Table of Contents
What are Natural Killer (NK) Cells?
- Natural killer (NK) cells are a subset of lymphocytes that play an important function in the immune system. They are part of the innate immune response and can target virus-infected cells as well as stressed cells like tumor cells. NK cells were previously described as bigger granular lymphocytes with tumor-killing capability. They are currently regarded as a separate lymphocyte lineage with both cytotoxic and cytokine-producing activities.
- NK cells, in contrast to B and T lymphocytes, express a limited set of receptors that are triggered by cellular damage caused by other cells. This distinct trait enables NK cells to locate and remove aberrant cells without prior activation. They are called “natural killers” because they can recognize and kill cells that lack MHC class 1 “self” signals.
- NK cells play an important role in the regulation of immunological responses and innate immunity. They release cytokines such as interferon, TNF, and chemokines, as well as other substances that influence the functioning of other immune cells. These chemicals released aid in the coordination and modulation of immune responses.
- Under normal physiological settings, NK cells are found in a variety of tissues. They can be found in the skin, uterus, kidney, joints, gastrointestinal tract, liver, and breast. NK cells account for around 5-20% of all circulating lymphocytes in the blood. Furthermore, they constitute a large proportion of lymphocytes in certain organs such as the liver and lungs.
- NK cell development includes several stages of maturation, expansion, and receptor acquisition. It was once thought that NK cells only developed in the bone marrow. Recent research has shown that they can also mature in secondary lymphoid tissues including the spleen and tonsils.
- NK cells have various surface markers that can be utilized to identify them. They are distinguished by the presence of CD56 and the lack of CD3 (CD56+, CD3). These markers enable researchers identify and study NK cells by distinguishing them from other lymphocytes.
- NK cells play a crucial part in the adaptive immune response in addition to their role in innate immunity. They may adapt to their surroundings and develop antigen-specific immunological memory. This skill is critical for dealing with secondary infections caused by the same antigen. As a result, NK cell activity is being investigated as a potential cancer and other disease therapy.
- To summarize, Natural Killer (NK) cells are a distinct subset of lymphocytes that have both cytotoxic and cytokine-producing abilities. They have the ability to identify and remove virus-infected and stressed cells, including tumor cells. NK cells are essential for innate immunity and immune response coordination. Their distribution spans multiple tissues, and their maturation and receptor acquisition occur at various times. Understanding the actions and properties of NK cells is critical for furthering immunology research and creating possible therapeutics.
Definition of NK Cells
NK cells, short for Natural Killer cells, are a type of lymphocyte that is part of the innate immune system. They have cytotoxic activity against virus-infected cells and stressed cells like tumor cells. NK cells can recognize and eliminate abnormal cells without the need for prior activation and play a crucial role in immune responses and innate immunity.
Properties of natural killer cells
Natural Killer (NK) cells possess several distinctive properties that set them apart from other lymphocytes:
- Large lymphocytes with granular structures: NK cells are characterized by their larger size compared to other lymphocytes, and they contain granules within their cytoplasm. These granules play a role in their cytotoxic activity against target cells.
- Lack of certain surface proteins: NK cells do not express T-cell receptor (TCR) or CD3 proteins, which are typically found on T lymphocytes. Additionally, they lack surface immunoglobulin M (IgM) and IgD, which are present on B lymphocytes. These differences in surface protein expression distinguish NK cells from other lymphocyte subsets.
- Lack of prior exposure effect: Unlike adaptive immune cells, NK cells do not require prior exposure to antigens to exert their activity. They are capable of recognizing and targeting abnormal cells, including virus-infected cells and tumor cells, without the need for prior sensitization. This property allows NK cells to mount a rapid immune response.
- Independence from the thymus: Unlike T lymphocytes, NK cells do not require the thymus for their development and maturation. The thymus is an organ involved in the production and selection of T lymphocytes. NK cells can develop and function normally even in individuals with thymus-related conditions.
- Normal numbers in severe combined immunodeficiency (SCID): SCID is a group of rare genetic disorders characterized by severe immune dysfunction. Interestingly, despite the overall compromised immune system in individuals with SCID, the number of NK cells remains normal. This suggests that NK cell development and maintenance are not dependent on the same factors that are disrupted in SCID.
Overall, NK cells possess unique properties that distinguish them from other lymphocytes. These characteristics contribute to their role as important effectors of the innate immune system and their ability to target and eliminate abnormal cells in various immune responses.
NK Cells and T Cells Share Some Features
NK cells and T cells, despite their distinct characteristics and functions, share some similarities and features:
- Common progenitor and lineage: Both NK cells and T cells originate from a common early progenitor in the bone marrow. Their precise lineage relationship is still being investigated, but it is clear that they share a developmental connection.
- Expression of certain membrane markers: NK cells express a combination of membrane markers that are characteristic of monocytes, granulocytes, and T cells. This overlap in marker expression suggests some shared molecular features between NK cells and T cells.
- Expression of specific membrane molecules: NK cells express unique sets of membrane-bound molecules. One example is CD2, which is also expressed by T cells and is involved in cell adhesion and activation. Another molecule is CD16 (or FcRIII), a receptor for the Fc portion of IgG. The expression of CD16 is nearly universal on NK cells and plays a role in their cytotoxic activity.
- Functional dependence on CD16: CD16 is crucial for NK cell activity, as depletion of CD16 using monoclonal antibodies significantly reduces NK cell function in peripheral blood. This indicates the importance of CD16-mediated signaling in NK cell activation and effector functions.
- Development independent of the thymus and gene rearrangement: Unlike T cells, NK cells do not primarily develop in the thymus. NK cell populations can be found and function in nude mice that lack a thymus and have few or no T cells. Furthermore, NK cells do not undergo receptor gene rearrangement, which is a characteristic feature of T and B cells. This is demonstrated by the development of NK cells in mice lacking the recombinase genes RAG-1 or RAG-2.
- Functional presence in SCID mice: SCID mice lack functional T and B cells due to immune deficiencies. However, these mice still possess functioning populations of NK cells. This highlights the capacity of NK cells and other components of the innate immune defense system to protect animals in the absence of adaptive immunity.
In summary, while NK cells and T cells have distinct features and functions, they share certain characteristics, including a common progenitor, overlapping membrane marker expression, dependence on specific membrane molecules, and the ability to function independently of the thymus and gene rearrangement. These shared features contribute to our understanding of the immune system’s complexity and its ability to mount effective responses against pathogens and abnormal cells.
Structure of Natural Killer (NK) Cells
The structure of Natural Killer (NK) cells exhibits several distinctive characteristics:
- Large and granular lymphocytes: NK cells are relatively larger in diameter compared to other similar lymphocytes. They have a granular cytoplasm filled with azurophilic granules. These granules contain hydrolytic and digestive enzymes, which play a role in the cytotoxic activity of NK cells.
- Absence of certain receptors: Unlike T and B cells, NK cells lack receptors typically found on other lymphocytes. This sets them apart and distinguishes their function and activation mechanisms from those of T and B cells.
- Percentage in peripheral circulating lymphocytes: NK cells constitute approximately 5% of the total circulating lymphocytes in the peripheral blood. Their presence can be observed through staining techniques like Wright-Giemsa staining.
- Variable shapes and microvilli: NK cells exist in various shapes and may express microvilli, particularly in the region where they come into contact with target cells. These surface structures aid in cell-to-cell interactions and target recognition.
- Granule compartments: The granules within NK cells consist of two distinct compartments. The outer compartment contains lysosome-associated acid enzymes and trumetaphosphatase. The inner compartment consists of structural components and does not possess enzymatic activity.
- Degranulation and vacuole formation: During the process of degranulation, the cytoplasm of NK cells forms multiple vacuole-like areas that contain granules and some granular debris. This is a part of the cytotoxic response of NK cells.
- Presence of cytoplasmic organelles: NK cells also contain other cytoplasmic organelles, such as mitochondria and polysomes, which are involved in cellular functions and protein synthesis.
- Nucleus and polarity: The nucleus of NK cells appears convoluted and exhibits distinct polarity in relation to the dense granules and pseudopodia. This structural arrangement is related to the movement and functionality of NK cells.
- Surface receptors: The surface of NK cells is characterized by the presence of different activating and inhibitory receptors. These receptors recognize various membrane proteins on target cells and play a crucial role in the activation and regulation of NK cell responses.
In summary, the structure of NK cells reflects their unique characteristics as large, granular lymphocytes with specific cytoplasmic components, distinctive organelles, and surface receptors that contribute to their cytotoxic and immunomodulatory functions.
Natural killer (NK) cell receptors
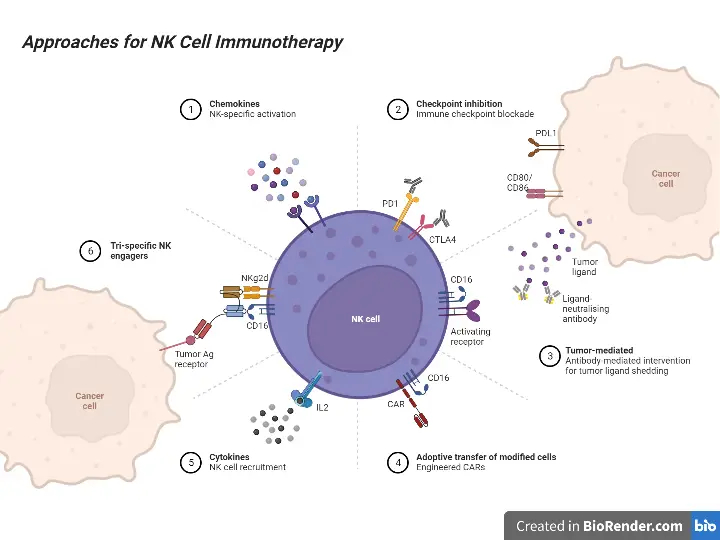
NK cell receptors can also be distinguished according to their function. Natural cytotoxicity receptors induce apoptosis (cell death) directly after binding to Fas ligands, which indicate infection of a cell. The MHC-independent receptors (described above) induce apoptosis in infected cells via an alternative pathway. The activation of natural killer cells is determined by the relative stimulation of inhibitory and activating receptors. If the inhibitory receptor signaling is more prominent, for example, NK cell activity will be inhibited; if the activating signal is more prominent, NK cell activity will be activated.
NK cell receptor types (including inhibitory and some activating members) are distinguished by their structures, as illustrated by the following examples:
Activating receptors
Activating receptors on natural killer (NK) cells play a crucial role in initiating their effector functions and immune responses. Here are some key activating receptors:
- Ly49 receptors: Ly49 receptors are relatively ancient, C-type lectin family receptors. In mice, they exist in a multigenic form, whereas humans have only one pseudogenic Ly49 receptor. These receptors specifically bind to classical (polymorphic) major histocompatibility complex class I (MHC I) molecules.
- NCR (natural cytotoxicity receptors): NCRs are type 1 transmembrane proteins of the immunoglobulin superfamily. Upon stimulation, NCRs mediate NK cell killing and the release of interferon-gamma (IFNγ). They can bind viral ligands such as hemagglutinins and hemagglutinin neuraminidases, as well as certain bacterial and cellular ligands associated with tumor growth, such as PCNA.
- CD16 (FcγIIIA): CD16 is involved in antibody-dependent cell-mediated cytotoxicity. These receptors specifically bind to the Fc portion of immunoglobulin G (IgG) antibodies, allowing NK cells to recognize and kill antibody-coated target cells.
- TLR (Toll-like receptors): Toll-like receptors are a group of receptors belonging to the pattern recognition receptor (PRR) family, which are typically expressed on cells of the innate immune system, including NK cells. TLRs recognize specific molecular patterns associated with pathogens (PAMPs) and tissue damage (DAMPs). Activation of TLRs on NK cells induces the production of inflammatory cytokines and chemokines, promoting immune responses. NK cells express various TLRs, such as TLR-1, TLR-2, TLR-3, TLR-4, TLR-5, TLR-7, TLR-8, TLR-9, and TLR-10, albeit at different levels. TLR signaling involves adaptor proteins, such as MyD88 and TRIF, leading to the activation of NF-κB and distinct activation of NK cell functions.
These activating receptors on NK cells enable them to recognize and respond to specific ligands associated with infected or stressed cells, tumor cells, and immune complexes, ultimately triggering their cytotoxicity, cytokine release, and immune activation.
Inhibitory receptors
Inhibitory receptors play a critical role in regulating the activity of natural killer (NK) cells and preventing them from attacking healthy cells. Here are some important inhibitory receptors:
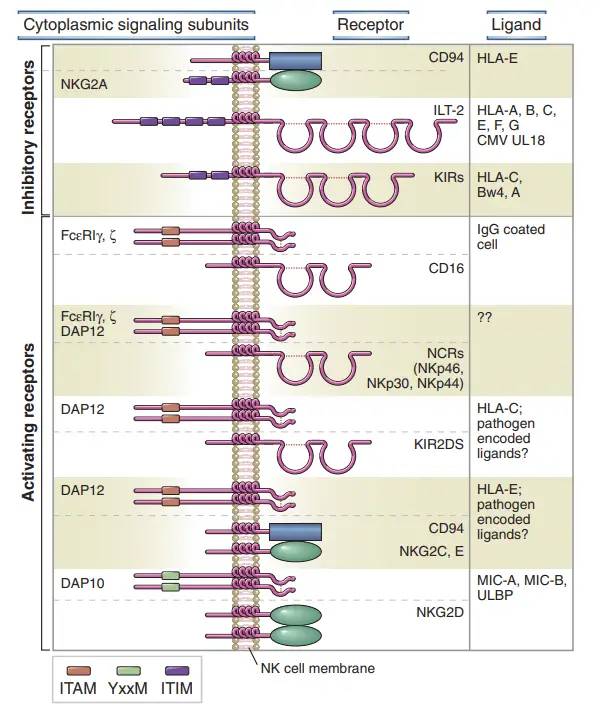
- Killer-cell immunoglobulin-like receptors (KIRs): KIRs belong to a multigene family of immunoglobulin-like receptors. They are present in nonhuman primates and are the main receptors for classical major histocompatibility complex class I (MHC I) molecules (HLA-A, HLA-B, HLA-C) and nonclassical MHC I molecules (Mamu-G) in primates. Some KIRs are specific for certain HLA subtypes. Most KIRs are inhibitory, meaning they dampen NK cell activity. When regular cells express MHC class I molecules on their surface, these molecules are recognized by KIR receptors, leading to the inhibition of NK cell killing.
- CD94/NKG2: CD94/NKG2 is a heterodimeric receptor belonging to the C-type lectin family. It is conserved in both rodents and primates and identifies nonclassical MHC I molecules, such as HLA-E. The expression of HLA-E on the cell surface depends on the presence of a nonamer peptide epitope derived from the signal sequence of classical MHC class I molecules. CD94/NKG2 indirectly monitors the levels of classical (polymorphic) HLA molecules and contributes to the regulation of NK cell activity.
- ILT or LIR (immunoglobulin-like receptor): ILT or LIR receptors are a recently discovered group of receptors belonging to the immunoglobulin receptor family. They also have inhibitory functions and help regulate NK cell activity.
- Ly49 receptors: Ly49 receptors are homodimeric receptors that have both activating and inhibitory isoforms. They are highly polymorphic and functionally analogous to KIRs in mice. While structurally unrelated to KIRs, they share a similar expression pattern and serve as receptors for classical (polymorphic) MHC I molecules.
These inhibitory receptors recognize specific ligands, such as MHC I molecules, and deliver inhibitory signals to NK cells, preventing them from attacking normal cells that express sufficient levels of MHC I. The balance between activating and inhibitory signals received by NK cells through these receptors is crucial for maintaining self-tolerance and preventing autoimmunity.
| Receptor | Type | Ligands | Function |
|---|---|---|---|
| Activating Receptors | |||
| Ly49 | C-type lectin | Unknown | Mediate NK killing and IFNγ release |
| NCR (natural cytotoxicity receptors) | Type 1 transmembrane proteins | Viral ligands, bacterial ligands, cellular ligands related to tumor growth | Mediate NK killing and release of IFNγ |
| CD16 (FcγIIIA) | Fc receptor | Immunoglobulin G (IgG) | Role in antibody-dependent cell-mediated cytotoxicity |
| TLR (Toll-like receptors) | Pattern recognition receptors | PAMPs, DAMPs | Induce immune response and activate NK cell functions |
| Inhibitory Receptors | |||
| Killer-cell immunoglobulin-like receptors (KIRs) | Immunoglobulin-like receptors | Classical MHC class I, nonclassical MHC I | Inhibit NK cell killing when regular cells express MHC class I |
| CD94/NKG2 | C-type lectin | Nonclassical MHC I (HLA-E) | Indirectly monitor levels of classical MHC molecules |
| ILT or LIR (immunoglobulin-like receptor) | Immunoglobulin-like receptors | Unknown | Regulate NK cell activity |
| Ly49 | C-type lectin | Classical MHC class I molecules | Both activating and inhibitory isoforms |
These receptors play important roles in modulating NK cell activity by either activating or inhibiting their functions. The activating receptors recognize specific ligands, such as viral or bacterial ligands, and trigger NK cell killing and release of cytokines. In contrast, the inhibitory receptors, such as KIRs and CD94/NKG2, recognize MHC class I molecules and deliver inhibitory signals to prevent NK cells from attacking healthy cells. The balance between activating and inhibitory signals received through these receptors is crucial for proper immune regulation and self-tolerance.
Recognition of Infected and Stressed Cells by NK Cells
Distinguish infected and stressed cells
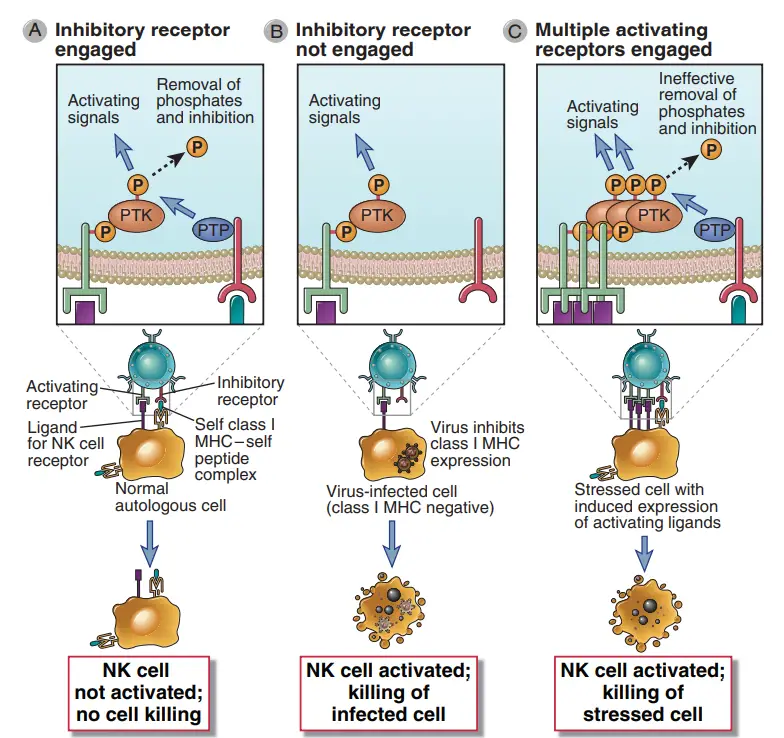
- NK cells discriminate infected and stressed cells from healthy cells, and NK cell activation is regulated by a balance of activating and inhibiting receptor signals.
- Multiple families of these receptors exist. These receptors identify chemicals on the surface of other cells and provide activating or inhibiting signals that stimulate or suppress NK responses, respectively.
- In general, activating receptors identify ligands on infected and damaged cells, whereas inhibitory receptors identify healthy normal cells.
- The outcome of an interaction between an NK cell and another cell is controlled by the integration of signals created by the NK cell’s array of inhibitory and activating receptors, which interact with ligands on the other cell.
- Due to the stochastic nature of their production, the spectrum of activating and inhibiting receptors expressed by various NK cells in a given individual is quite diverse.
- Consequently, an individual’s NK cells will react to various types of bacteria or infected cells.
- In addition, the genes encoding the majority of these receptors are polymorphic, meaning that there are multiple variants of the genes in the population, so that each individual expresses a slightly different form of the receptors.
Expression of inhibitory receptors
- The majority of natural killer (NK) cells carry inhibitory receptors that detect class I major histocompatibility complex (MHC) molecules, which are ordinarily found on the cell surface of virtually all healthy cells in the body.
- In addition to their involvement in regulating NK cell activation, class I MHC molecules show on the cell surface peptides originating from cytoplasmic proteins, including microbial proteins, for identification by CD8+ T lymphocytes.
- For the time being, it is essential to realise that NK cells and T cells use fundamentally different types of receptors to recognise class I MHC molecules. In contrast to T cells, the majority of NK receptors for class I MHC suppress NK activation.
- This is advantageous because normal cells express class I MHC molecules, but many viruses and other causes of cell stress inhibit class I MHC expression on the cell surface.
- Consequently, NK cells perceive the presence of class I MHC molecules as indicators of a normal, healthy self, whereas their absence indicates infection or harm.
- Infected or stressed cells will not transmit inhibitory signals to NK cells. NK cells are also likely to receive activating signals via activating receptors from the same infected cells.
- In the end, the NK cell will be activated to emit cytokines and kill the infected or stressed cell. This capacity of NK cells to become activated by host cells lacking class I MHC is referred to as “recognition of missing self.”
Immunoreceptor tyrosine-based inhibition motif (ITIM)
- Immunoreceptor tyrosine-based inhibition motif (ITIM) is a structural motif in the cytoplasmic tails of NK cell inhibitory receptors that interacts with substances that impede the signalling pathways of activating receptors.
- ITIMs contain phosphorylated tyrosine residues upon ligand interaction to the inhibitory receptor. This results in the recruitment and activation of phosphatases, which remove phosphates from a number of signalling proteins or lipids produced by signalling pathways downstream of NK activating receptors.
- In the end, the signalling functions of activating receptors are inhibited. ITIMs are located in the cytoplasmic tails of receptors other than NK inhibitory receptors.
- The main group of NK inhibitory receptors is composed of killer cell immunoglobulin-like receptors (KIRs), which are immunoglobulin (Ig) superfamily members.
- Antibody (also known as Ig) molecules were the first to possess a structural domain called an Ig fold, which is shared by all members of this family. KIRs bind diverse molecules of class I MHC.
- As noted previously, a second major group of NK inhibitory receptors belongs to the C-type lectin family, which contains proteins with carbohydrate-binding capabilities.
- One of these receptors is the heterodimer CD94/NKG2A, which detects the HLA-E molecule of class I MHC. Intriguingly, HLA-E shows peptides generated from other class I MHC molecules, so CD94/NKG2A is essentially a surveillance receptor for numerous class I MHC molecules.
- Leukocyte Ig-like receptors (LIRs), a third family of NK inhibitory receptors, are likewise Ig superfamily members that bind class I MHC molecules, albeit with lesser affinity than the KIRs, and are more abundantly expressed on B cells than NK cells.
Recognition of a heterogeneous group of ligands
- Activating receptors on NK cells identify a heterogeneous array of ligands, some of which may be expressed on normal cells and others of which are produced primarily on stressed, infected, or altered cells.
- For several of these receptors, the molecular characteristics of the ligands are inadequately defined.
- The enhanced expression of ligands on unhealthy cells that bind to activating receptors on NK cells may result in signals that overwhelm the signals from inhibitory receptors, particularly if class I MHC is also diminished or absent from the unhealthy cell.
Promote target cell killing and cytokine secretion
- The majority of activating NK receptors share a structural motif in their cytoplasmic tails known as an immunoreceptor tyrosine-based activation motif (ITAM) that engages in signalling processes that boost target cell death and cytokine production.
- In some of these receptors, the ITAM as well as the extracellular ligand-binding region are included on a single polypeptide chain.
- In other receptors, such as FcεRIγ, ζ,, and DAP12, the ITAMs are in distinct polypeptide chains that do not bind ligand but are noncovalently connected with the ligand-binding chain.
- ITAMs are also found in the cytoplasmic tails of other multichain immunological signalling receptors, such as the antigen receptors on T and B cells.
- After ligand attachment to NK cell activating receptors, cytoplasmic kinases phosphorylate tyrosine residues inside the ITAMs, other protein kinases are attracted to the changed ITAMs and activated, and these kinases contribute to further signalling by phosphorylating other proteins.
- As previously noted, numerous NK cell activating receptors are members of the C-type lectin or KIR families, which also contain inhibitory receptors. Some of the activating receptors appear to bind class I MHC molecules, similar to the inhibitory receptors, although it is unknown how infected or injured cells selectively activate these receptors.
How NK Cells Kill infected cells and phagocytosed microbes?
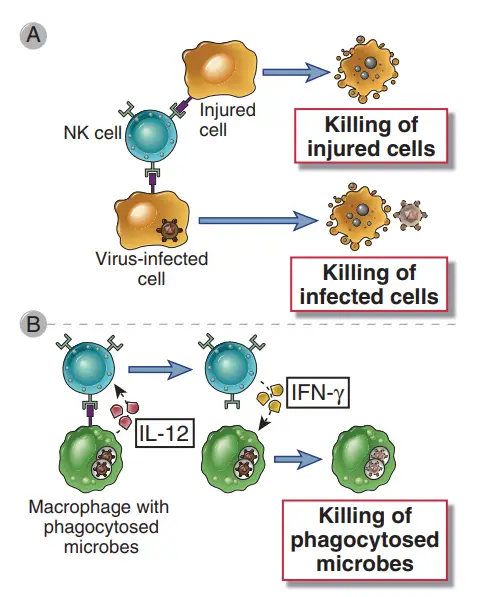
- NK cells have the effector activities of destroying infected cells and activating macrophages to eliminate phagocytosed microorganisms.
- The NK cell–mediated cytotoxic mechanism is largely identical to that of CD8+ CTLs.
- Similar to CTLs, NK cells contain granules carrying substances that facilitate the death of target cells. Granule exocytosis releases these proteins near to the target cells when NK cells are activated.
- Perforin, a granule protein of NK cells, enhances the entry of granzymes, another granule protein, into the cytoplasm of target cells. Granzymes are enzymes that trigger a series of signalling events that result in the apoptotic death of target cells.
- The signalling pathways responsible for apoptosis. By destroying infected viral and intracellular bacterial cells, NK cells eradicate infection reservoirs.
- Some cancers, particularly those of hematopoietic origin, are targets for natural killer (NK) cells, possibly because tumour cells do not exhibit typical quantities or types of class I MHC molecules.
- Similarly to IFN- produced by T cells, IFN- produced by NK cells activates macrophages and enhances their ability to destroy phagocytosed bacteria.
- IFN- generated by NK cells in lymph nodes can also direct naive T cells to differentiate into TH1 cells.
- NK cells play various critical roles in defence against intracellular pathogens. They eliminate virally infected cells before antigen-specific CTLs may become completely active, i.e., within the initial few days of viral infection.
- Early in the course of a viral infection, IL-12 and IL-15 stimulate the expansion and activation of NK cells, which then destroy infected cells, particularly those with diminished amounts of class I MHC molecules.
- Moreover, NK cell-secreted IFN-γ stimulates macrophages to kill phagocytosed microorganisms. This IFN-γ–dependent NK cell–macrophage response can control an intracellular bacterial infection, such as Listeria monocytogenes, for several days or weeks, allowing T cell–mediated immunity to develop and remove the infection.
- NK cell depletion increases vulnerability to infection by some viruses and intracellular microorganisms. In the absence of T cell–mediated immunity, the NK cell response of T-cell-deficient mice may be adequate to control infection with such microorganisms for a time, but the animals eventually perish.
- By eliminating infected cells that have evaded CTL-mediated immune attack by lowering production of class I MHC molecules, NK cells may also play a crucial role later in the body’s response to infection.
- Because NK cells are capable of killing specific tumour cells in vitro, it has been hypothesised that they also serve to eliminate malignant clones in vivo.
How do NK Cells work against pathogens? (Immunity)
1. Cytotoxic Immune Response
The cytotoxic immune response mediated by natural killer (NK) cells involves several steps and mechanisms to eliminate target cells. Here is a description of the cytotoxic response of NK cells:
- Degranulation: NK cells undergo degranulation, which involves the release of cytotoxic molecules stored in their granules, such as perforin and granzymes. During this process, proteins like lysosomal-associated membrane protein-1 and -2 (LAMP-1 and LAMP-2) are expressed on the surface of NK cells.
- Perforin-mediated pore formation: Released perforin molecules polymerize and form pores on the surface of target cells. These pores allow the entry of granzymes into the target cells.
- Granzyme-induced apoptosis: Granzymes are serine proteases that enter the target cells through the perforin pores. Once inside, granzymes can activate caspase molecules, initiating a cascade of events that leads to the induction of apoptosis in the target cells.
The process of degranulation can be further divided into four steps:
- Formation of immunological synapse: The target cell and NK cell form an immunological synapse, which involves the interaction of specific receptors and ligands. This leads to the reorganization of the actin cytoskeleton.
- Polarization of microtubule-organizing center: The microtubule-organizing center of the NK cell polarizes towards the lytic synapse, the region of contact with the target cell.
- Docking of secretory lysosome: The secretory lysosome, containing cytotoxic molecules, docks with the plasma membrane of the NK cell at the lytic synapse.
- Fusion of secretory lysosome: The secretory lysosome fuses with the plasma membrane of the target cell, releasing its contents, including perforin and granzymes, into the target cell.
Apart from the degranulation process, NK cells can also induce target cell apoptosis through death receptor-mediated pathways. NK cells express TNF receptor ligands that can bind to their corresponding receptors on the target cells. This binding activates the death receptors, leading to conformational changes and the recruitment of adaptor proteins responsible for initiating apoptosis in the target cells.
Overall, the cytotoxic immune response of NK cells involves degranulation, perforin-mediated pore formation, granzyme-induced apoptosis, and death receptor-induced target cell apoptosis. These mechanisms enable NK cells to eliminate target cells and contribute to immune defense against infections and tumor development.
2. Effector Immune Response of NK cells
The effector immune response of natural killer (NK) cells involves the release of various cytokines and chemokines, which play a crucial role in antiviral, antibacterial, and antitumor activities. Here is an overview of the effector immune response of NK cells:
- Cytokine release: Activated NK cells release a variety of cytokines, including interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin-10 (IL-10), interleukin-5 (IL-5), and interleukin-13 (IL-13). These cytokines have diverse functions and contribute to the immune response against pathogens and tumors.
- IFN-γ modulation: IFN-γ produced by NK cells plays a crucial role in modulating the immune response. It can influence the expression of caspase, Fas ligand (FasL), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which are involved in the activation of antitumor activity. By promoting apoptosis of tumor cells, IFN-γ enhances the cytotoxic immune response of NK cells.
- IL-8 and tumor regression: IL-8, a chemokine released by NK cells, has been found to contribute to the regression of melanoma tumors. It inhibits tumor metastasis in a perforin-dependent manner, suggesting its involvement in the suppression of tumor growth and spread.
The effector immune response of NK cells is not solely dependent on their cytotoxic activity but is also influenced by the cytokines and chemokines released by other immune cells and stromal cells in the tumor microenvironment. These factors enhance the overall immune response against pathogens and tumors, contributing to the elimination of infected or malignant cells.
3. Inhibitory action
- NK cells possess inhibitory receptors that play a crucial role in preventing the inappropriate killing of normal cells in the body. These inhibitory receptors recognize specific membrane proteins expressed on normal cells and dampen the cytotoxic activity of NK cells toward these cells. This inhibitory action is essential to maintain immune balance and prevent autoimmunity.
- The inhibitory receptors on NK cells function by transmitting inhibitory signals upon binding to their corresponding ligands on normal cells. These ligands are often molecules involved in cell surface recognition and self-recognition, such as major histocompatibility complex class I (MHC-I) molecules. Normal cells express MHC-I molecules on their surface, which serve as a marker of self-identity.
- When an NK cell encounters a normal cell expressing MHC-I molecules, the inhibitory receptor on the NK cell recognizes and binds to the MHC-I molecules. This interaction triggers inhibitory signaling pathways within the NK cell, leading to the suppression of cytotoxic activity against the normal cell. By doing so, NK cells avoid attacking healthy cells and limit their cytotoxic responses to infected or cancerous cells that may have reduced or altered MHC-I expression.
- The inhibitory action of NK cells is crucial for maintaining immune tolerance and preventing autoimmune reactions. It ensures that NK cells selectively target cells that exhibit signs of infection, transformation, or stress, while sparing normal cells from unnecessary damage. This balance between activating and inhibitory signals allows NK cells to provide an appropriate immune response, eliminating threats without causing harm to healthy tissues.
- In summary, NK cells possess inhibitory receptors that recognize specific membrane proteins, particularly MHC-I molecules, on normal cells. These inhibitory receptors transmit signals that inhibit NK-mediated cytotoxic killing of normal cells. This mechanism is essential for preventing autoimmunity and maintaining immune homeostasis by allowing NK cells to distinguish between healthy cells and those requiring immune attack.
Functions of Natural Killer (NK) Cells
Natural Killer (NK) cells perform various functions that contribute to the immune response. Some of the key functions of NK cells are as follows:
- Cytolytic Activity: NK cells exhibit cytolytic activity against target cells. They release granules containing cytotoxic molecules, such as perforin and granzymes, which can induce target cell death through apoptosis or lysis.
- Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC): NK cells can participate in ADCC, where they recognize and bind to target cells that are coated with antibodies. Through the interaction of their Fc receptors, particularly CD16, with the Fc portion of antibodies, NK cells can initiate the killing of antibody-bound target cells.
- Activation by Cytokines: NK cells can be activated by cytokines, such as interferons, released by cells undergoing viral infection or stress. This activation enhances the cytotoxic potential of NK cells and promotes their interaction with other immune cells, such as cytotoxic T cells and macrophages.
- Rapid Response to Antigens: Unlike many other immune cells, NK cells lack antigen-specific receptors and can recognize and respond to antigens without prior exposure. This allows NK cells to mount an immediate response against infected or transformed cells, contributing to the early defense against pathogens.
- Immunological Surveillance of Tumor Cells: NK cells play a crucial role in immunosurveillance, monitoring the presence of tumor cells. They can directly induce cell death in tumor cells, even in the absence of specific adhesion molecules or antigenic recognition. This function helps in controlling tumor growth and preventing the spread of cancer.
- Clearance of Dead and Senescent Cells: NK cells, along with other cells of the innate immune system such as macrophages, participate in the clearance of dead cells and senescent cells. They recognize and eliminate these cells, contributing to tissue homeostasis and removal of potentially harmful cellular debris.
- Adaptive Features: While traditionally considered part of the innate immune system, recent studies have revealed adaptive features of NK cells. They can exhibit expansion of specific subsets, increased longevity, and a more potent response upon subsequent exposures. This adaptive behavior enhances their ability to respond to recurring infections or challenges.
- Uterine NK Cells: Uterine NK cells are a specialized subset of NK cells found in the uterus during pregnancy. They have lower cytotoxicity compared to peripheral NK cells and play a critical role in establishing and maintaining successful pregnancy. Uterine NK cells help modulate the maternal immune response to prevent rejection of the fetal tissue.
In summary, NK cells perform diverse functions in immune responses, including cytolytic activity, ADCC, cytokine-mediated activation, rapid response to antigens, immunosurveillance of tumor cells, clearance of dead and senescent cells, adaptive features, and specialized roles in reproductive biology. These functions highlight the importance of NK cells in immune defense, tissue homeostasis, and reproductive processes.
Applications of Natural Killer (NK) Cells
Natural Killer (NK) cells have several applications in the field of immunotherapy and cancer treatment. Here are some of the key applications of NK cells:
- Anticancer Therapy: NK cells have shown promise in targeting and killing cancer cells. Tumor-infiltrating NK cells have been found to play a critical role in promoting cell death in certain types of cancers, such as triple-negative breast cancer. To harness the therapeutic potential of NK cells, researchers are exploring the use of allogeneic NK cell infusions from peripheral blood. These cells need to be purified and expanded in culture before infusion into patients.
- CAR-NK Cells: Similar to CAR T cells, CAR-NK cells are genetically modified NK cells that express chimeric antigen receptors (CARs). CARs enhance the binding of NK cells to specific cell surface antigens, enabling them to recognize and kill cancer cells more effectively. CAR-NK cells have several advantages over CAR T cells, including the ability to use allogeneic cells and the absence of graft-versus-host disease (GvHD) risk. They also retain the expression of NK cell activating receptors, allowing them to target tumor cells even when the target antigen expression is downregulated.
- NK-92 Cells: NK-92 cells are a highly active NK cell line that can be expanded in large numbers for clinical use. These cells exhibit broad and potent cytotoxicity against tumor cells. Clinical studies have demonstrated the safety and anti-tumor activity of NK-92 cells in patients with various types of cancer, including lung cancer, pancreatic cancer, melanoma, and lymphoma. Efforts are being made to engineer NK-92 cells to eliminate the need for irradiation before infusion and enhance their therapeutic potential.
- NKG2D-Fc Fusion Protein: NKG2D is an activating receptor present on NK cells that can recognize stress-induced ligands on cancer cells. Researchers have fused NKG2D with a stimulatory Fc portion of an antibody to create the NKG2D-Fc fusion protein. This fusion protein has shown promising results in reducing tumor growth and prolonging survival in animal models of lymphomas and Hodgkin lymphoma.
- TLR Ligands: Toll-like receptor (TLR) ligands can effectively activate NK cell effector functions and enhance their anti-tumor immune response. Combining TLR ligands with other therapeutic agents, such as monoclonal antibodies, can enhance the efficacy of NK cell-mediated immunotherapy. For example, combining TLR ligands with trastuzumab, an antibody used in the treatment of HER2+ breast cancer, has shown improved anti-tumor effects.
These are some of the notable applications of NK cells in cancer therapy and immunotherapy. Further research and clinical trials are underway to explore the full potential of NK cells and optimize their use in various disease settings.
New findings on Natural Killer (NK) Cells
New findings on Natural Killer (NK) cells have provided valuable insights into their functions and potential applications in immunotherapy. Some of the recent discoveries include:
- Recognition of Tumor Cells: NK cells can identify and eliminate tumor cells, even when they lack traditional tumor antigens. This is because NK cells can detect changes in the surface proteins of tumor cells, such as those induced by stress or damage. This broadens the scope of tumor recognition by NK cells.
- Activation Mechanisms: NK cells can be activated through various signals, including cytokines, stress molecules, and physical contact with other cells. This adaptability enables NK cells to respond to a wide range of threats and enhances their versatility as immune effectors.
- Laboratory Production and Expansion: NK cells can be generated and expanded in laboratory settings, making them potential candidates for cancer immunotherapy. This capability allows for the development of NK cell-based therapies that can be tailored to individual patients.
- Involvement in Autoimmune Diseases: NK cells have been found to contribute to autoimmune diseases like rheumatoid arthritis. They can release inflammatory molecules that lead to tissue damage in healthy cells. Understanding this role is crucial for developing targeted therapies to regulate NK cell activity in autoimmune disorders.
Specific recent findings include:
- Mechanosurveillance: A 2022 study published in Nature revealed that NK cells possess the ability to kill tumor cells through mechanosurveillance. They can sense physical properties, such as stiffness, of tumor cells and employ this information to eliminate them.
- Gut Microbiota Influence: A 2022 study in Science Immunology demonstrated that NK cells can be activated by butyrate, a molecule produced by the gut microbiota. This discovery suggests a potential role of the gut microbiome in regulating NK cell activity.
- CAR-NK Cells: A 2022 study published in Cell highlighted the engineering of NK cells to express chimeric antigen receptors (CARs). CARs are proteins that enable targeted recognition of specific tumor cells. This finding opens up possibilities for developing CAR-based cancer therapies utilizing NK cells.
These findings contribute to our growing knowledge of NK cells and pave the way for the development of novel NK cell-based therapies for cancer and other diseases. As research continues, further breakthroughs in NK cell research can be expected, leading to more effective treatment options in the future.
Natural killer (NK) cells Summary
- Immunologists found natural killer cells by accident while assessing the in vitro activity of tumor-specific cells isolated from animals with tumours.
- Mice that had not been inoculated and mice with unrelated tumours served as negative controls.
- To the investigators’ consternation, the controls also demonstrated considerable tumour cell lysis. Characterization revealed that a population of big granular lymphocytes was responsible for this nonspecific tumour cell death.
- The cells, dubbed natural killer (NK) cells due to their nonspecific cytotoxicity, account for 5–10% of the recirculating lymphocyte population.
- These cells contribute to the immune system’s defence against viruses and malignancies. Due to the fact that NK cells produce a variety of immunologically significant cytokines, they play crucial functions in immune regulation and have a significant impact on both innate and adaptive immunity.
- Specifically, IFN-γ production by NK cells can influence macrophage participation in innate immunity by stimulating phagocytic and microbicidal activities.
- IFN-γ generated from NK cells can affect the TH1 vs TH2 commitment of helper T cell populations by inhibiting TH2 expansion and stimulating TH1 development via macrophage and dendritic cell production of IL-12.
- The Chediak-Higashi syndrome described in the Clinical Focus exemplifies the catastrophic effects of an NK cell deficiency.
- NK cells participate in the first immune response to some viruses and intracellular bacteria. IFN-, IFN-, and IL-12 each promote NK activity.
- In the course of a viral infection, the concentration of these cytokines increases rapidly, followed by a wave of NK cells that reaches its peak in around three days.
- NK cells are the initial line of defence against virus infection, limiting viral replication for approximately 7 days while CTL-P cells undergo activation, proliferation, and differentiation into functional CTLs.
- A young woman who was completely devoid of NK cells exemplifies the significance of these cells in the fight against viral infections. Despite having normal T- and B-cell counts, this patient suffered from severe varicella virus and life-threatening CMV infections.
Natural killer cells vs Cytotoxic t cells
Natural killer (NK) cells and cytotoxic T cells (CTLs) are two types of immune cells that play important roles in defending the body against infections and cancer. While both NK cells and CTLs are capable of killing infected or abnormal cells, there are some key differences between the two cell types.
- Origin and Development: NK cells are a type of lymphocyte that originates from the bone marrow and matures in the peripheral blood and lymphoid tissues. In contrast, CTLs originate from T cells and mature in the thymus.
- Cell Surface Receptors: NK cells are characterized by the presence of surface receptors that allow them to recognize and kill infected or abnormal cells without prior exposure. In contrast, CTLs require prior exposure to specific antigens before they can recognize and kill infected or abnormal cells.
- Specificity: NK cells have a broad specificity and can recognize and kill a wide variety of target cells, including virus-infected cells and cancer cells. In contrast, CTLs have a narrow specificity and can only recognize and kill cells that present a specific antigen.
- Killing Mechanism: NK cells use a variety of mechanisms to kill target cells, including the release of cytotoxic granules and the production of cytokines. In contrast, CTLs use cytotoxic granules that contain molecules like perforin and granzyme to induce cell death in target cells.
- Role in Immune Response: NK cells are an important part of the innate immune system and are involved in the early defense against infections and cancer. In contrast, CTLs are part of the adaptive immune system and are involved in the elimination of specific pathogens or abnormal cells.
In summary, while both NK cells and CTLs are capable of killing infected or abnormal cells, they differ in their origin and development, cell surface receptors, specificity, killing mechanisms, and role in the immune response.
What kills cancer cells in the body naturally?
The body has several natural mechanisms to kill cancer cells, including:
- Natural Killer (NK) Cells: NK cells are a type of white blood cell that can recognize and directly kill cancer cells. They do this by recognizing specific proteins on the surface of cancer cells that are not present on normal, healthy cells.
- Cytotoxic T Cells: Cytotoxic T cells are another type of white blood cell that can kill cancer cells. They do this by recognizing cancer cells that are displaying abnormal proteins on their surface, which are often the result of DNA mutations that occur during the development of cancer.
- Apoptosis: Apoptosis is a process of programmed cell death that occurs in normal cells as well as cancer cells. In normal cells, apoptosis helps to eliminate damaged or old cells from the body. In cancer cells, apoptosis can be induced by various treatments like chemotherapy, radiotherapy, and immunotherapy.
- Immune System Modulation: The immune system can also be modulated to target cancer cells more effectively. For example, immune checkpoint inhibitors can block certain proteins on cancer cells that inhibit the immune response, allowing the immune system to better recognize and attack the cancer cells.
Overall, the body has several natural mechanisms to kill cancer cells. However, in some cases, these mechanisms may not be sufficient to eliminate all cancer cells, and additional treatments like chemotherapy, radiation therapy, and immunotherapy may be necessary.
FAQ
Where Are Natural Killer Cells Found?
Natural killer cells are sentinels of the immune system that patrol the body in search of contaminated and malignant cells. Consequently, NK cells are ubiquitous and present in the majority of human organs. The largest quantities are found in the bloodstream, the uterus, the lungs, and the liver.
From where NK cells are Originated?
Stem cells in the bone marrow, lymph nodes, spleen, or tonsils differentiate into natural killer cells. 5 to 20% of circulating white blood cells in the human body are mature NK cells.
What are natural killer cells?
Natural killer (NK) cells are a type of lymphocyte, which is a white blood cell that plays a crucial role in the immune system. NK cells are called “natural killers” because they are able to recognize and kill infected or abnormal cells without prior exposure to a specific antigen.
NK cells are part of the innate immune system, which provides the first line of defense against infections and cancer. They are found in the blood, lymphoid tissues, and various organs throughout the body. NK cells are capable of killing a wide range of target cells, including virus-infected cells, tumor cells, and cells that have been damaged or stressed.
NK cells use several mechanisms to recognize and kill target cells. They have surface receptors that can detect changes in the expression of molecules on the surface of infected or abnormal cells, allowing them to distinguish between healthy and unhealthy cells. Once activated, NK cells can release cytotoxic granules that contain molecules like perforin and granzyme, which induce cell death in the target cell. NK cells can also produce cytokines that help to activate other cells of the immune system.
NK cells play an important role in immunosurveillance, which is the process by which the immune system identifies and eliminates abnormal cells before they develop into cancer. In addition, NK cells are involved in the response to viral infections and in the regulation of immune responses during pregnancy.
Overall, natural killer cells are a critical component of the immune system that helps to defend the body against infections and cancer by recognizing and killing infected or abnormal cells.
Are natural killer cells innate or adaptive?
Natural killer (NK) cells are generally considered a part of the innate immune system, which is the first line of defense against infections and cancer. Unlike adaptive immune cells such as T cells and B cells, which require specific recognition of antigens and clonal expansion to become activated, NK cells can respond to a wide variety of pathogens and abnormal cells without prior exposure.
However, recent studies have suggested that NK cells may also possess some adaptive features. For example, some studies have shown that NK cells can undergo clonal expansion in response to certain viral infections or cytokine stimulation, similar to what is seen with adaptive immune cells. In addition, certain subsets of NK cells have been found to display memory-like properties, which allows for a more rapid and potent response to subsequent infections.
Overall, while natural killer cells are typically considered to be innate immune cells, their ability to display some adaptive features suggests a more complex role in the immune system.
Are natural killer cells lymphocytes?
Yes, natural killer (NK) cells are a type of lymphocyte, which is a white blood cell that plays a crucial role in the immune system. Lymphocytes are divided into two main types: B cells and T cells, which are part of the adaptive immune system, and NK cells, which are part of the innate immune system.
NK cells share many characteristics with other lymphocytes, including the ability to recognize and respond to foreign antigens. However, unlike B cells and T cells, NK cells do not possess antigen-specific receptors and are able to recognize and kill target cells without prior exposure to a specific antigen.
Overall, natural killer cells are a specialized type of lymphocyte that play an important role in the innate immune response to infections and cancer.
What do natural killer cells do?
Natural killer (NK) cells are a type of white blood cell that play an important role in the immune system. They are part of the body’s innate immune response and serve as the first line of defense against infections and cancer.
NK cells are primarily known for their ability to identify and kill abnormal cells in the body, including virus-infected cells, cancer cells, and cells that have been damaged or stressed. They do this by recognizing specific proteins on the surface of these cells, which are often absent or reduced in normal healthy cells.
In addition to their cytotoxic activity, NK cells also secrete cytokines and chemokines, which help to activate and recruit other immune cells to the site of infection or inflammation. They also play a role in regulating the immune response by interacting with other immune cells and modulating their activity.
NK cells are particularly important for immunosurveillance, which is the process by which the immune system constantly monitors the body for abnormal or foreign cells. They are able to identify and eliminate these cells before they can cause harm or spread throughout the body.
Overall, natural killer cells play a crucial role in the immune system by identifying and eliminating abnormal or infected cells and helping to regulate the immune response.
are natural killer cells phagocytes?
No, natural killer (NK) cells are not phagocytes. Phagocytes are a type of immune cell that are able to engulf and destroy foreign particles, such as bacteria and debris, by a process called phagocytosis.
In contrast, NK cells do not engulf or digest foreign particles. Instead, they are able to directly recognize and kill abnormal cells, such as virus-infected or cancerous cells, through a process called cytotoxicity. NK cells accomplish this by releasing specialized granules that contain molecules like perforin and granzymes, which can cause the target cell to undergo apoptosis (programmed cell death).
While NK cells are not phagocytes, they do work in conjunction with other immune cells, such as macrophages, which are phagocytes. NK cells can activate macrophages to phagocytose and clear debris or dead cells from the body. This cooperation between different types of immune cells is an important aspect of the immune system’s ability to fight off infections and maintain tissue homeostasis.
How do natural killer cells recognize their targets?
Natural killer (NK) cells recognize their targets through a complex system of activating and inhibitory receptors on their surface. These receptors allow NK cells to distinguish between healthy, normal cells and abnormal or infected cells, including cancer cells.
NK cells have both activating and inhibitory receptors on their surface. Activating receptors recognize specific molecules on the surface of target cells, which trigger a signaling cascade that leads to the activation of the NK cell and the release of cytotoxic granules. Inhibitory receptors, on the other hand, recognize “self” molecules on healthy cells, which prevent the NK cell from attacking these cells.
The balance between activating and inhibitory signals is critical for NK cell function. If the activating signals are stronger than the inhibitory signals, the NK cell will become activated and attack the target cell. If the inhibitory signals are stronger, the NK cell will not attack the healthy cell.
One important activating receptor on NK cells is called NKG2D. This receptor recognizes stress-induced molecules that are often upregulated on the surface of tumor cells, infected cells, and other abnormal cells. When NKG2D binds to its ligands on the target cell, it triggers the activation of the NK cell and the release of cytotoxic granules.
NK cells also have other activating receptors, including NKp46, NKp44, and NKp30, which can recognize a wide variety of ligands on target cells, including viral proteins, bacterial molecules, and stress-induced molecules.
Overall, NK cells use a sophisticated system of activating and inhibitory receptors to recognize and attack abnormal or infected cells, while sparing healthy cells.
How do natural killer cells kill?
Natural killer (NK) cells are capable of killing target cells through a process called cytolysis or apoptosis. NK cells have several mechanisms for inducing cell death in their target cells.
One of the primary ways that NK cells kill their target cells is by releasing cytotoxic granules, which contain proteins called perforin and granzymes. Perforin creates pores in the membrane of the target cell, while granzymes enter the cell and trigger a cascade of events that lead to cell death. This process is known as the perforin/granzyme pathway.
NK cells can also induce target cell death through the activation of death receptors on the surface of the target cell. One such receptor is called Fas, and when it is bound by its ligand, it triggers a signaling cascade that leads to apoptosis or programmed cell death.
In addition, NK cells can induce target cell death by secreting cytokines, which are signaling molecules that can activate various cellular pathways leading to apoptosis.
Overall, NK cells have multiple mechanisms for killing their target cells, which allows them to respond to a wide variety of infectious or abnormal cells in the body. By selectively targeting cells that are infected or transformed, NK cells play a crucial role in immune surveillance and protecting the body against cancer and viral infections.
References
- Paul, S., & Lal, G. (2017). The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Frontiers in Immunology, 8. doi:10.3389/fimmu.2017.01124
- Addou-Klouche, L. (2017). NK Cells in Cancer Immunotherapy. In (Ed.), Natural Killer Cells. IntechOpen. https://doi.org/10.5772/intechopen.71217
- Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front Immunol. 2019 May 31;10:1205. doi: 10.3389/fimmu.2019.01205. PMID: 31214177; PMCID: PMC6554437.
- Vivier, E., Tomasello, E., Baratin, M. et al. Functions of natural killer cells. Nat Immunol 9, 503–510 (2008). https://doi.org/10.1038/ni1582
- Abel, A. M., Yang, C., Thakar, M. S., & Malarkannan, S. (2018). Natural Killer Cells: Development, Maturation, and Clinical Utilization. Frontiers in Immunology, 9. doi:10.3389/fimmu.2018.01869
- Diefenbach, A. (2014). Natural Killer Cells. Antibody Fc, 75–93. doi:10.1016/b978-0-12-394802-1.00004-2
- Bryceson, Y. T., Björkström, N. K., Mjösberg, J., & Ljunggren, H.-G. (2014). Natural Killer Cells. The Autoimmune Diseases, 187–199. doi:10.1016/b978-0-12-384929-8.00013-7
- Cichocki, Frank & Miller, Jeffrey & Anderson, Stephen & Bryceson, Yenan. (2013). Epigenetic regulation of NK cell differentiation and effector functions. Frontiers in immunology. 4. 55. 10.3389/fimmu.2013.00055.
- https://www.news-medical.net/health/What-are-Natural-Killer-Cells.aspx
- https://courses.lumenlearning.com/wm-biology2/chapter/natural-killer-cells/
- https://www.thermofisher.com/in/en/home/life-science/cell-analysis/cell-analysis-learning-center/immunology-at-work/cytotoxic-t-cell-overview.html
- https://en.wikipedia.org/wiki/Natural_killer_cell
- https://biologydictionary.net/natural-killer-cells/
- https://microbenotes.com/natural-killer-nk-cells/
