Table of Contents
What is the Ninhydrin Test?
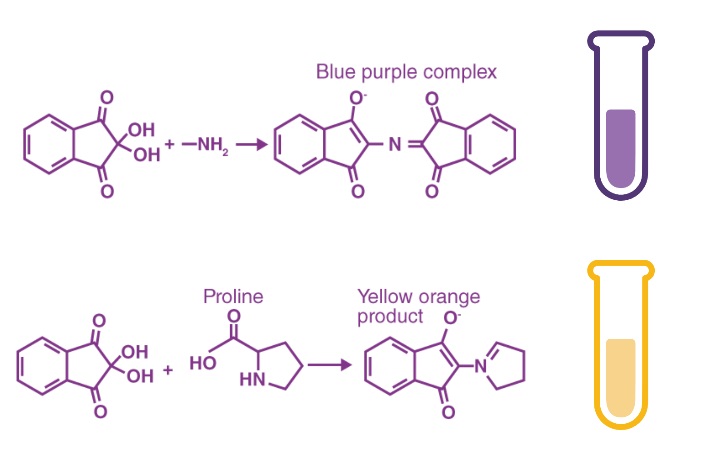
The ninhydrin chemical test is used for determining whether an analyte contains any amines or amino acids. In this test, ninhydrin (a chemical compound with the formula C9H6O4; IUPAC name: 2,2-dihydroxyindane-1,3-dione) is added to a test solution of the analyte. The formation of a deep blue color within the test sample is an indication that the analyte contains ammonia, primary/secondary amino acids, or both.
Ninhydrin Test Highlight
- Ninhydrin is used in many bioanalytical processes, including the amino acid analysis method.
- SSDs use the ninhydrin test to detect residual protection in reusable surgical tools.
- Discoloration occurs when the amino acids react with ninhydrin.
- The ninhydrin testing can be used for quantitative and qualitative purposes, i.e. chromatographic visualization and peptide sequencin. You can also order research peptides from licensed stores that offer premium-quality products for your research whenever you require them.
- Amino acids a can cause discoloration ranging from blue to purple, while secondary amines such as proline can produce yellow to orange discoloration.
- The ninhydrin test can be used to see fingerprints.
- Ninhydrin is the preferred chemical for visualizing fingerprints on porous materials and papers, as it reacts with the sweat-retained amino acids.
- Ruhemann purpura is the name for the strong compound which is formed by ninhydrin.
- Ninhydrin reacts to compounds that contain an amine, such as blood proteins.
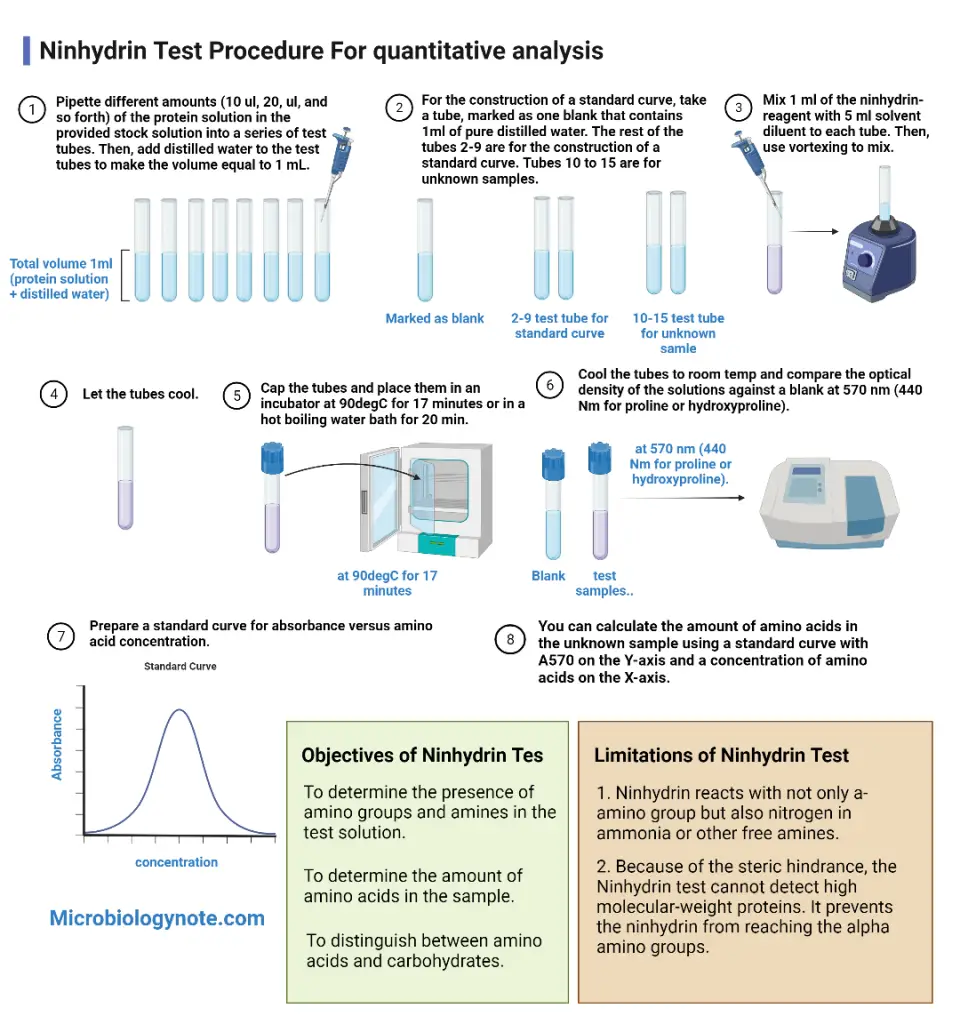
Objectives of Ninhydrin Test
- To determine the presence of amino groups and amines in the test solution.
- To determine the amount of amino acids in the sample.
- To distinguish between amino acids and carbohydrates.
Ninhydrin Test Principle
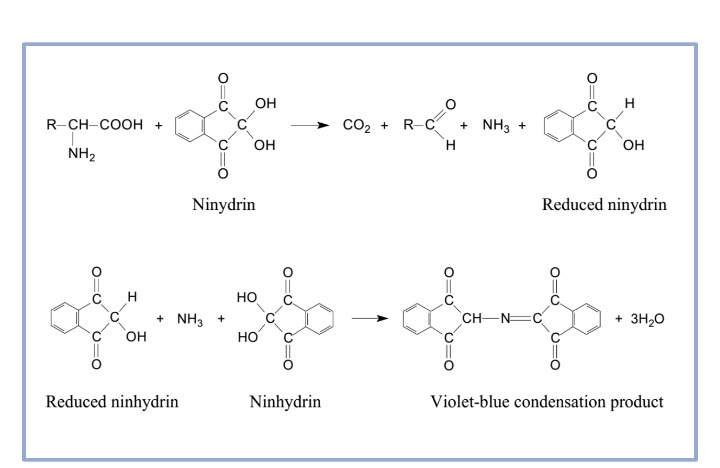
All amino acids can perform a ninhydrin-test. This test is the result of the reaction of the amino group of the unbound amino acid with ninhydrin. You know that ninhydrin can be a powerful oxidizing agent. Its presence causes the amino acids to undergo oxidative deamination, releasing ammonia, and reduces the formation of ninhydrin. The NH3 reacts with the ninhydrin molecule, resulting in the formation a blue substance. Some amino acids, such as proline or hydroxyproline, do not produce blue or purple substances. They generally yield to a brown product.
Ninhydrin Test Reaction
Requirements
Reagents
- Ninhydrin reagent: Dissolve 0.35g ninhydrin into 100 ml of ethanol (isopropanol or a 1:1 mixture of butanol/acetone may be substituted for ethanol).
- For the quantitative test, dilute solvent is required: Combine equal amounts of water with n-propanol.
- Standard solution (1% protein solution)
- Example solution
Materials required
- Test tubes
- Test tube stand
- Pipette
- Equipment
- Water bath
- Spectrophotometer
Procedure of Ninhydrin Test
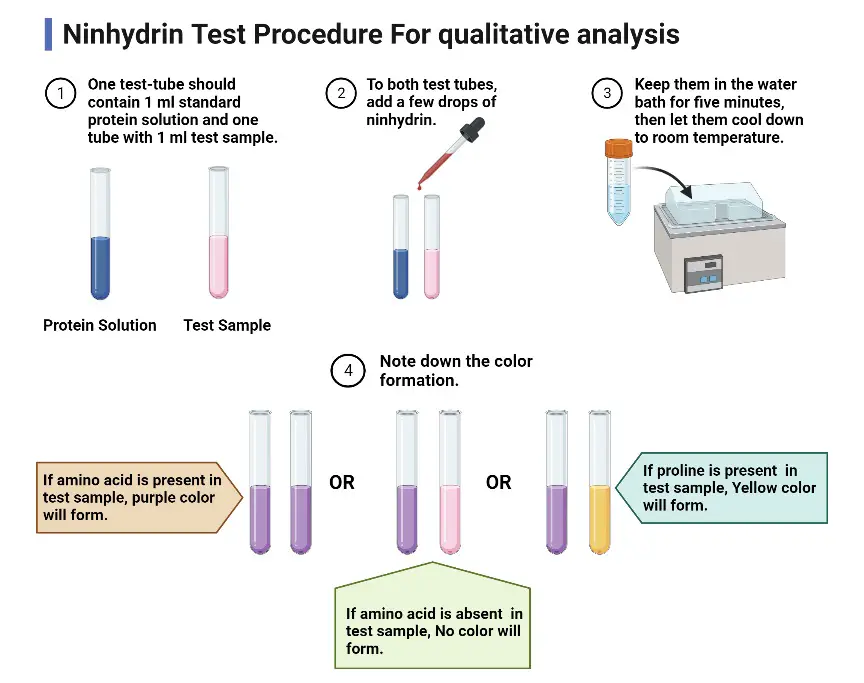
Ninhydrin Test Procedure For qualitative analysis
- One test-tube should contain 1 ml standard protein solution and one tube with 1 ml test sample.
- To both test tubes, add a few drops of ninhydrin.
- Keep them in the water bath for five minutes, then let them cool down to room temperature.
- Note down the color formation.
Ninhydrin Test Procedure For quantitative analysis
- Pipette different amounts (10 ul, 20, ul, and so forth) of the protein solution in the provided stock solution into a series of test tubes. Then, add distilled water to the test tubes to make the volume equal to 1 mL.
- For the construction of a standard curve, take a tube, marked as one blank that contains 1ml of pure distilled water. The rest of the tubes 2-9 are for the construction of a standard curve. Tubes 10 to 15 are for unknown samples.
- Mix 1 ml of the ninhydrin-reagent with 5 ml solvent diluent to each tube. Then, use vortexing to mix.
- Let the tubes cool.
- Cap the tubes and place them in an incubator at 90degC for 17 minutes or in a hot boiling water bath for 20 min.
- Cool the tubes to room temp and compare the optical density of the solutions against a blank at 570 nm (440 Nm for proline or hydroxyproline).
- Prepare a standard curve for absorbance versus amino acid concentration.
- You can calculate the amount of amino acids in the unknown sample using a standard curve with A570 on the Y-axis and a concentration of amino acids on the X-axis.
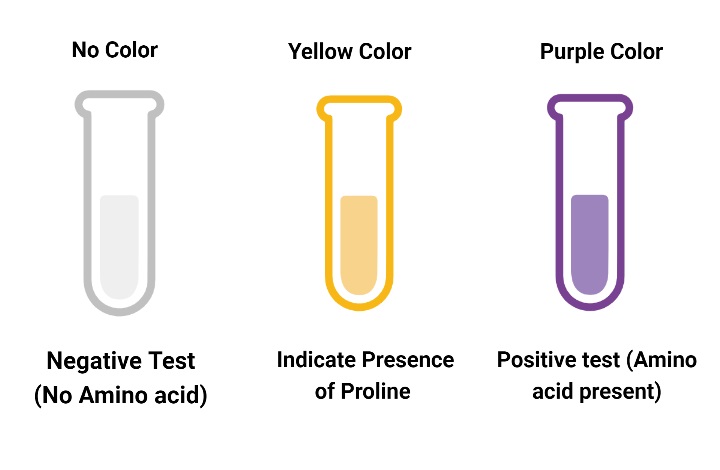
Result of Ninhydrin Test
- Ninhydrin Test Positive result: A purple-colored complex found in the tube is a positive result. This indicates that there are amino acids in the sample.
- Ninhydrin Test Negative Result :A negative result is when the tube does not contain the complex. This indicates that there are no amino acids in the sample.
From the graph we can calculate the concentration of unknown samples.
Application of Ninhydrin Test
- It plays an important role in monitoring deprotection in solid phase peptide synthesis. If nitrogen is deprotected, the ninhydrin test turns blue.
- It is used in the analysis of amino acids in proteins. Most amino acids are hydrolyzed and react with ninhydrin with the exception of proline. Some amino acid chains degrade. Therefore, a separate analysis is needed to identify amino acids that may react or not react with ninhydrin.
- It is used to verify a solution suspected of having ammonium ions. A treatment with ninhydrin would result in a dramatic purple color.
- It is usually used by forensic investigators in the analysis of fingerprints on porous surfaces. A finger mark containing amino acids is treated with a ninhydrin solution, which results in a purple amino acid finger crest pattern. Therefore, making the fingerprint visible.
Limitations of Ninhydrin Test
- Ninhydrin reacts with not only a-amino group but also nitrogen in ammonia or other free amines.
- Because of the steric hindrance, the Ninhydrin test cannot detect high molecular-weight proteins. It prevents the ninhydrin from reaching the alpha amino groups.
FAQ
Why is Ninhydrin used in chromatography?
There are many types of reagents that can be used to detect amino acids on thin posterior chromatographic plates. One of these is ninhydrin. Because of its high sensitivity, it is the most popular. With all amino acids except proline and hydroproline, the resultant color is blue/purple/violet.
What does ninhydrin test for?
The ninhydrin test is used for testing if a protein has been digested or broken down.
Amino acids gives which color with ninhydrin test?
Amino acids gives Deep purple colour with ninhydrin test
What is considered a positive control for ninhydrin test?
A dibasic amino acid, arginine, is recommended in guidelines as the positive control and a solution is supplied with ninhydrin-based test kits. Arginine reacts readily with ninhydrin but it is not a protein and would be considered inappropriate as a control by most analysts.
What color is a positive ninhydrin test?
The marker for a positive ninhydrin test is a deep blue colouration obtained in the solution. This reaction indicates the presence of amino acids, other amines and ammonia in the test sample.
What part of leucine is responsible for the color development in a ninhydrin test?
α-amino group is responsible for the color development in a ninhydrin test. This part react with the ninhydrin and forms a color compound.
What groups in the amino acid or protein are responsible for a positive ninhydrin test?
The amine functional group of α-amino acids reacts with ninhydrin to form purple-colored compounds.
Why doesn’t the amino acid proline give a positive ninhydrin test?
Ninhydrin is a strong oxidising agent when it reacts with alpha amino acids gives purple color which is known as ruhemanns purple complex, this is the result of the reaction of ninhydrin and amines present in the primary amino acids, prolin has a ring structure so nitrogen is not free to react with the ninhydrin as it is locked in the ring structure hence unable to produce purple complex and gives yellow color. And also, Proline and hydroxyproline give a yellow spot upon reaction with Nihhydrin, This color difference is due to the lack of a primary amine that all of the other protein amino acids have.
In order for the ninhydrin test to work what functional group must be present?
Amine functional group of α-amino acids
Why does ninhydrin test color change?
Ninhydrin reacts with the α-amino group of primary amino acids producing ‘Ruhemann’s purple’. The chromophore formed is the same for all primary amino acids.
A negative ninhydrin test will turn what color?
If no colour change is observed, the analyte does not contain amino acids, amines, or ammonia, Which means it is a negative ninhydrin test.
References
- https://microbenotes.com/ninhydrin-test/
- https://en.wikipedia.org/wiki/Ninhydrin
- https://www.onlinebiologynotes.com/ninhydrin-test-principle-requirements-procedure-and-result/
- https://www.chem.ucalgary.ca/courses/350/Carey5th/Ch27/ch27-3-3.html
- https://pubs.acs.org/doi/10.1021/jf030490p
- https://www.sciencedirect.com/topics/chemistry/ninhydrin-reaction
- https://unacademy.com/content/jee/study-material/chemistry/ninhydrin-test/
- http://biocheminfo.com/2020/04/03/ninhydrin-test-principle-reaction-reagents-procedure-and-result-interpretation/
