Table of Contents
What is Periodic Acid-Schiff (PAS) Staining?
Periodic Acid-Schiff (PAS) staining is a laboratory staining technique used to detect the presence of specific sugars and carbohydrates in tissue samples. The PAS stain highlights the presence of sugars, glycoproteins, and mucopolysaccharides in biological tissues and cells. The staining process is often used in the diagnosis of diseases related to abnormal carbohydrate metabolism and the analysis of tissue samples in medical research.
- The PAS staining system provides both conventional and microwave techniques for demonstrating lymphocytes and mucopolysaccharides.
- In cases of lymphocytic leukaemia that have reached an advanced stage, the cells’ staining pattern is useful for guiding therapy decisions. The PAS reaction is excellent for demonstrating mucopolysaccharides in tissue slices.
- Diastase (-amylase) digestion, followed by the PAS stain, is a valuable diagnostic tool for glycogen storage disorder. PAS reagents are intended for “In Vitro Diagnostic Use.” The PAS staining technique can also be used to identify fungi in tissue sections.
- When glycols are exposed to periodic acid, they are oxidised to aldehydes. After reaction with Schiff’s reagent (a combination of pararosaniline and sodium metabisulfite), a pararosaniline adduct that stains glycol-containing cellular components is produced.
- This reaction is applicable to blood and bone marrow films, tissue touch preparations, and tissue sections.
- This test may be useful in detecting some cases of erythroleukemia and acute lymphoblastic leukaemia when applied to blood or bone marrow films.
- Diastase (-amylase) digestion can be utilised as a diagnostic tool for glycogen storage disorder. Diastase hydrolyzes tissue-bound starch, glycogen, and breakdown products derived from these polysaccharides. Prior to PAS staining, the byproducts of the digestive process are washed away. Included is a method for quick PAS staining utilising microwave ovens.
Principle of Periodic Acid-Schiff (PAS) Staining
Schiff’s reaction with carbohydrates is an oxidative process in which polysaccharides react with periodic acid to produce an aldehyde, an oxidised molecule. Due to the fixation of colourless Schiffs fuchsin, aldehydes are disclosed by their red, pink, or magenta colour. PAS is associated with diastase enzymes, which are responsible for the transformation of starch into maltose and then glucose. During glucose conversion, the stain turns pink, indicating the intracellular or extracellular persistence of mucins. Nuclei are stained with hematoxylin or methyl green.
Preparation of Solutions and Reagents
Schiffs Reagent is a mixture of periodic acid and aniline, used in histology to stain carbohydrates in tissue sections. It is a vital tool for pathologists and histologists, who rely on accurate staining techniques to make critical diagnoses.
A high-quality Schiffs reagent is essential for accurate results in histology. Poor-quality reagents can lead to incorrect diagnoses and incorrect treatment decisions. Therefore, it is essential to use a Schiffs reagent that has been tested and verified to be of high quality.
One simple method to test the quality of a Schiffs reagent is to add 10 ml of 37% formalin to the reagent. A good Schiffs reagent will turn red-purple in color immediately, while a poor-quality reagent will have a delayed reaction, producing deep blue-purple coloration.
Periodic Acid solution (0.5%): Periodic acid- 0.5g and Distilled water- 100ml
Testing Schiff’s Reagent
- 10 ml of 37% formalin
- Add Schiff’s reagent to be tested.
- A good Schiffs reagent turns red-purple, but a bad Schiffs reagent has a delayed reaction that produces a deep blue-purple hue.
Mayer’s Hematoxylin Solution
Procedure for Periodic Acid-Schiff (PAS) Staining
Specimen Collection
No current test procedure can provide absolute confidence that blood or tissue samples are not infectious. Consequently, all blood derivatives and tissue specimens must be considered infectious. Blood or bone marrow films are freshly made using whole, EDTA, or heparinized blood or bone marrow. As quickly as feasible, repair. Tissues treated in 10% neutral buffered formalin, Zenker’s or Bouin’s can be used to detect polysaccharides. It is important to note that certain carbohydrates are water-soluble. Carnoy’s fluid, Gendre’s fluid, or acid alcoholic formalin are advised for demonstrating glycogen. When tissue is fixed with a picric acid-containing fixative, the extraction of diastase may take longer. Cut tissue sections at 5 microns.
If using a microwave, please refer to the Owner’s Manual for instructions. For control purposes, blood films prepared from clinically healthy persons may be included. The cytoplasm of polymorphonuclear leukocytes will exhibit a strong red stain. Every time a stain sequence is done, sections of tissue that are known to be PAS-positive and/or to contain glycogen must be included. The data received from this method should be analysed in conjunction with the results of other clinical diagnostic tests or information.
BLOOD, BONE MARROW, OR TISSUE TOUCH PREPARATIONS
Standard Procedure
- Fix air-dried blood films in Formalin-Ethanol Fixative Solution for one minute at room temperature.
- Rinse slides 1 minute in steadily running tap water.
Immerse slides for five minutes in Periodic Acid Solution at room temperature. - Rinse slides in numerous changes of distilled water.
Immerse slides for 15 minutes at room temperature in Schiff’s Reagent. NOTE: Immediately after usage, cover Schiff’s Reagent and refrigerate at 2–8 degrees Celsius.
Wash slides for five minutes under running water. - Counterstain slides for 90 seconds with Hematoxylin Solution, Gill No. 3.
- Rinse slides in running tap water for 15 to 30 seconds, allow to air-dry, then inspect microscopically with a 900x oil immersion lens. To mount slides, toluene or xylene-based mounting medium may be used.
Microwave Procedure:
- Fix air-dried films for 1 minute at room temperature in Formalin-Ethanol Fixative Solution.
- Rinse slides for one minute under a steadily running faucet.
- Place slides in a 40 ml plastic Coplin jar containing Periodic Acid Solution.
- Microwave for 10 seconds at 800 watts.
- Rinse thoroughly with multiple changes of deionized water.
Place slides in a plastic Coplin jar containing 40 cc of Schiff’s Reagent. - Microwave for 15 seconds at 800 watts. Incubate for one minute after mixing the solution with a beral pipette or applicator stick.
- Rinse for five minutes in warm, gently running tap water.
- Place slides in a plastic Coplin jar containing 40 cc of Hematoxylin Solution Gill No. 3.
- Microwave for 10 seconds at 800 watts.
- 1–2 minutes of rinsing with running tap water, followed by soaking in Working Scott’s Tap Water Substitute at room temperature.
- Rinse in running tap water. Air dry.
- To mount slides, toluene or xylene-based mounting medium may be used.
II. TISSUE SECTIONS
Standard Procedure:
- Deparaffinize and hydrate with deionized water parts.
- Immerse slides for five minutes in Periodic Acid Solution at ambient temperature (18–26 degrees Celsius).
- Rinse the slide with distilled water in multiple changes.
- Immerse slides for 15 minutes at room temperature (18–26°C) in Schiff’s Reagent.
- Wash slides for five minutes under running water.
- Counterstain slides for 90 seconds with Hematoxylin Solution, Gill No. 3.
- Rinse slides with running tap water.
Dehydrate, clarify, and mount portions in mounting media based on toluene or xylene.
Microwave Procedure:
- Deparaffinize and then hydrate with deionized water.
- Place the slides in a 40 ml plastic Coplin jar containing Periodic Acid Solution. Cover the jar loosely with the lid, or use lids with holes drilled in them.
- Microwave for 10 seconds at 800 watts.
- 4.Rinse thoroughly with multiple changes of deionized water.
- Place slides in a plastic Coplin jar containing 40 cc of Schiff’s Reagent.
- Microwave for 15 seconds at 800 watts. Incubate for one minute after mixing the solution with a beral pipette or applicator stick.
- Rinse for five minutes in warm, gently running tap water.
- Place slides in a plastic Coplin jar containing Hematoxylin, Gill No. 3.
- Microwave for 10 seconds at 800 watts.
- 10 minutes of rinsing with running tap water, followed by soaking in Scott’s Tap Water Substitute at room temperature. Rinse in running tap water. Dehydrate, then clear and ascend.
Microwave Procedure for Diastase (α-Amylase) Digestion:
- Use duplicate test slides. Label one for diastase digestion and the other for PAS staining alone. NOTE: Tissue adhesive-coated slides are suggested. When utilising diastase digestion, portions should not be celloidinized.
- Deparaffinize and hydrate the glass slides with deionized water.
- Prepare Diastase (-amylase) Working Solution by dissolving 0.2 grammes of -Amylase (Catalog No. A 3176) in 40 millilitres of deionized water. Mix thoroughly and store in a plastic Coplin jar. Prepare immediately before use.
- Microwave for 25 seconds at 600 watts.
- Remove slides from Coplin jar and rinse digested slide in running tap water for 5 minutes.
- Using both digested and undigested slides, continue with step 2 of the Microwave Procedure for Tissue.
PAS-positive compounds discolour pink to red, whereas nuclei are blue. A Diastase (α-Amylase) Extraction slide will lack detectable glycogen PAS staining when compared to the positive control slide containing undigested glycogen.
Results and Interpretation
- Other carbohydrate components, including glycogen, mucin, elastic fibres, basophilic granules of the pituitary gland, reticular fibres, thyroid colloids, basement membranes, and bone cartilage, stain pink or purple.
- Depending on the dye employed, the nuclei stain green or blue.
- The background has a blue stain.
The substances positive for PAS are:
- Polysaccharides: Glycogen, cellulose, and starch are three examples of polysaccharides. Numerous leucocytes include glycogen, fungi (Candida albicans, Histoplasma capsualtum, Cryptococcus, and Blastomycosis), bacteria, and actinomycosis.
- Glycoproteins: Mucins, mucoid secretions of intestinal tracts, uterine glands, ducts, tracheobronchial tress, hormones (TSH), megakaryocytes, etc. are examples of glycoproteins.
- Glycolipids: Gangliosides, primarily fatty acid-based grey matter.
- Substances that do not contain carbohydrates: unsaturated lipids, phospholipids, and phosphoinositides.
- Certain pigments and substances: Certain pigments and chemicals, including Ceroid, lipofucsin, melanosis coli pigment, and Dubin-Johnson pigment.
- Plasmogens: They are acetyl phospholipids, such as Russell bodies.
- Miscellaneous: Amyloid, cartilage matrix, colloid and ocular lens substance.
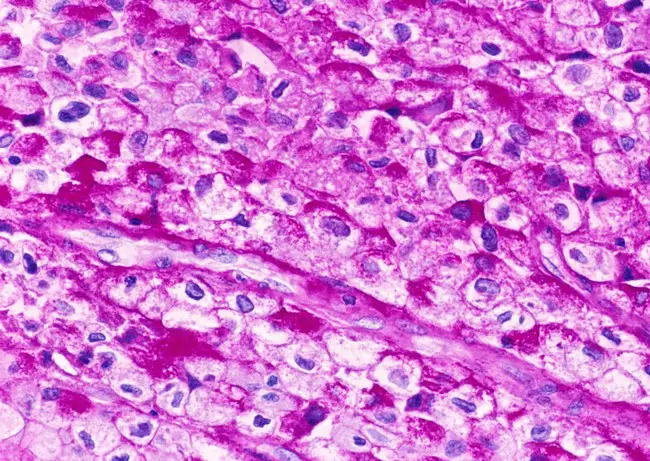
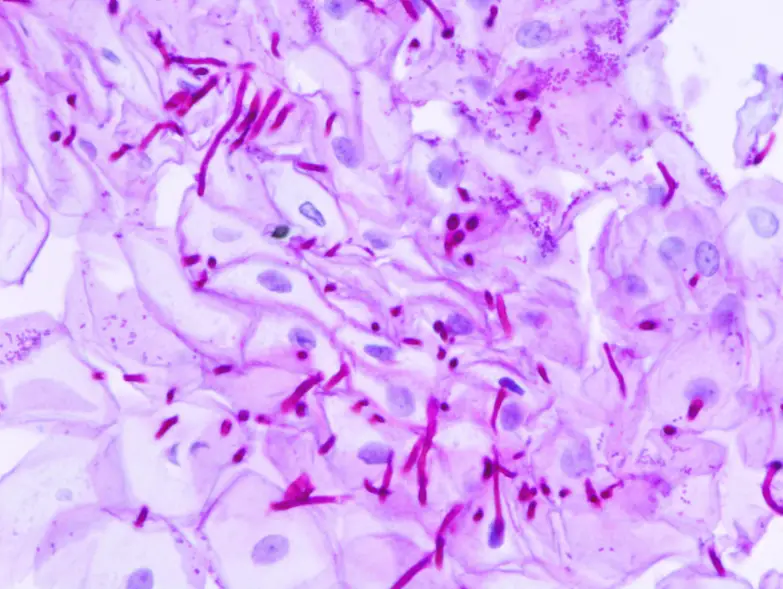
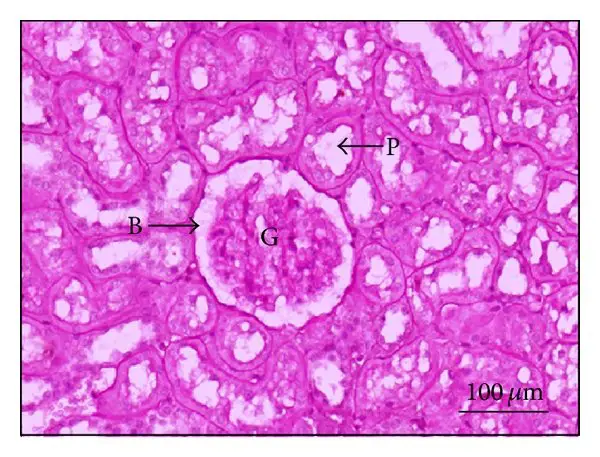
Applications of Periodic Acid-Schiff (PAS) Staining
- PAS facilitates the demonstration of glycogen, cellulose, and starch. When glycogen storage disorder is suspected, this is useful to detect glycogen deposits in the liver.
- Through the PAS stain, the basement membrane of numerous tissues can also be observed. When assessing renal disease, the PAS is most typically used to measure the thickness of the glomerular basement membrane.
- PAS stain can identify Cryptococcus neoformans, Histoplasma capsulatum, Aspergillus fumigatus, Blastomyces, etc. in tissue samples because to the high carbohydrate content of their cell wall/capsule.
- PAS demonstrates mucin, namely acidic mucin. The endocervical glands, intestinal glands, and bronchial glands are crucial.
- PAS facilitates the demonstration of cerebrosides and gangliosides. It aids in the diagnosis of disorders such as Gaucher’s, Krabbe’s, and lysosomal storage diseases.
- PAS stain demonstrates pigments such as lipofuchsin and pigments of Dubin-Johnson syndrome.
- PAS imparts a stain to the russell bodies of plasma cells.
Precautions
When performing Periodic Acid-Schiff (PAS) staining, it is important to take the following precautions:
- Handle hazardous chemicals with care: PAS staining requires the use of hazardous chemicals, such as periodic acid, which can cause skin and eye irritation, and should be handled with gloves and eye protection.
- Wear appropriate personal protective equipment (PPE): Laboratory personnel should wear appropriate PPE, such as laboratory coats and gloves, to minimize exposure to hazardous chemicals.
- Follow proper disposal procedures: Used solutions and waste from PAS staining should be properly disposed of according to local regulations to minimize the risk of environmental contamination.
- Store reagents properly: Reagents used in PAS staining should be stored in a secure, well-labeled location, away from food and drink, and out of reach of children.
- Avoid contamination: Care should be taken to avoid contamination of reagents and samples, as this can affect the accuracy of the results.
- Follow established protocols: Established protocols for PAS staining should be followed carefully to ensure consistent results and to minimize the risk of errors.
- Monitor the duration and temperature of incubation: The duration and temperature of incubation during PAS staining can affect the results, so it is important to monitor these conditions carefully and make any necessary adjustments.
- Read and follow the instructions of the reagents: The instructions of the reagents used in PAS staining should be read and followed carefully to ensure optimal results and to minimize the risk of errors.
FAQ
What is Periodic Acid-Schiff (PAS) staining?
PAS staining is a histochemical technique used to visualize and identify certain carbohydrates, glycogen, and mucopolysaccharides in tissues and cells.
What kind of samples can be stained using PAS?
PAS staining can be used on a variety of tissue samples, including biopsy specimens, blood smears, and urine samples.
What are some of the applications of PAS staining?
PAS staining is commonly used to diagnose a variety of conditions, such as fungal infections, liver and kidney diseases, tumors, mucopolysaccharidoses, and metabolic storage diseases.
Why is PAS staining considered a sensitive method?
PAS staining is highly sensitive and can detect minute amounts of carbohydrates, glycogen, and mucopolysaccharides, making it an effective method for detecting these substances in tissues and cells.
Is PAS staining specific for a single type of carbohydrate?
No, PAS staining is not specific for a single type of carbohydrate and can stain other structures, leading to false positive results.
Can other substances interfere with PAS staining results?
Yes, the presence of other substances in tissues and cells, such as heme and other pigments, can interfere with PAS staining and affect the results.
How long does the PAS staining procedure take?
The PAS staining procedure is time-consuming and the results may be affected by the duration and temperature of incubation and the concentration of the stain.
Are there any hazardous chemicals involved in PAS staining?
Yes, PAS staining requires the use of hazardous chemicals, such as periodic acid, that must be handled with care in the laboratory to avoid health risks to laboratory personnel.
Is PAS staining easy to perform in a laboratory setting?
Yes, the PAS staining procedure is simple and straightforward, making it easy to perform in a laboratory setting.
Does PAS staining provide clear visualization of carbohydrates, glycogen, and mucopolysaccharides?
Yes, the PAS stain provides clear and intense staining, making it easy to visualize and identify the presence of carbohydrates, glycogen, and mucopolysaccharides in tissues and cells.
References
- Peffault de Latour R, Legrand O, Moreau D, Perrot JY, Blanc CM, Chaoui D, Casadevall N, Marie JP. Comparison of flow cytometry and enzyme cytochemistry for the detection of myeloperoxydase in acute myeloid leukaemia: interests of a new positivity threshold. Br J Haematol. 2003 Jul;122(2):211-6. doi: 10.1046/j.1365-2141.2003.04384.x. PMID: 12846888.
- Zugibe, F.T. Positive periodic acid-Schiff staining of acid mucopolysaccharides. Histochem J 2, 191–197 (1970). https://doi.org/10.1007/BF01003468
- https://www.pathologyoutlines.com/topic/stainspas.html
- http://www.ihcworld.com/_protocols/special_stains/masson_trichrome.htm
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/171/727/395.pdf
- http://www.histology.leeds.ac.uk/what-is-histology/histological_stains.php
