Table of Contents
What is the Lysine Iron Agar (LIA) Test?
The solid medium Lysine Iron Agar (LIA) is indicated for use in qualitative techniques for the differentiation of microorganisms based on the generation of lysine decarboxylase and hydrogen sulphide.
- Edwards and Fife created Lysine Iron Agar to identify lactose-fermenting Salmonellae.
- Salmonellae are known to rapidly decarboxylate lysine and generate substantial quantities of hydrogen sulphide.
- This medium is susceptible to lactose fermenting and lactose non-fermenting Salmonella species.
- Numerous bacteria in this category rapidly ferment lactose, hence inhibiting H2S generation on Triple Sugar Iron Agar (M021).
- Therefore, it is possible to overlook the organisms usually associated with food poisoning outbreaks. Thatcher and Clark described the isolation of Salmonella species from food using selective agar and inoculation on Lysine Iron Agar and Triple Sugar Iron (M021) in combination.
- Using these two media allows for stronger distinction between coliform organisms, such as E. coli. Escherichia coli and Shigella species.
- Peptone and yeast extract supply important nutrients. Dextrose is a fermentable carbohydrate source.
- The presence of ferric ammonium citrate and sodium thiosulphate indicates the production of H2S.
- The blackening of the media is caused by the synthesis of ferrous sulphide by cultures that create hydrogen sulphide.
- Lysine decarboxylation results in an alkaline reaction (purple hue) that produces the amine cadaverine, whereas organisms that do not decarboxylate lysine create acid butt (yellow colour).
- Organisms that deaminate lysine produce alpha-ketocarboxylic acid, which under the influence of oxygen combines with iron salt on the surface of the medium to form a reddish-brown product.
- The medium is pierced to the base of the butt and slanted.
Purpose of Lysine Iron Agar (LIA) Test
- This test is used to differentiate gram-negative bacilli based on decarboxylation or deamination of lysine and hydrogen sulphide production (H2S).
Principle of Lysine Iron Agar (LIA) Test
- Gelatin peptone and yeast extract provide bacterial development with nitrogen, amino acids, and vitamins.
- Dextrose is a fermentable carbohydrate source, while bromocresol purple is a pH indicator.
- As indicators, sodium thiosulfate and ferric ammonium citrate form a dark precipitate at the bottom of the tube when H2S is produced.
- Lysine serves as the substrate for lysine decarboxylase and lysine deaminase detection.
- When lysine is decarboxylated, such as in Salmonella spp., the amine changes to cadaverine, producing a purple butt (alkaline).
- When lysine is deaminated, as is the case with Proteus spp., the amine is converted to -ketocarboxylic acid and the slant turns red.
Lysine Iron Agar Composition
| Ingredients | Gms/liter |
| Peptone | 5.000 |
| Yeast extract | 3.000 |
| Dextrose (Glucose) | 1.000 |
| L-Lysine | 10.00 |
| Ferric ammonium citrate | 0.500 |
| Sodium thiosulphate | 0.040 |
| Bromocresol purple | 0.020 |
| Agar | 15.000 |
Final pH (at 25°C): 6.7±0.2
Lysine Iron Agar Preparation
- Suspend 34.56 grammes in 1000 ml purified/distilled water.
- To completely dissolve the medium, bring to a boil.
- Dispense into tubes and autoclave at 15 pounds of pressure (121 degrees Celsius) for 15 minutes.
- The tubes are cooled in an inclined position to generate slants with deep butts.
Lysine Iron Agar test Procedure
- Allow the medium to reach room temperature before inoculating.
- Collect the centre of a well-isolated colony from a pure culture plate using a straight inoculating needle.
- Inoculate the LIA Slant by piercing the centre of the tube twice to the bottom and using a fishtail motion to streak the slant.
- At 35°C, incubate the tubes.
- After 18 to 24 hours of incubation, examine tubes and analyse results.
Result of Iron Agar test Procedure
Lysine Decarboxylation (detected in butt)
- Positive Test – Purple slant/purple butt (alkaline), butt reaction could be obscured by H2S generation
- Negative Test – Purple slant/yellow butt (acid), just glucose fermentation
Lysine Deamination (detected on slant)
- Positive Test – Red slant
- Negative Test – Slant remains purple
H2S Production
- Positive Test – Black precipitate
- Negative Test – No black color development
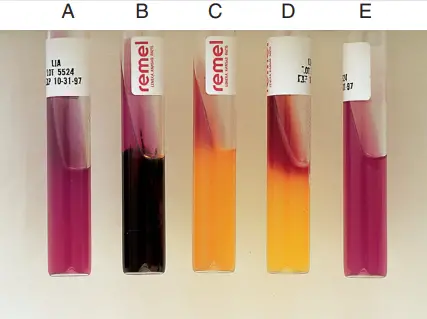
B, Alkaline slant/alkaline butt, H2 S positive (K/K H2S1). C, Alkaline slant/acid butt (K/A). D, Red slant/acid butt (R/A). E, Uninoculated tube.
Cultural characteristics after 18-24 hours at 35-37°C
| Organisms | Butt | H2S | Slant |
| Escherichia coli ATCC® 25922 | alkaline reaction, purple or no colour change | negative reaction | alkaline reaction, purple or no colour change |
| Proteus mirabilis ATCC® 12453 | acidic reaction, yellowing of the medium | positive reaction, blackening of medium | deep red,lysine deamination |
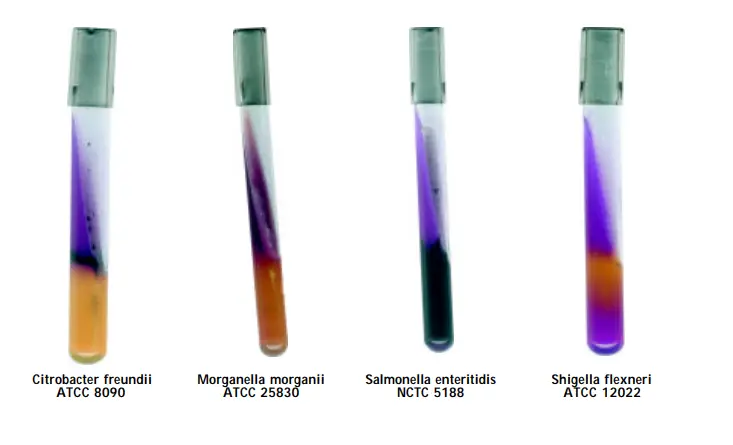
| Citrobacter freundii ATCC 8090 | acidic reaction, yellowing of the medium | positive reaction, blackening of medium | alkaline reaction, purple or no colour change |
| Salmonella Arizonae ATCC 13314 | alkaline reaction, purple or no colour change | positive reaction, blackening of medium | alkaline reaction, purple or no colour change |
| Salmonella Enteritidis ATCC 13076 (00030*) | alkaline reaction, purple or no colour change | positive reaction, blackening of medium | alkaline reaction, purple or no colour change |
| Salmonella Typhimurium ATCC 14028 (00031*) | alkaline reaction, purple or no colour change | positive reaction, blackening of medium | alkaline reaction, purple or no colour change |
| Shigella flexneri | acidic reaction, yellowing of the medium | negative reaction | alkaline reaction, purple or no colour change |
| Salmonella choleraesuis subsp. arizonae | purple | positive reaction | purple |
Characteristic reactions of some Enterobacteriaceae cultured on Lysine Iron Agar:
| Organisms | Butt | Slant surface | H2S Production |
| Arizona | violet | violet | positive |
| Salmonella (except Salm. paratyphi A; no lysine decarboxylase production, butt = yellow, slant surface violet) | violet | violet | positive |
| Proteus mirabilis (except some strains do not deaminate lysine) | yellow | red-brown | positive |
| Proteus vulgaris, Proteus morganii, Proteus rettgeri | yellow | red-brown | negative |
| Providencia | yellow | red-brown | negative |
| Citrobacter | yellow | violet | positive |
| Escherichia | yellow | violet | negative |
| Shigella | yellow | violet | negative |
| Klebsiella | violet | violet | negative |
Limitations
- Proteus spp. Those that generate hydrogen sulphide will not discolour the medium. Additional examinations, include triple sug.
- Since acid in the butt may inhibit H2S creation, species that do not make lysine decarboxylase, such as Proteus spp., may not produce H2S.
- LIA is incompatible with TSI and Moeller Decarboxylase media.
- After 24 hours of incubation, the slant reaction with Morganella morganii may be varied and may require extended incubation.
- With species other than Citrobacter spp., gas production may be erratic or diminished.
- A small precipitate may be visible on the slant prior to inoculation. This will not alter the medium’s performance.
- In contrast to other Salmonella spp., Salmonella paratyphi A does not manufacture lysine decarboxylase, resulting in an alkaline lean and an acid bend.
Quality Control
- Alkaline slant and butt: H2S positive: Citrobacter freundii (ATCC8090)
- Alkaline slant and butt: Escherichia coli (ATCC25922)
- Alkaline slant and butt: H2S positive: Salmonella typhimurium (ATCC14028)
- Red slant, acid butt: Proteus mirabilis (ATCC12453)
References
- http://www.biolab.rs/wp-content/uploads/2018/01/Lysine-Iron-LIA-Agar.pdf
- https://catalog.hardydiagnostics.com/cp_prod/product/r22-lia-lysine-iron-agar-slant-45ml-13x100mm-tube-order-by-the-package-of-20-by-hardy-diagnostics-media-prepared
- https://atlas.sund.ku.dk/microatlas/food/pheno_tests/LIA/
- https://legacy.bd.com/europe/regulatory/Assets/IFU/US/L007465(07)(0107).pdf
- https://www.nelabservices.com/pdf/tech-sheets/lysine-agar-2016.pdf
- http://faculty.collin.edu/dcain/CCCCD%20Micro/lysine_iron_agar.htm
- https://www.humeau.com/media/blfa_files/TC_Lysine-Iron-Agar_14501164054_EN_280920.pdf
- https://assets.thermofisher.com/TFS-Assets/MBD/Instructions/IFU61300.pdf
- http://www.dalynn.com/dyn/ck_assets/files/tech/TL97.pdf
- https://himedialabs.com/td/m377.pdf
- https://microbiologynote.com/lysine-iron-agar-lia-composition/
- https://assets.fishersci.com/TFS-Assets/MBD/Instructions/IFU453772.pdf
- https://microbenotes.com/lysine-iron-agar-test/


