision: HPLC is extremely precise in determining the concentration of components in a sample. It allows for accurate quantification of target compounds at low levels, even in complex matrices. This precision is essential for applications such as pharmaceutical potency testing, determining impurity levels, and assessing sample purity.
- Selectivity: HPLC provides high selectivity, enabling the separation and analysis of compounds with similar chemical properties. Different column chemistries, stationary phases, and mobile phase compositions can be employed to achieve selective separations, even in complex matrices. This selectivity is particularly important in pharmaceutical analysis, food testing, and forensic analysis.
- Sample Compatibility: HPLC can accommodate a wide range of sample matrices, including liquids, solids, gases, and complex mixtures. It offers flexibility in sample preparation techniques and allows for direct injection or extraction-based sample preparation methods, depending on the nature of the sample.
- Sensitivity: HPLC can achieve high sensitivity, allowing for the detection and quantification of compounds at low concentrations. Sensitivity can be enhanced through the use of sensitive detectors such as UV-Vis detectors, fluorescence detectors, and mass spectrometers. This is advantageous in trace analysis, pharmacokinetic studies, and environmental monitoring.
- Automation and Robustness: HPLC systems can be automated, providing enhanced precision and reproducibility. Automated sample handling, injection, and data acquisition reduce manual errors and increase sample throughput. HPLC systems are robust and can handle a wide range of sample loads and variations in operating conditions.
- Method Development and Validation: HPLC offers a well-established methodology with extensive resources for method development and validation. There are established guidelines and protocols for method development, ensuring that the developed methods are reliable, reproducible, and compliant with regulatory requirements.
Limitations of High-Performance Liquid Chromatography (HPLC)
While High-Performance Liquid Chromatography (HPLC) offers several advantages, there are also certain limitations to consider. Here are some key limitations of HPLC:
- Cost: HPLC can be a costly technique to implement and maintain. It requires specialized equipment, including high-pressure pumps, detectors, and columns. Additionally, the use of organic solvents and consumables can contribute to the overall cost of running HPLC analyses.
- Complexity: HPLC is a complex technique that requires expertise in method development, instrument operation, and data interpretation. Optimizing separation conditions, selecting appropriate stationary phases and mobile phases, and troubleshooting potential issues can be challenging and time-consuming, especially for complex samples.
- Sensitivity: While HPLC can achieve high sensitivity for many compounds, it may have limitations in detecting certain compounds. Some compounds may have low detectability due to poor response with the chosen detector or interference from the sample matrix. This can be overcome by using more sensitive detectors or sample preparation techniques.
- Irreversible Adsorption: In some cases, certain compounds may irreversibly adsorb to the stationary phase or column packing material, leading to poor recovery and detection. This can result in incomplete separations or loss of analytes of interest. Special care must be taken to select suitable stationary phases and optimize the method to mitigate this limitation.
- Limited Applicability for Volatile Substances: HPLC is not the method of choice for the analysis of highly volatile substances. Gas Chromatography (GC) is typically more suitable for separating and analyzing volatile compounds due to its ability to vaporize the sample and carry it through the separation column.
- Long Analysis Time: HPLC analysis can sometimes require longer run times compared to other chromatographic techniques. This is especially true for complex samples or when resolving closely eluting peaks. While advancements in column technology and instrument efficiency have reduced analysis times, it may still be a consideration for high-throughput applications.
- Sample Matrix Interference: HPLC can be susceptible to interference from complex sample matrices, such as biological fluids or environmental samples. Co-elution of sample components or matrix effects can affect the separation and detection of analytes, leading to inaccurate results. Sample preparation techniques or additional sample cleanup steps may be required to mitigate matrix interference.
FAQ
What is High-Performance Liquid Chromatography (HPLC)?
High-Performance Liquid Chromatography (HPLC) is an analytical technique used to separate, identify, and quantify components in a mixture. It involves the passage of a sample mixture through a column containing a stationary phase and a mobile phase, with different components in the sample interacting differently with the stationary phase, resulting in their separation.
How does HPLC differ from other chromatographic techniques?
HPLC differs from other chromatographic techniques, such as Gas Chromatography (GC), in that it utilizes a liquid mobile phase instead of a gas. This makes HPLC suitable for the separation and analysis of a wider range of compounds, including non-volatile and thermally labile substances.
What are the common applications of HPLC?
HPLC has diverse applications in various fields, including pharmaceutical analysis, environmental monitoring, food and beverage analysis, forensic science, clinical diagnostics, and more. It is used for analyzing drugs, separating complex mixtures, determining impurities, quantifying analytes in biological samples, and quality control of industrial products, among other applications.
What are the different types of HPLC columns?
There are various types of HPLC columns, including normal phase, reverse phase, ion exchange, and size exclusion columns. Normal phase columns use a polar stationary phase and a non-polar mobile phase, while reverse phase columns use a non-polar stationary phase and a polar mobile phase. Ion exchange columns contain ionic groups for separating anions and cations, while size exclusion columns separate compounds based on their size.
Which detector is commonly used in HPLC?
The most commonly used detector in HPLC is the UV-Vis detector, which measures the absorbance of analytes at specific wavelengths. Other detectors used in HPLC include fluorescence detectors, refractive index detectors, and mass spectrometers, which offer additional selectivity and sensitivity for specific applications.
How can HPLC analysis be optimized for better results?
To optimize HPLC analysis, several factors can be adjusted, including the choice of column, mobile phase composition, flow rate, temperature, and detector settings. Method development experiments can be performed to find the most suitable conditions for achieving efficient separations and desired analytical results.
What is the significance of sample preparation in HPLC?
Sample preparation is crucial in HPLC to ensure accurate and reliable results. It involves the extraction, purification, and concentration of analytes from the sample matrix, removing interferences, and preparing the sample for injection into the HPLC system. Proper sample preparation techniques contribute to enhanced sensitivity, reduced matrix effects, and improved separation efficiency.
What are the advantages of using HPLC over other analytical techniques?
HPLC offers several advantages, including high separation efficiency, versatility in analyzing different compound types, the ability to handle complex sample matrices, and accurate quantification of analytes. It is also suitable for both qualitative and quantitative analysis, and it can be automated for high-throughput applications.
Can HPLC be used for chiral separations?
Yes, HPLC can be used for chiral separations. Chiral compounds have molecules that are non-superimposable mirror images of each other, and HPLC with chiral stationary phases can selectively separate and quantify these enantiomers.
What are the key parameters to consider for HPLC method validation?
HPLC method validation involves assessing various parameters, including specificity, linearity, accuracy, precision, robustness, limit of detection (LOD), and limit of quantitation (LOQ). Method validation ensures that the developed HPLC method is reliable, reproducible, and fit for its intended purpose.
References
- https://www.shodex.com/en/kouza/a.html
- https://www.alphacrom.com/en/hplc-basics
- https://laboratoryinfo.com/hplc/
- https://sciencing.com/disadvantages-advantages-hplc-5911530.html
- https://www.slideshare.net/krakeshguptha/hplc-26970638
- https://www.chemguide.co.uk/analysis/chromatography/hplc.html
