Table of Contents
- The Hippurate Hydrolysis Test measures an organism’s ability to create hippuricase, an enzyme that hydrolyzes the substrate hippurate.
- It identifies Streptococcus agalactiae, Campylobacter jejuni, Listeria monocytogenes, and Gardnerella vaginalis.
- The final products of hippuric acid hydrolysis by hippuricase are glycine and benzoic acid.
- The oxidising agent ninhydrin deaminates glycine and is reduced in the process.
- The results of ninhydrin oxidation combine to generate a purple-colored compound.
- Because ninhydrin could react with free amino acids contained in growth media and other broths, the test medium must contain only hippurate.
- In 1922, Ayers and Rupp examined the ability of -hemolytic streptococci to convert hippuric acid into benzoic acid and glycine using an enriched medium containing hippuric acid.
- Leuthardt identified hippuricase as the enzyme responsible for hydrolysis of hippurate in 1951.
- Braunstein et al. discovered in 1969 that the hippurate hydrolysis test was useful for identifying Streptococcus agalactiae (Lancefield group B).
- Facklam et al. demonstrated that identification of -hemolytic Streptococcus groups A, B, and D could be accomplished by combining hippurate hydrolysis with bile esculin and 6.5% sodium chloride.
Purpose of Hippurate Hydrolysis Test
- A number of bacteria can be tentatively identified through the production of the enzyme hippuricase.
Principle of Hippurate Hydrolysis Test
- Group B streptococci (Streptococcus agalactiae) and certain enterococci are capable of hydrolyzing 1% aqueous sodium hippurate to generate glycine and sodium benzoate.
- The oxidising agent ninhydrin deaminates glycine, which is then reduced and turns purple.
- Since ninhydrin interacts with all free amino acids, the test medium must consist solely of hippurate.
- Thus, Group B streptococci can be separated from Groups A, C, F, and G, which are incapable of hydrolyzing sodium hippurate. A small number of Group D and viridans streptococci can also hydrolyze sodium hippurate.
Hippurate → Glycine + Benzoic acid
Glycine + Ninhydrin → purple-colored complex
Composition of Hippurate Hydrolysis Broth
| Ingredients | Gms / Litre |
| HI powder# | 10.000 |
| Peptone | 10.000 |
| Sodium chloride | 5.000 |
| Sodium hippurate | 10.000 |
| Final pH ( at 25°C) | 7.4±0.2 |
Equivalent to Heart infusion powder
Preparation of Hippurate Hydrolysis Broth
- Suspend 35 grammes in 1000 cc distilled water.
- If necessary, apply heat to dissolve the medium entirely.
- In tubes, dispense 5 ml volumes.
- Autoclave at 15 pounds of pressure (121 degrees Celsius) for 15 minutes.
Ninhydrin for Hippurate Hydrolysis Test Composition: Formulation per 10 mL
- Ninhydrin-0.35 g
- Acetone-5.0 mL
- Butanol-5.0 mL on
Procedure of Hippurate Hydrolysis Test
Method 1 – Classical Method
- Inoculate sterile sodium hippurate broth with the test organism as a first step.
- After that, incubate at 35°C overnight.
- The broth is then centrifuged to remove debris.
- Add ferric chloride reagent to the supernatant as a last step.
- After 10 minutes, if the residue remains, benzoic acid is detected. The test results are favourable for hippurate hydrolysis.
Method 2 – Ninhydrin Method
- Add 0.2ml (three to four drops) of water with a pH between 6.8 and 7.2 to a hippurate test tube.
- Then, using a heavy inoculum from an 18 to 24 hour culture, create a heavy suspension of the organism in the hippurate reagent using a standard inoculating loop and a heavy inoculum from the culture (the tube should be cloudy looking after inoculation, turbid).
- After that, incubate the tube at 35-37°C for two hours.
- During the incubation time, replenish the ninhydrin indicator solution by adding 2ml of pH 6.8-7.2 distilled water to the dropper bottle. Replace the cap tip and cap, and shake the bottle vigorously for one minute. Allow the substrate to stand at room temperature for 30 minutes, or until it has completely dissolved.
- After two hours of incubation, add two drops of the ninhydrin indicator solution to the organism and hippurate reagent mixture.
- Then, re-incubate for 30 minutes at 35-37°C.
- Observe the tubes at 10-minute intervals for the appearance of a dark blue tint, which indicates a successful test. Typically, the colour change will occur 10 to 15 minutes after adding the ninhydrin indicator solution.
Method 3- Using Hippurate Disks
- Add 0.4 mL of sterile distillated water and one Hippurate Disk to a tiny, sterile test tube (13 x 100 mm)
- The tube should be heavily inoculated with a few colonies from a pure, overnight (24-hour) culture of the test organism produced on solid media. Shake or vortex the suspension to ensure homogeneity.
- Incubate at 37°C for 2 hours (a 24 hour incubation period is required for Legionella).
- Slowly add 0.2 mL (five drops) of Ninhydrin Reagent (Catalog Number RN70) down the tube’s side to create an overlay. Do not shake.
- Reincubate the tube at 37°C for 10 to 20 minutes and observe for colour change. Tubes should not be incubated for longer than 30 minutes.
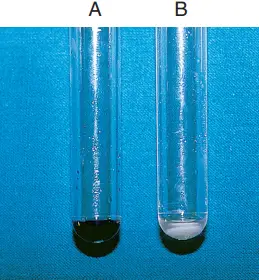
Interpretations and results of Hippurate Hydrolysis Test
- Within 30 minutes, a deep blue tint (about the colour of crystal violet) indicates a positive ninhydrin reaction test.
- In contrast, a negative reaction is indicated by a slight purple hue or no colour change.
| Organisms | Results |
| Enterococcus faecalis | negative |
| Streptococcus agalactiae | positive |
| Streptococcus pyogenes | negative |
| Campylobacter jejuni | positive |
| Campylobacter coli | negative |
Control organisms
Using the following quality control organisms, all lot numbers of Hippurate Hydrolysis Reagent have been evaluated and determined to be acceptable. The testing of a positive and negative control should adhere to the laboratory’s defined quality control protocols. Patient outcomes should not be published if abnormal quality control results are observed.
- Streptococcus agalactiae: hippurate positive.
- Streptococcus pyogenes: hippurate negative.
Reporting results
- S. agalactiae organisms are Gram-positive, catalase-negative cocci that have a characteristically narrow beta-hemolysis zone and are hippurate-positive.
- Tiny Gram-positive rods that are catalase-positive, motile at 25°C, beta-hemolytic, CAMP-positive, and hippurate-positive characterise L. monocytogenes organisms.
- G. vaginalis organisms are Gram-variable, catalase-negative, hemolytic on human blood agar, and hippurate-positive rods.
- C. jejuni organisms are curved, Gram-negative rods that are distinguished by a positive oxidase and catalase reaction, lack of aerobic growth at 35°C, and a positive hippurate reaction.
Limitations
- Incubation with ninhydrin for more than 30 minutes may produce false-positive results.
- A negative test result does not exclude the possibility of identifying G. vaginalis because the biotypes that cause bacterial vaginosis might be hippurate negative.
- Hippurate can be positive for streptococci of the Viridans group; additional tests are required to confirm the identification of non-hemolytic colonies.
- A few enterococci are beta-hemolytic and may hydrolyze hippurate, yet they are positive for pyrrolidinyl—naphthylamide (PYR) (S. agalactiae is PYR negative)
- In addition, a tiny proportion of C. jejuni organisms are hippurate-negative, necessitating the adoption of additional identifying techniques.
- The inoculum must be prepared using a solid agar medium, such as sheep blood agar.
- Positive and negative controls must be tested simultaneously with the test isolates when Hippurate Hydrolysis Reagent is employed. If a Hippurate Broth without inoculation is used as a negative control, the tube must be incubated with the test isolates.
- If utilising the hippurate hydrolysis test for the presumptive identification of group B streptococci, only test -hemolytic colonies that are catalase-negative, gram-positive cocci with streptococci-like morphology.
- This test is simply a portion of the overall group B streptococcus identification method. For final identification of the test isolate, additional testing may be required. Refer to the appropriate sources for additional instructions.
- Shake the tubes after adding the Hippurate Hydrolysis Reagent before evaluating the results. Shaking facilitates the breakdown of soluble hippurate and glycinate precipitate, resulting in a negative outcome. Not shaking tubes may result in false-positive readings.
- Positive and negative controls must be evaluated concurrently with test isolates when employing 12% Ferric Chloride (Hippurate Hydrolysis Reagent). Negative controls consisting of uninoculated Hippurate Broth must be cultured with the test isolates.
Uses of Hippurate Hydrolysis
- The hippurate test is used to identify and differentiate the species listed below.
- Additionally, it helps distinguish -hemolytic Streptococcus agalactiae from other -hemolytic streptococci.
- The hippurate hydrolysis test is crucial for distinguishing Campylobacter jejuni (+) from other campylobacter species (–), and more specifically Campylobacter coli (–) and Campylobacter laridis (–)
- Streptococcus agalactiae (+) from other human β-hemolytic streptococci (usually –)
- Legionella pneumophila (+) and L. feeleii (V) from other Legionella and Legionella-like species (–)
- Actinobacillus lignieresii (+) from Actinobacillus equuli (–)
- Listeria grayi (–) from L. innocua (+), L. ivanovii (+) and L. monocytogenes (+)
- Brevibacterium iodinum (–) from B. casei (+), B. epidermidis (+) and B. linens (+)
- Mobiluncus mulieris (–) from M. curtisii subsp. curtisii (+) and M. curtisii subsp. holmesii (+)
- Aid in the identification of Gardnerella vaginalis (+)
References
- Adzitey F, Corry J. A Comparison between Hippurate Hydrolysis and Multiplex PCR for Differentiating Campylobacter coli and Campylobacter jejuni. Trop Life Sci Res. 2011 May;22(1):91-8. PMID: 24575212; PMCID: PMC3819090.
- https://www.vetbact.org/popup/popup.php?id=36&LANG=en
- https://assets.thermofisher.com/TFS-Assets/MBD/Instructions/IFU21221.pdf
- https://assets.thermofisher.com/TFS-Assets/LSG/manuals/IFU61150.pdf
- http://www.dalynn.com/dyn/ck_assets/files/tech/DH45.pdf
- https://exodocientifica.com.br/_technical-data/M1054.pdf
- https://www.clinmicronow.org/doi/abs/10.1128/9781683670438.CMPH.ch3.17-21
- https://universe84a.com/hippurate-hydrolysis-test-introduction/
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/161/707/53275dat.pdf
- https://himedialabs.com/td/dd035.pdf
- https://microbenotes.com/hippurate-hydrolysis-test-principle-procedure-and-result-interpretation/
- https://microbeonline.com/hippurate-hydrolysis-test-principle-procedure-uses-results/
