Table of Contents
What is Innate Immunity ?
- Innate immunity serves as the initial line of defense in the human body, acting against invading microorganisms even before the adaptive immune system has a chance to fully develop. This natural defense mechanism is in place even before exposure to microbes occurs.
- The cellular components of the innate immune system encompass various elements such as epithelial barriers and leukocytes, including neutrophils, macrophages, NK cells, lymphocytes with invariant antigen receptors, and mast cells. These components work together to provide a coordinated response against potential threats.
- One of the key mechanisms of the innate immune system involves pattern recognition receptors that are present on plasma and endosomal membranes, as well as within the cytosol. These receptors play a crucial role in identifying specific structures known as pathogen-associated molecular patterns (PAMPs). PAMPs are shared by various microbes, are absent in mammalian cells, and often play a vital role in the survival of these microorganisms. This recognition mechanism limits the ability of microbes to evade detection by mutating or suppressing the expression of these molecules. Additionally, these receptors can also identify molecules produced by the host that indicate cellular damage, which are referred to as DAMPs (Damage-associated molecular pattern molecules).
- Among the pattern recognition receptors, Toll-like receptors (TLRs) are particularly significant. They are found on the cell surface as well as in endosomes, and they recognize a wide range of ligands, including components of bacterial cell walls and microbial nucleic acids.
- Apart from TLRs, other cytosolic pattern recognition receptors exist. For example, the RIG-I-like receptors (RLRs) are capable of recognizing viral RNA, while CDSs can detect microbial DNA. NOD-like receptors (NLRs) recognize constituents of bacterial cell walls and also serve as components of various inflammasomes, which are specialized caspase-1-containing enzyme complexes that form in response to a diverse array of PAMPs and DAMPs. Inflammasomes often include NLR family proteins as recognition structures, along with an adaptor molecule and the enzyme caspase-1. The main function of caspase-1 is to produce active forms of inflammatory cytokines such as IL-1 and IL-18.
- In addition to cellular receptors, the innate immune system also utilizes soluble pattern recognition and effector molecules present in the plasma. Examples of such molecules include pentraxins like CRP, collectins like MBL, and ficolins. These soluble molecules have the ability to bind to microbial ligands, thereby enhancing clearance of the pathogens through both complement-dependent and complement-independent mechanisms.
- Furthermore, there are specialized cells called innate lymphoid cells (ILCs), which resemble lymphocytes in terms of their morphology and function but do not possess clonally distributed T cell antigen receptors. ILCs can be classified into three helper subsets that secrete cytokines similar to those produced by Th1, Th2, and Th17 helper T cells.
- In summary, innate immunity provides the initial defense against microbial invaders by employing various cellular components, pattern recognition receptors, soluble molecules, and specialized cells. This innate immune response is crucial in limiting the spread of infections and creating an environment conducive to the subsequent activation of the adaptive immune system.
Definition of Innate Immunity
Innate immunity refers to the body’s natural, non-specific defense mechanisms that provide an immediate response to invading pathogens without prior exposure. It serves as the first line of defense against infections and is present from birth.
Features of Innate immunity
Innate immunity possesses distinctive features that contribute to its vital role in the immune system. These features can be summarized as follows:
- Genetic and Constitutional Makeup: Innate immunity is determined by an individual’s genetic and constitutional characteristics. It is an inherent defense mechanism that does not rely on prior exposure to specific bacteria or their byproducts. This means that the effectiveness of innate immunity is not improved through repeated exposures.
- First Line of Defense: The innate immune system serves as the primary line of defense for the host’s immune response. It acts swiftly to counteract invading pathogens before the adaptive immune system has a chance to develop a specific response.
- Pre-Existing Mechanisms: The mechanisms of innate immunity are already in place prior to exposure to foreign agents. They provide immediate protection against a wide range of pathogens without the need for prior recognition. Unlike the adaptive immune system, innate immunity is not specific to any particular pathogenic agent.
- Key Players: Phagocytic cells, such as macrophages and neutrophils, play crucial roles in innate immunity. These cells have the ability to engulf and destroy invading microorganisms. Additionally, barriers like the skin and mucous membranes act as physical barriers, preventing the entry of pathogens into the body. Furthermore, the host produces a variety of antimicrobial chemicals that contribute to the innate immune response.
Overall, innate immunity possesses distinct features that enable it to provide rapid and nonspecific defense against pathogens. It relies on pre-existing mechanisms, key cell types, and physical barriers to protect the host from infections, playing a critical role in maintaining overall immune system function.
Types of Innate immunity
Individual immunity, racial immunity, and species immunity are classifications of innate immunity.
1. Individual immunity
- Individual immunity signifies resistance to infection, which is genetically determined and differs among individuals of the same race and species.
- For instance, if one homozygous twin develops tuberculosis, the likelihood that the other twin will also develop tuberculosis is extremely high.
- But among heterozygous twins, the likelihood of the other twin developing tuberculosis is quite low.
2. Racial immunity
- Racial immunity refers to variations in susceptibility or resistance to infection between races of the same species.
- For instance, the prevalence of sickle cell anaemia along the Mediterranean coast confers immunity to the malaria parasite Plasmodium falciparum.
- This is due to an erythrocyte genetic defect that results in sickle-shaped erythrocytes that impede P. falciparum parasitization.
- Individuals with an inherited impairment in glucose-6-phosphatase dehydrogenase are also less susceptible to P. falciparum infection.
3. Species immunity
- Species immunity refers to the entire or relative resistance to a pathogen displayed by all members of a certain species.
- For instance, poultry are resistant to Bacillus anthracis and rats to Corynebacterium diphtheriae, whereas humans are susceptible.
- The precise cause of this form of immunity is unknown.
Factors influencing innate immunity
Age and nutritional health of the host are among the elements that may influence the host’s innate immunity.
1. Age
- Age extremes increase a person’s susceptibility to certain illnesses. This is partially explained by the undeveloped immune systems of extremely young children and ageing persons.
- Typically, the placental barrier protects the foetus in pregnancy from mother illnesses. HIV, rubella virus, CMV, and Toxoplasma gondii, however, are able to pass the placental barrier and induce congenital illnesses.
- Extremely elderly persons are more prone to disease (such as pneumonia) and have a high mortality rate.
- Measles, measles, poliomyelitis, and chickenpox are a few examples of infections that induce more severe clinical symptoms in adults than in children.
- This may be the result of an adult’s more aggressive immunological response, which causes higher tissue damage.
2. Nutritional status
- The nutritional state of the host is crucial for innate immunity.
- In malnutrition, both humoral and cell-mediated immunity are diminished.
- Examples are:
- In protein–calorie malnutrition, neutrophil activity, interferon response, and C3 and factor B of the complement are diminished.
- A deficiency in vitamin A, vitamin C, and folic acid increases a person’s susceptibility to infection by numerous microorganisms.
3. Hormonal levels
- Certain hormonal problems increase an individual’s susceptibility to infection.
- Patients with diabetes mellitus, hypothyroidism, and adrenal insufficiency, for instance, are more prone to staphylococcal infection, streptococcal infection, candidiasis, aspergillosis, zygomycosis, and numerous other microbial illnesses.
- Similarly, pregnant women are more susceptible to a variety of infections due to their elevated steroid levels.
Mechanisms of innate immunity
It kills invading microorganisms and stimulates acquired (adaptive) immune processes. Innate immunity, in contrast to adaptive immunity, lacks memory and does not improve upon re-exposure to the same microbe. The innate immune system depends primarily on four types of protective barriers: (a) anatomic barriers, (b) physiologic barriers, (c) phagocytosis, and (d) inflammatory responses.
1. Anatomic barriers
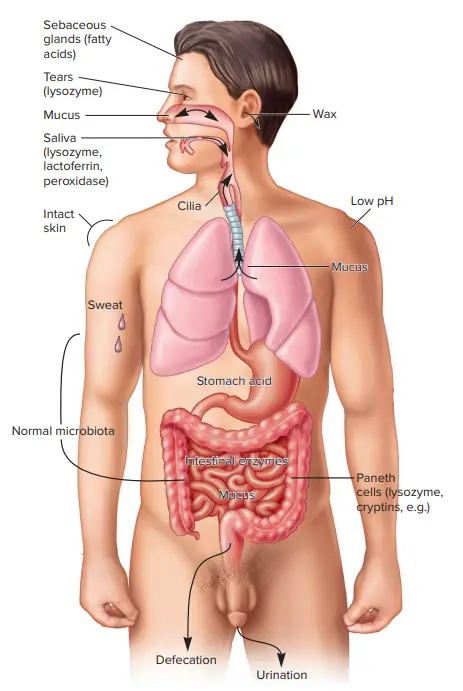
Anatomical barriers play a crucial role in the body’s defense against pathogens, encompassing physical, chemical, and biological defense mechanisms. These barriers act as the first line of defense, providing protection against invading organisms. Here are some key features of anatomical barriers:
- Epithelial Surfaces: Epithelial surfaces, such as the skin, form a physical barrier that is impermeable to most infectious agents. This barrier prevents the entry of pathogens into the body. The shedding of skin epithelium, known as desquamation, aids in the removal of bacteria and other infectious agents that may have adhered to the surface. Additionally, the lack of blood vessels in the epidermis, the inability to retain moisture, and the presence of sebaceous glands in the dermis create an environment that is unfavorable for microbial survival.
- Gastrointestinal Tract: In the gastrointestinal tract, various defense mechanisms are at play. Peristalsis, the rhythmic contraction of muscles, helps move infectious agents through the digestive system, aiding in their removal. Gastric acid and bile acids present in the stomach and intestines, respectively, create an acidic environment that inhibits the growth of pathogens. Digestive enzymes break down potential pathogens. Mucus traps infectious agents, preventing their entry into the body. Defensins, antimicrobial peptides, provide additional protection against pathogens. Moreover, the gut flora, the naturally occurring microorganisms in the intestines, compete with pathogenic bacteria for nutrients and attachment sites, limiting their colonization.
- Respiratory Airways and Lungs: The respiratory system employs various defense mechanisms to protect against airborne pathogens. The mucociliary escalator, composed of cilia and mucus-producing cells, helps to trap and move infectious agents out of the respiratory tract, preventing their entry into the lungs. Surfactant, a substance produced in the lungs, contributes to the maintenance of lung function and defense against pathogens. Defensins, present in the respiratory tract, exhibit antimicrobial properties.
- Nasopharynx: The nasopharynx, located at the back of the nose, employs defense mechanisms such as mucus, saliva, and lysozyme. Mucus traps infectious agents, while saliva contains antimicrobial properties. Lysozyme, an enzyme found in various bodily secretions, helps to break down the cell walls of bacteria.
- Eyes: Tears play a crucial role in protecting the eyes from infection. Tears contain antimicrobial substances that help to flush out potential pathogens and prevent their colonization.
- Blood-Brain Barrier: The blood-brain barrier is a specialized anatomical barrier formed by endothelial cells in the blood vessels of the brain. It regulates the entry of substances into the brain, including pathogens. The tight junctions between endothelial cells prevent the passive diffusion of most molecules, while active transport mechanisms, such as P-glycoprotein, further control the movement of substances.
Overall, anatomical barriers provide a frontline defense against pathogens by employing various physical, chemical, and biological mechanisms. These barriers are critical in preventing the entry and colonization of infectious agents, contributing to the overall immune defense of the body.
2. Physiological barriers
Physiological barriers are essential components of innate immunity, contributing to the body’s defense against pathogens. These barriers utilize various mechanisms to prevent and combat infections. Here are some key examples of physiological barriers:
- Gastric Acidity: The low pH of stomach contents serves as a natural physiological barrier against infection. The acidic environment in the stomach makes it difficult for most ingested microbes to survive, providing protection against gastrointestinal infections.
- Lysozyme, Interferon, and Complement: Soluble innate immune mediators play a crucial role in physiological barriers. Lysozyme, an enzyme found in various bodily secretions, exerts antimicrobial effects by targeting the bacterial cell wall. Interferons, produced by cells in response to viral contamination, inhibit the production of viral structural proteins, thereby exhibiting broad antiviral activity. Complement refers to a group of serum-soluble chemicals that, when activated, can damage the cell membrane of pathogens.
- Microorganism-Specific Compounds: Certain compounds are exclusive to microorganisms and are not observed in multicellular organisms. These unique microbial molecules serve as strong markers of innate immunity. The host’s immune system can rapidly recognize and target invaders expressing these specific molecules, initiating a prompt immune response.
These physiological barriers contribute to the early detection and elimination of pathogens. Gastric acidity, lysozyme, interferon, and complement all play vital roles in preventing infection and promoting the body’s defense against invading microorganisms. Additionally, the recognition and targeting of microorganism-specific compounds by the immune system highlight the remarkable ability of innate immunity to identify and respond to potential threats.
3. Phagocytosis
- Phagocytosis is a crucial process employed by cells to engulf and eliminate various particles, including bacteria, fungi, parasites, dead cells, and debris. It involves a series of molecular events and plays a vital role in the body’s innate immune response.
- The process of phagocytosis begins when foreign particles, such as bacterial cells, bind to specific receptors present on the surface of phagocytes. These receptors recognize the particles and initiate the phagocytic process. The phagocyte extends its membrane around the particle, engulfing it and forming a structure called a phagosome. Within a minute, the phagosome fuses with a lysosome or a granule, forming a phagolysosome. This fusion leads to the activation of various killing mechanisms, ultimately resulting in the destruction of the engulfed particle.
- Different types of phagocytes exist, including neutrophils, macrophages, dendritic cells, and mast cells. Neutrophils, for example, are rapid phagocytes that can engulf bacteria within approximately nine minutes. Macrophages and dendritic cells are slower in their phagocytic activity, and the process can take several hours in these cells. Macrophages, known for their voracious appetite, engulf large amounts of material and release some undigested debris back into the surrounding tissues. This released debris acts as a signal to recruit more phagocytes from the blood, amplifying the immune response.
- Phagocytes possess a variety of surface receptors that aid in the recognition and binding of particles. These receptors include opsonin receptors, scavenger receptors, and Toll-like receptors. Opsonin receptors enhance phagocytosis of bacteria that have been coated with antibodies or complement proteins. Scavenger receptors bind to a broad range of molecules on the surface of bacterial cells, while Toll-like receptors specifically recognize foreign DNA, RNA, and other molecules. Activation of Toll-like receptors not only increases phagocytosis but also triggers the release of inflammatory hormones, contributing to the immune response.
- Phagocytes are vital components of the immune system, protecting the body by ingesting harmful particles and contributing to subsequent immunity. They are involved in fighting infections by chemotaxis, a process in which chemical signals attract phagocytes to the site of infection. Upon contact with bacteria or other pathogens, the phagocytes bind to them and proceed to engulf and destroy them. Additionally, phagocytes, such as macrophages and dendritic cells, can present antigens from the engulfed material to other immune cells, facilitating the development of immunity.
- Phagocytosis is an intricate process that plays a critical role in maintaining the body’s defense against pathogens. While pathogens have evolved mechanisms to evade phagocytic attacks, phagocytes remain essential in the body’s immune response, contributing to the overall protection and health of tissues and organs.
4. Inflammatory responses
- Damage to tissue induced by a wound or an invading pathogenic bacterium triggers a complicated chain of events known as the inflammatory responses.
- Inflammation may result in the activation of a specific immune response to the invader or the elimination of the invader by innate immune system components.
- The four primary characteristics of inflammatory responses are rubor (redness), calor (temperature increase), dolor (pain), and tumor (swelling).
Mediators of inflammatory reactions
The primary mediators of inflammatory reactions are histamine, kinins, acute-phase proteins, and defensin.
- Histamine: Histamine is a chemical molecule released in reaction to tissue injury by a number of cells. It is an important mediator of the inflammatory response. It causes vasodilation and a rise in permeability by binding to receptors on surrounding capillaries and venules.
- Kinins: Kinins are additional crucial mediators of the inflammatory response. Normal blood plasma contains them in an inactive state. The activation of these tiny peptides by tissue damage results in vasodilation and increased permeability of capillaries. Additionally, bradykinin stimulates pain receptors in the skin. This response likely serves a defensive function, as pain generally prompts a person to protect an injured area.
- Acute-phase proteins: C-reactive proteins and mannose-binding proteins are components of the innate immune system. As a nonspecific response to pathogens and other forms of tissue injury, these proteins are generated at a higher concentration in the plasma during acute-phase reaction. In response to proinflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tissue necrosis factor, they are generated in the liver (TNF). They are referred to as proinflammatory cytokines because they amplify inflammatory reactions.
- Defensins: Defensins are an additional essential component of the innate immune system. They are cationic peptides that kill bacteria by creating pores in their membranes. They are primarily found in the lower respiratory system and digestive tract. In contrast to the respiratory system, the gastrointestinal tract contains -defensins. Additionally, -defensins demonstrate antiviral action. They attach to CXCR4 receptors and prevent HIV virus entrance into cells. It is unknown how these defensins distinguish bacteria from other cells.
Innate Resistance Relies on Chemical Mediators
- Mammalian hosts are equipped with a chemical arsenal to combat the continual assault of microbes.
- Importantly, the normal microbiota also contribute their own molecules to the host’s resilience by warding off rivals.
- The next issue is how chemical defences created by the host guard against microbial invasion.
- Lysozyme (muramidase) is an enzyme that lyses bacteria by hydrolyzing the connection between N-acetylmuramic acid and N-acetylglucosamine in the peptidoglycan of the bacterial cell wall, particularly in Gram-positive bacteria.
- Lactoferrin, an iron-binding protein, is also present in substantial levels in tears and other mucous secretions. Similar to transferrin, lactoferrin is present in breast milk and mucous secretions.
- Both proteins bind iron, restricting the capacity of invading microorganisms to proliferate by lowering the availability of iron.
- Additionally, mucous membranes create lactoperoxidase, an enzyme that catalyses the generation of superoxide radicals, a reactive oxygen species that is harmful to numerous microbes.
Antimicrobial Peptides
- Peptides with broad-spectrum antibacterial activity are usually believed to be the oldest form of basic animal defence.
- Many are positively charged and so electrostatically attracted to the cell surfaces of microorganisms.
- Antimicrobial peptides are amphipathic; hence, they are soluble in both aqueous and lipid-rich environments, as they contain both hydrophobic and hydrophilic components.
- Upon engaging with the membranes of their targets, antimicrobial peptides destroy their prey through a variety of methods.
- Cationic peptides and bacteriocins are two primary classes of antimicrobial peptides.
Cationic Peptides
- Humans manufacture three kinds of generic cationic peptides whose biological activity is correlated with their potential to destroy bacterial plasma membranes.
- Most form holes, affecting membrane permeability and frequently resulting in lysis. The first group of cationic peptides consists of -helical, linear peptides. Examples include the cathelicidins.
- Approximately 30 members of this family are present in numerous animals, including humans. They are secreted as big proteins that undergo proteolytic cleavage to become active.
- Cathelicidins possess antibacterial activity against bacteria, viruses with envelopes, and fungus. Human cells that produce cathelicidin peptides include neutrophils, respiratory and urogenital epithelial cells, and alveolar macrophages.
- A second group, known as defensins, is also generated from precursor proteins that mature via protease action.
- Defensins, unlike cathelicidins, are found in both invertebrates and vertebrates.
- There are two types of defensins in humans: and. The granules of neutrophils (phagocytic cells), intestinal Paneth cells, and intestinal and respiratory epithelial cells contain defensins, which aid in bacterial breakdown.
- defensins are commonly found in epithelial cells and are known to trigger the production of immune system chemical mediators by mast cells.
- Similar to cathelicidins, they are active against numerous bacteria, fungi, and viruses with envelopes. Histatins are a third type of cationic peptides.
- Histatins are bigger peptides discovered in the saliva of humans and other primates with fungicidal properties.
- In contrast to cathelicidins and defensins, which damage the membranes of microorganisms, histatins impair mitochondrial activity and produce oxidative and osmotic stress, resulting in the death of fungi.
Bacteriocins
- In addition to host cells releasing antimicrobial peptides, some members of the bacterial microbiome release bacteriocins, which kill closely related bacteria.
- Bacteriocins are peptides that may provide an adaptive advantage to their producers, which are immune to their bacteriocins, against other bacteria.
- However, they occasionally improve bacterial virulence by causing damage to the host’s immune cells. Escherichia coli is an example of a bacteriocin-producing bacterium. It synthesises colicins, which are bacteriocins encoded on several Col plasmids.
- Some colicins connect to specific receptors on the cell envelope of sensitive target bacteria and cause cell lysis, while others attack intracellular locations such as ribosomes or interfere with energy synthesis.
- The lantibiotics generated by Streptococcus, Bacillus, Lactococcus, and Staphylococcus are more examples.
Complement Is a Cascading Enzyme System
- The complement system is an old form of innate immunity that is present in both primitive invertebrates and mammals.
- When discovered, this group of more than 30 heat-labile serum proteins was determined to “supplement” the antimicrobial activities of other immune system components.
- The complement system can be activated by three distinct types of signals, but they all result in the same three final effects: (1) stimulating an inflammatory response by promoting the recruitment of white blood cells (leukocytes); (2) penetrating microbial cell membranes, thereby causing cell lysis; and (3) defending against microbial invasion by promoting phagocytosis via opsonization.
- Opsonization (Greek opson, to prepare victims for) is the term used when bacteria or other non-self antigens are coated with specific complement proteins, antibodies, or both.
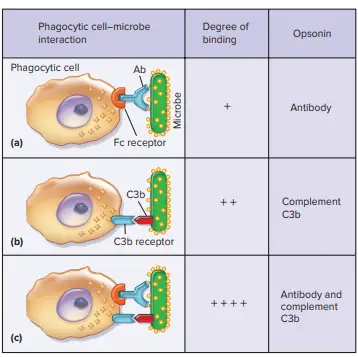
- These proteins are known as opsonins in this context, and the microbe is said to be opsonized. On the surface of antigens, the presence of opsonins acts as a beacon or target that facilitates recognition by phagocytic cells.
- This is because phagocytic cells have receptors on their surface that identify opsonins specifically. When phagocytes are coated with opsonins, their ability to eliminate pathogens significantly increases.
- Complement proteins are the first opsonins to be produced, hence they play a crucial role in phagocytic microbe elimination.
- Produced in an inert state, the activity of complement proteins is regulated by a cascade of proteolytic cleavage.
- Initial activation of important complement proteins results in their proteolytic activity, which is used to activate further complement proteins.
- Because activation routes are complex, we present a simplified perspective. The three activation pathways of the complement are the alternative, lectin, and classical pathways.
The alternative complement pathway
- In response to bacterial molecules having repeated structures, such as the lipopolysaccharide (LPS) of Gram-negative bacteria, the alternative complement pathway is activated.
- These compounds induce complement (C) protein 3 (C3) to fragment into C3a and C3b.
- C3b is generally unstable, but when it binds to microbial cell surfaces, it becomes stable. Membrane-bound C3b functions as an opsonin, hence facilitating phagocytosis.
- C3b also interacts with another complement protein termed factor B, which, once attached to C3b, is broken by the complement protease factor D to produce the remaining two consequences (inflammation and microbial cell lysis).
- The smaller cleavage product is released, whereas factor Bb remains linked to C3b, forming the C3bBb complex.
- C3bBb serves as the C3 convertase of the alternative complement pathway, a protease.
- This C3 convertase enhances the route by cleaving additional C3 into C3a and C3b, increasing the quantity of C3b that coats microbial structures.
- C3bBb not only serves to enhance C3b, but it also activates another complement protein, C5, via proteolysis. Because C3bBb cleaves both C3 and C5, it is both a C3 and C5 convertase.
- As a C5 convertase, it causes the formation of C5a and C5b cleavage products. C5b quickly forms a complex with two additional complement proteins, C6 and C7, to create C5b67, which is maintained by attaching to microbial membranes.
- Then, C8 and C9 bind to form the membrane attack complex (MAC), which forms a pore in the plasma membrane or outer membrane of the target cell.
- MAC is highly efficient against Gram-negative bacteria but less so against Gram-positive bacteria and fungi, as their membranes are protected by strong cell walls.
- Importantly, host cells possess multiple methods to defend their membranes against complement attack.
- The protein factor H and decay accelerating factor (DAF) on the surface of host cells are the most important.
- Factor H inhibits Bb, whereas DAF binds to C4b and C3b and displaces Bb and C2a. As we will see, this disarms both the lectin and classical pathways, as well as the alternative pathway.
- Both C3a and C5a synthesis result in significant pro-inflammatory effects. For instance, the binding of C3a and C5a to receptors on particular host cells causes the release of other biological mediators.
- As a result, phagocytic cells, particularly neutrophils, might be attracted to the site of the infection.
- Neutrophils are voracious phagocytes that are crucial for the elimination of extracellular infections. The inflammatory signals generated as a result of C3a and C5a activation also excite other innate immune cells (dendritic cells and macrophages), which in turn assist trigger an adaptive immune response.
The lectin complement pathway
- In addition to the activation of a C3 convertase, the lectin complement pathway requires the activation of a C3 convertase. In this instance, however, the proteolytic cascade is initiated by a soluble host lectin—a protein that binds to certain carbohydrates.
- An important lectin is mannose-binding protein (MBP). This protein binds mannose, a key sugar found in bacterial, fungal, and some viral envelopes.
- MBP is capable of forming a complex with mannose-binding lectin-associated serine protease-1 (MASP-1), MASP-2, and MASP-3 when it binds to mannose on pathogens.
- The MASPs are then activated as proteases. First, MASP proteases cleave the complement protein C4 into C4a and C4b. C4b is similar to C3b in that it also attaches to microbial surfaces.
- After C4b binds to the target microorganism, a second complement protein, C2, forms a complex with it.
- A MASP then cleaves C2 to liberate C2a and C2b. Then, C2a binds to C4b to create C3 convertase. This C3 convertase now creates C3a and C3b, similar to the C3 convertase of the alternate pathway.
- In addition to serving as an opsonin, a portion of C3b combines with C4b and C2a to generate a C5 convertase (C3b plus C4bC2a) that enables the assembly of MAC components and the induction of inflammation by C5a and C3b.
- Thus, despite the usage of distinct C3 and C5 convertases, the alternate process yields the same three outcomes.
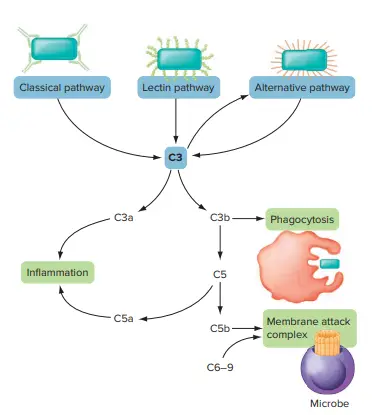
Classical complement pathway
- Typically, pathogen-specific antibodies are required for activation of the classical complement system.
- Antibodies are glycoproteins produced as an adaptive response by activated B cells.
- Every antibody binds to a distinct antigen. When this occurs in conjunction with complement activation, the antibody-antigen complex contacts C1, a three-protein complement component (q, r, s).
- This produces a protease-active trimolecular complex (C1qrs + antibody + antigen).
- As reported for the lectin route, the active C1s subcomponent cleaves C4 and C2 to form the C3 convertase C4b2a.
- C4b2a binds to the antigen-antibody-C1 complex as opposed to a microbiological cell or other antigenic particle.
- C4b2a serves as a C3 convertase to cleave C3 into a pathogen-bound subcomponent C3b and a soluble C3a component, as observed in the lectin pathway.
- C5 convertase of the classical pathway is identical to C5 convertase of the lectin pathway (C3b + C4bC2a).
- Thus, the primary distinctions between the three pathways are (1) the types of molecules that trigger initial activation, (2) the mechanism by which the C3 and C5 convertases are activated, and (3) the identity of the C3 and C5 convertases, although these are identical for the lectin and classical pathways.
- It is essential to notice that the three routes are not active simultaneously.
- Microbes arriving for the first time at a local tissue site engage with components of the alternative pathway and subsequently the lectin pathway, resulting in the production of C3a and C5a to help stimulate inflammation, opsonization of the microbes with C3b, and commencement of the lytic sequence by MAC.
- In the event that viruses persist or re-invade the host, antibody responses will also activate the classical complement system.
Cytokines Are Chemical Messages Between Cells
- A highly specialised communication system is required for an effective and coordinated immune system.
- The “language” of the mammalian immune system is composed of tiny, soluble proteins that, when attached to their cellular receptors, create a signal transduction pathway that regulates the transcription of genes and the activation of proteins required for an adequate immunological response.
- Due to the fact that each cytokine induces a unique cellular response or collection of responses, the immunological activity is dependent on which signals are received, how many receptors become occupied, and any external or internal interference.
- Cytokine (Greek cyto, cell; kinesis, movement) refers to any soluble, low-molecular-weight protein or glycoprotein that is secreted by one cell group and functions as an intercellular (between cells) mediator or signalling molecule. Many different types of cells create cytokines.
- Once released, cytokines have a broad spectrum of effects on cells, resulting in several cellular responses.
- Infection by a virus, bacterium, or parasite, malignancy, and inflammation are stimuli that induce cytokine synthesis, which is carefully regulated. Additionally, the release of particular cytokines by one kind of immune cell might stimulate the production of other cytokines by other cells.
- Cytokines can only exert their biological effects when attached to specific receptors on the surface of target cells.
- The majority of cells contain hundreds to a few thousand cytokine receptors; yet, cytokines are extremely strong molecules, and a maximal cellular response occurs when just a small fraction of receptors are occupied by cytokine molecules.
- Although there are numerous ways to classify cytokines, they can be grouped into three basic categories: (1) cytokines that regulate innate immunity, (2) cytokines that regulate adaptive immunity, and (3) cytokines that drive hematopoiesis.
- The naming of cytokines has been a case of the left hand not knowing what the right hand has done.
- Historically, proteins were named based on their function. As the precision of protein biochemistry increased, scientists classified proteins based on their structures.
- This resulted in a list of proteins with distinct names but identical functions and proteins with distinct names but same structures.
- For convenience, the nomenclature of cytokines can be separated into four functional groups:
- Chemokines are cytokines that drive cell movement (i.e., chemotaxis and chemokinesis).
- Interleukins are cytokines released by one leukocyte that act on another lymphocyte.
- Interferons are regulatory cytokines released in response to an infection.
- Colony-stimulating factors are cytokines that stimulate the development and differentiation of immature leukocytes in the bone marrow (CSFs).
- Based on early discoveries of their in vitro action, the last significant family of cytokines produced by immune cells is known as tumour necrosis factors (TNF); their immunological purpose is to promote an inflammatory response.
- Although these phrases are a convenient shorthand for identifying function, they do not exclude one another. Interferons produced by one kind of leukocyte influence the behaviour of other leukocytes; interferons also function as interleukins.
Acute-Phase Proteins
- In addition to chemical mediators from leukocytes, a number of physiologically active chemicals from other somatic cells can produce profound physiological changes in the host. An interesting example happens when macrophages respond to locations of damage.
- They release pro-inflammatory cytokines that induce rapid production of acute-phase proteins in the liver.
- These mediators assist in the prevention of blood loss and ready the host for microbial invasion. C-reactive protein (CRP), mannose-binding protein (MBP), and surfactant proteins A (SP-A) and D (SP-D) are all opsonins that attach to bacterial surfaces.
- CRP also interacts with C1q to activate the conventional complement pathway, whereas MBP binds to bacteria and fungus to activate complement via the lectin pathway.
- SP-A, SP-D, and C1q are also known as collectins, which are proteins with a collagen-like motif coupled to globular binding sites by α-helices.
- These proteins (together with others) police host tissues by binding to (“collecting”) foreign items, so assisting in the elimination of bacteria and their products.
Cells of Innate Immunity
- The process of hematopoiesis happens in the bone marrow of mammals to produce blood cells. All leukocytes (Greek: leukos, white; kytos, cell) are derived from hematopoietic precursor cells in the foetal liver, bone marrow, and thymus.
- Some leukocytes that are prompted to undergo additional development become tissue-dwelling cells that respond to local damage. These cells notify the entry of alien organisms by sounding the alarm.
- After the alarm has been raised, other leukocytes circulate in the body’s fluids and are recruited to the areas of infection.
- Between 4,500 to 10,000 leukocytes per cubic millimetre of blood are present in the ordinary adult.
- During an immunological response, its average value changes drastically. In most bacterial and fungal infections, for instance, the white blood cell count might rise as leukocytes travel from the bone marrow to the circulation and subsequently to the site of invasion.
- This transitory increase in circulating leukocytes (leukocytosis) is a useful diagnostic for detecting an infectious condition for doctors. Now, we will discuss these cells’ unique functions.
Mast Cells
- Mast cells are produced from bone marrow and differentiate in connective tissue.
- Granules are specialised organelles found in the cytoplasm of mast cells, which feature an indented nucleus and a cytoplasm loaded with granules.
- Mast cells are not phagocytic, but when activated, they rapidly degranulate, releasing their granule contents into the extracellular environment.
- Histamine, prostaglandins, serotonin, heparin, dopamine, platelet-activating factor, and leukotrienes are found in mast cell granules.
- These substances are known as vasoactive mediators because they affect the tone and diameter of blood vessels.
- Additionally, mast cells include high-affinity receptors for the antibody type associated with allergic responses (IgE).
- When IgE binds to these receptors in sufficient amounts, preformed vasoactive mediators are released.
Granulocytes
- The nuclei of granulocytes are also unevenly shaped, having two to five lobes.
- Their cytoplasm is composed of granules containing reactive chemicals that destroy bacteria and exacerbate inflammation.
- There are three types of granulocytes: neutrophils, basophils, and eosinophils.
Basophils
- Basophils (Greek: basis, base; philein, to love) contain nuclei with an uneven form and two lobes.
- The granules contain similar molecules to those found in mast cells, including histamine.
- Basophils, like mast cells, play a key role in the development of allergies and hypersensitivities because they release prepared vasoactive mediators and possess high-affinity receptors for the type of antibody linked with allergic responses.
Eosinophils
- Eosinophils (Greek: ; philein) contain a two-lobed nucleus linked by a thin chromatin filament.
- In addition to peroxidases and a caustic protein called major basic protein, eosinophil granules contain hydrolytic enzymes (e.g., nucleases and glucuronidases).
- When attracted by soluble chemotactic mediators, eosinophils circulate in low quantities and move from the bloodstream into tissue spaces, especially mucous membranes. They guard against parasitic protozoa and helminths (worms).
- These organisms are too large to be phagocytosed, therefore when eosinophils release hydrolytic enzymes, cationic peptides, and reactive oxygen species into extracellular fluid, the plasma membrane of the parasite is destroyed and (hopefully) the organism is killed.
- Eosinophils contribute to allergic reactions because their granules contain histaminase. Consequently, their numbers frequently increase during parasite infections and allergic reactions.
Neutrophils
- Neutrophils (Latin neuter, neither; philein) are also known as polymorphonuclear cells (PMNs) due to the fact that their nuclei feature three to five lobes joined by thin chromatin strands.
- Neutrophils are “phagocytic machines” that include primary and secondary granules, which are inconspicuous organelles.
- Peroxidase, lysozyme, defensins, and different hydrolytic enzymes are found in primary granules, whereas secondary granules contain collagenase, lactoferrin, cathelicidin, and lysozyme.
- After phagocytosis, these enzymes and other substances assist breakdown foreign material. Mature neutrophils leave the bone marrow and circulate in the blood in order to travel rapidly to sites of tissue damage and infection, where they serve as the primary phagocytic responders.
- Neutrophils move to places where C3a and C5a complements have been released, where they discover opsonized particles that are more readily phagocytosed. Neutrophils have a lifespan of around one week.
- In the context of phagocytosis and the inflammatory response, neutrophils and their antimicrobial chemicals are discussed in more depth.
Monocytes, Macrophages, and Dendritic Cells
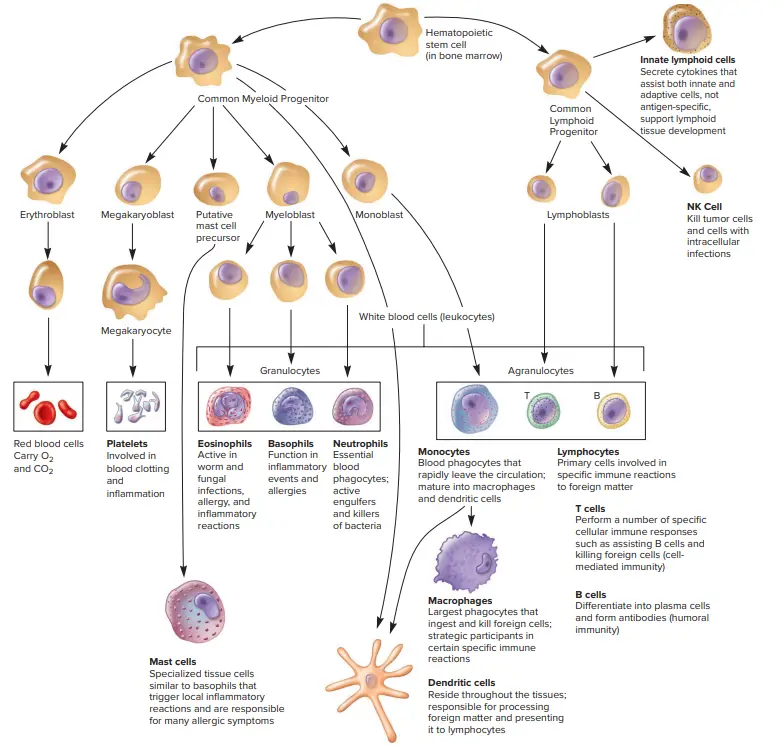
Monocytes
- Monocytes (Greek monos, single; cyte, cell) are leukocytes having granules in the cytoplasm and an oval or kidney-shaped nucleus.
- They are generated in the bone marrow, enter the blood, circulate for approximately eight hours, expand, migrate into tissues, and develop into macrophages or dendritic cells.
Macrophages
- Macrophages (Greek:, macros, large;, phagein, to consume) are larger than their progenitor cells, the monocytes.
- Macrophages reside in particular tissues, where they are referred to as resident or fixed macrophages.
- There, they can function as sentinel cells, emitting a chemical warning when a bacterium invades. In addition to activating complement, macrophages release chemokines that draw neutrophils to the site of infection so that the pathogen can be effectively phagocytosed and its spread to other body sites is prevented.
- Due to the fact that macrophages and neutrophils are strongly phagocytic, their role in innate resistance is studied in greater depth in the context of phagocytosis.
Dendritic cells (DCs)
- Dendritic cells (DCs) are characterised by the presence of many protrusions. They sample antigens within the host using the extensive surface area provided by their cellular projections.
- The majority of DCs reside in tissues, particularly in the skin and mucous membranes of the nose, lungs, and intestines, where they constantly monitor their surroundings.
- DCs are highly specialised cells that are trained to identify and phagocytose infections for the sole goal of displaying a piece of pathogen-derived peptide on their surface via specific receptors.
- DCs travel to lymphoid tissues to “present” the antigen to lymphocytes, conveying important information about invading pathogens and generating an adaptive immune response.
- DCs can trigger immunological responses from T cells that have never encountered a specific antigen by presenting antigen.
- Thus, DCs bridge the gap between the innate reaction to all pathogens and the adaptive response to a particular pathogen.
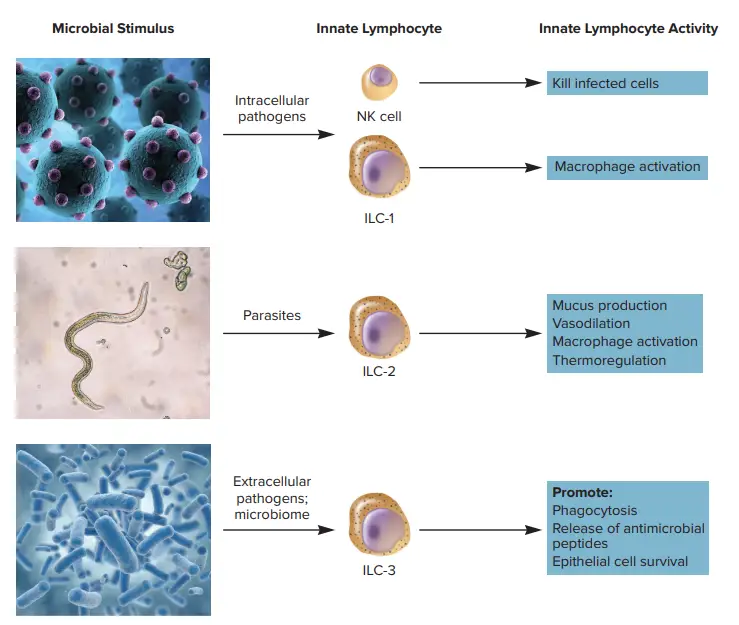
Innate Lymphoid Cells
- All innate immune cells studied thus far arise from common myeloid progenitor cells. When it was discovered that natural killer (NK) cells originate from common lymphoid progenitor cells, many scientists were perplexed because these cells had an intrinsic immunological activity.
- More recently, it was discovered that common lymphoid progenitor cells also generate innate lymphoid cells (ILCs), which include lymphoid tissue inducer (LTi) cells.
- Although these cells are all lymphocytes, two distinguishing characteristics separate them apart from adaptive immunity lymphocytes (T and B cells).
- First, unlike T and B cells, ILCs (which include NK cells) do not experience the same complex developmental processes that result in antigen-specific receptor expression. This results in the second significant distinction.
- ILCs lack receptors that respond to and “remember” specific infections, unlike adaptive immune cells. As innate immune cells, they are triggered by the same stress signals that activate neutrophils, macrophages, and dendritic cells.
- Included in this category are opsonized microorganisms, foreign antigens, and cytokines. After maturation, each cell has a unique purpose.
- As their name implies, lymphoid tissue inducer (LTi) cells promote the growth of lymph node and Peyer’s patches tissue (specialized lymphoid tissue in the small intestine).
- Other ILCs can be split into three functional kinds, ILCs 1, 2, and 3, each of which responds to a distinct antigenic trigger.
- ILC-1s help activate macrophages when activated by intracellular pathogens (such as viruses). ILC-2s cause vasodilation, the release of increased mucus, and macrophage activity in response to external pathogens such as parasites (especially those that reside in the colon).
- The role of ILC-2s in thermoregulation is not fully understood. ILC-3s have been shown to be crucial for immunological homeostasis because they respond to our normal microbiota as well as pathogenic microorganisms.
- ILC-3s contribute to the maintenance of the barrier function along mucous membranes by encouraging the production of antimicrobial peptides, inhibiting an immune response to normal gut microbiota, and promoting phagocytosis and the survival of epithelial cells lining the mucosa.
- Sometimes, natural killer (NK) cells are referred to as cytotoxic ILCs. These big, granular cells recognise and eliminate stressed, cancerous, or virus-infected cells.
- NK cells examine neighbouring cells by contacting them through many surface receptors. These interactions give NK cells with both kill and non-kill messages.
- The fate of the target cell is determined by the balance of these signals. If the target cell is malignant or contaminated, the kill signals overwhelm the survival signals, and the NK cell becomes lethal.
- Once the NK cell is activated to kill the target cell, it mobilises its cytoskeletal proteins to stick firmly to the target cell and releases a cargo of lethal chemicals. These include the pore-forming protein perforin and the granzyme enzymes.
- Collectively, these proteins induce the death of the target cell (apoposis). In addition to responding to the ratio of kill to survival signals provided by the target cell, NK cells can also detect cells that need to be eliminated using a second mechanism.
- NK cells have antibody receptors and can therefore attack cells that have been opsonized by antibodies. Remember that self-cells should not be antigenic and so should not be antibody-coated.
- However, malignant cells may create mutant proteins that the adaptive immune system recognises as foreign.
- Additionally, infected host cells may bind viral proteins to their surfaces. In both instances, antibodies develop and attach.
- Antibody-dependent cell-mediated cytotoxicity describes the process by which NK cells recognise and kill such cells (ADCC).
Functions of Innate Immunity
Innate immunity carries out various essential functions that contribute to the overall defense of the host. These functions can be described as follows:
- Recruitment of Immune Cells: Innate immunity plays a crucial role in recruiting immune cells to the sites of infection. It accomplishes this by producing chemical factors, including cytokines, which act as chemical mediators. These cytokines attract and activate immune cells, such as macrophages and neutrophils, to the infected area, thereby initiating the immune response.
- Activation of the Complement Cascade: The innate immune system activates the complement cascade, a series of proteins, to identify and eliminate bacteria, stimulate immune cells, and facilitate the clearance of antibody complexes or dead cells. This process enhances the immune response and helps in the eradication of pathogens.
- Identification and Removal of Foreign Substances: Specialized white blood cells, such as phagocytes (e.g., macrophages and neutrophils), are involved in the identification and removal of foreign substances present in organs, tissues, blood, and lymph. These cells engulf and eliminate pathogens, damaged cells, and other foreign materials, contributing to the overall defense against infections.
- Activation of the Adaptive Immune System: Innate immunity plays a critical role in activating the adaptive immune system. It achieves this through a process known as antigen presentation. Antigen-presenting cells, such as macrophages and dendritic cells, capture and present antigens from pathogens to T cells, which then initiate a specific immune response. This interaction between the innate and adaptive immune systems is vital for effective pathogen clearance and long-term immunity.
- Physical and Chemical Barrier: Innate immunity acts as a physical and chemical barrier against infectious agents. Physical measures, such as the skin, serve as a physical barrier that prevents the entry of pathogens into the body. Chemical measures, such as clotting factors in the blood, are released in response to injuries that breach the initial physical barrier. These factors promote clotting to minimize further pathogen invasion. However, it’s important to note that the blood-brain barrier is an example of a separate second-line physical or chemical barrier, protecting the nervous system from pathogens that have already gained access to the host.
In summary, innate immunity performs vital functions in the immune response. It recruits immune cells to infection sites, activates the complement cascade, identifies and eliminates foreign substances, activates the adaptive immune system, and acts as a physical and chemical barrier. These functions collectively contribute to the early detection, containment, and elimination of pathogens, maintaining the overall health and defense of the host.
FAQ
What is innate immunity?
Innate immunity refers to the body’s immediate and general defense mechanism against pathogens and foreign substances. It is present from birth and provides the first line of defense for the immune system.
How does innate immunity differ from adaptive immunity?
Innate immunity is non-specific and does not require prior exposure to a specific pathogen. It provides immediate protection and is the first response to infection. In contrast, adaptive immunity is specific to a particular pathogen and develops over time through exposure and recognition of antigens.
What are the key components of innate immunity?
The key components of innate immunity include physical barriers (such as the skin and mucous membranes), chemical barriers (such as antimicrobial proteins and enzymes), phagocytic cells (such as macrophages and neutrophils), natural killer (NK) cells, and the complement system.
How do phagocytic cells contribute to innate immunity?
Phagocytic cells, such as macrophages and neutrophils, play a crucial role in innate immunity by engulfing and destroying pathogens through a process called phagocytosis. They recognize and bind to foreign particles, internalize them, and break them down using antimicrobial substances.
What is the complement system and its role in innate immunity?
The complement system is a group of proteins that circulate in the blood and contribute to innate immunity. When activated, these proteins can identify and destroy pathogens, recruit immune cells to the site of infection, and enhance the effectiveness of phagocytosis.
How do natural killer (NK) cells function in innate immunity?
NK cells are a type of lymphocyte that can recognize and eliminate infected or abnormal cells, such as virus-infected cells or cancer cells. They provide rapid responses to these cells without prior sensitization.
What are some examples of physical barriers in innate immunity?
Physical barriers in innate immunity include the skin, which acts as a physical barrier preventing the entry of pathogens, and mucous membranes in the respiratory, gastrointestinal, and genitourinary tracts that trap and remove pathogens.
How do antimicrobial proteins contribute to innate immunity?
Antimicrobial proteins, such as defensins and lysozyme, are secreted by various cells and tissues. They have the ability to directly kill or inhibit the growth of pathogens, providing an additional layer of defense against infections.
Can innate immunity be enhanced?
While innate immunity is largely genetically determined, certain factors can enhance its effectiveness. These include maintaining a healthy lifestyle, proper nutrition, regular exercise, and adequate sleep. Vaccines also play a role in stimulating innate immune responses.
How does innate immunity interact with adaptive immunity?
Innate immunity and adaptive immunity work together to provide comprehensive immune protection. Innate immunity provides the initial response to infection, activating adaptive immunity. Adaptive immunity, in turn, generates a targeted response to the specific pathogen, leading to long-term immunity and memory of the pathogen for future encounters.
References
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Innate Immunity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26846/
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. The innate and adaptive immune systems. [Updated 2020 Jul 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279396/
- Biron, C. A. (2016). Innate Immunity. Viral Pathogenesis, 41–55. doi:10.1016/b978-0-12-800964-2.00004-5
- Mak, T. W., & Saunders, M. E. (2006). Innate Immunity. The Immune Response, 69–92. doi:10.1016/b978-012088451-3.50006-5
- Actor, J. K. (2012). Innate Immunity. Elsevier’s Integrated Review Immunology and Microbiology, 43–51. doi:10.1016/b978-0-323-07447-6.00006-5
- Burrell, C. J., Howard, C. R., & Murphy, F. A. (2017). Innate Immunity. Fenner and White’s Medical Virology, 57–64. doi:10.1016/b978-0-12-375156-0.00005-9
- Eckmann, L. (2006). Innate Immunity. Physiology of the Gastrointestinal Tract, 1033–1066. doi:10.1016/b978-012088394-3/50045-3
- https://journals.sagepub.com/home/ini
- https://www.technologynetworks.com/immunology/articles/innate-vs-adaptive-immunity-335116
- https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323532662000394
- https://www.khanacademy.org/test-prep/mcat/organ-systems/the-immune-system/a/innate-immunity
- https://ciiid.washington.edu/content/what-innate-immunity
- https://en.wikipedia.org/wiki/Innate_immune_system
- https://microbenotes.com/innate-immune-system-an-introduction/
