Table of Contents
What is Plasmodium Vivax?
- Plasmodium vivax is the parasite species that causes malaria in humans. Plasmodium knowlesi is one of the five Plasmodium species that can cause malaria in humans, along with Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi.
- Plasmodium vivax is transmitted to humans by female Anopheles mosquitoes that are infected with the parasite. Once in the bloodstream, the parasite attacks red blood cells, causing flu-like symptoms such as fever, chills, and headache. P. vivax, unlike Plasmodium falciparum, can generate dormant liver stages known as hypnozoites, which can reactivate months or years after the initial infection, causing malaria relapses.
- Mostly found in South and Southeast Asia, Latin America, and the Horn of Africa, P. vivax is a species of Plasmodium. It is a major public health concern in many parts of the world, particularly in regions where the mosquito vector is plentiful and treatment options are limited.
Discovery
- 1885 saw the description of the genus Plasmodium by Ettore Marchiafava and Angelo Celli. There are currently more than 200 species classified within this genus.
- It is sometimes referred to as the malaria parasite. At least 11 species of the nearly 200 known Plasmodium species infect humans.
- Several species, including primates, rodents, birds, and reptiles, infect other creatures. Throughout its life cycle, the parasite always has two hosts: a vector, typically a mosquito, and a vertebrate host.
- Four species of Plasmodium are known to cause human malaria: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae and Plasmodium ovale. Plasmodium falciparum is the most fatal of the four species of the parasite.
Characteristics of Plasmodium Vivax
- Morphology: Plasmodium vivax is a eukaryotic, unicellular parasite that belongs to the class Apicomplexa. Its life cycle is comprised of numerous phases in both the human host and the mosquito vector.
- Size: The parasite’s diameter ranges from 1 to 4 microns, which is smaller than a red blood cell.
- Genome: P. vivax’s genome is roughly 48 megabases in size and contains around 5,600 predicted genes. Its genetic variety adds to its capacity to escape the human immune system.
- Transmission: P. vivax is transmitted to humans by female Anopheles mosquitoes that are infected with the parasite. P. vivax, unlike certain other Plasmodium species, can be transmitted by both daytime and nighttime mosquito bites.
- Life Cycle: P. vivax’s life cycle consists of several phases, including sporozoites, merozoites, and gametocytes. After being transmitted by an infected mosquito to a human host, the sporozoites migrate to the liver, where they infect hepatocytes and multiply to generate hundreds of merozoites. These merozoites enter the bloodstream and infect red blood cells, resulting in malaria symptoms. Certain merozoites transform into gametocytes, which can be ingested by a mosquito during a blood meal to conclude the sexual phase of the parasite’s life cycle.
- Hypnozoites: P. vivax is capable of forming hypnozoites, which are dormant liver stages that can persist in the liver for months or even years before reactivating and causing malaria relapses.
- Drug Resistance: P. vivax has developed resistance to certain antimalarial medications, especially in Southeast Asia. This has complicated the treatment of P. vivax malaria, as effective medications must be able to target both the parasite’s blood and liver stages.
- Geographical Distribution: Plasmodium vivax is the most globally dispersed of all malaria parasites, having the highest incidence in Asia and the Pacific. It is also widespread in portions of Africa, Latin America, and the Middle East.
- Symptoms: P. vivax malaria shares symptoms with other kinds of malaria, including fever, chills, headache, muscle pains, and weariness. Typically, symptoms occur 10 to 15 days following infection.
- Treatment: Treatment options for P. vivax include antimalarial medicines such as chloroquine, primaquine, and artemisinin-based combination treatments. However, the existence of hypnozoites, which can trigger relapses of the illness, can complicate treatment.
- Prevention: The most effective method for preventing P. vivax malaria is to avoid mosquito bites by using insect repellent, wearing protective clothes, and sleeping under a mosquito net. Additional preventative strategies include eliminating standing water where mosquitoes grow and taking antimalarial medication before travelling to malaria-endemic regions.
- Impact: Malaria caused by P. vivax is a severe threat to public health, producing significant morbidity and mortality, especially among children under the age of 5. In addition, it has a huge impact on the economy due to lost production and medical expenses. The presence of hypnozoites, which can trigger relapses of the infection months or even years after the initial infection, has hampered control and eradication attempts.
- Vector: Plasmodium vivax is transmitted to humans predominantly through the bite of female Anopheles mosquitoes, which serve as the parasite’s vector. These mosquitoes are mainly found in tropical and subtropical regions of the world and bite between dusk and dawn.
- Host: Plasmodium vivax infects humans and other primates, such as macaques and chimpanzees. The principal reservoir for the parasite is the human host, and transmission occurs when an infected mosquito feeds on human blood and injects sporozoites into the bloodstream.
- Incubation Time: Plasmodium vivax normally incubates between 10 and 15 days after the bite of an infected mosquito. This indicates that the infection’s symptoms, including fever, chills, headache, muscle pains, and weariness, typically manifest within this timeframe. However, in rare instances, the incubation period might be longer or shorter depending on factors such as the individual’s immunological condition, the number of transmitted parasites, and the parasite strain. It is also crucial to note that P. vivax is capable of producing hypnozoites, which can linger in the liver for months or even years before reactivating and causing malaria relapses. This can complicate the identification and treatment of P. vivax infections and highlights the significance of recurrence surveillance after therapy.
Various forms in the life cycle of Plasmodium
- Sporozoite – The sporozoite of Plasmodium vivax is elongated and around 15 µm in length. The virus is kept in the salivary glands of mosquitoes and is transmitted to humans. They contain thick pellicles and fibres on their periphery that aid in movement.
- Schizont — The adult liver schizont has a diameter of 40-80 µm. Upon rupture, merozoites are released. Erythrocytic schizonts are 7-8 µm in length and are the same size as RBCs. They have several nuclei and multiple merozoites.
- Immature trophozoites – The Plasmodium vivax immature trophozoites are ring-shaped and are formed in the erythrocytes. About 1-2 µm in diameter.
- Mature Plasmodium trophozoites – The form of mature Plasmodium trophozoites is uneven (amoeboid). They include haemozoin, a dark pigment generated from the haemoglobin of the infected RBCs.
- Gametocytes -The gametocytes range in size from 7 to 14 µm. The form and size of gametocytes vary amongst Plasmodium species. In the RBCs, gametocytes are generated.
- Zygote – Resulting from the fusion of micro and mega gametocytes.
- Ookinete – The zygote transforms into mobile ookinetes. They are around 15-20 µm in length and are elongated. They move through the mosquito’s midgut wall and transform into oocysts.
- Oocyst – It has a diameter of around 50 µm. The oocysts transform into sporozoites that enter and are retained in the salivary glands.
Life Cycle of Plasmodium vivax
Life cycle of Plasmodium vivax is divided into:
- Asexual life cycle or schizogony in man
- Sexual life cycle or sporogony in female Anopheles mosquito
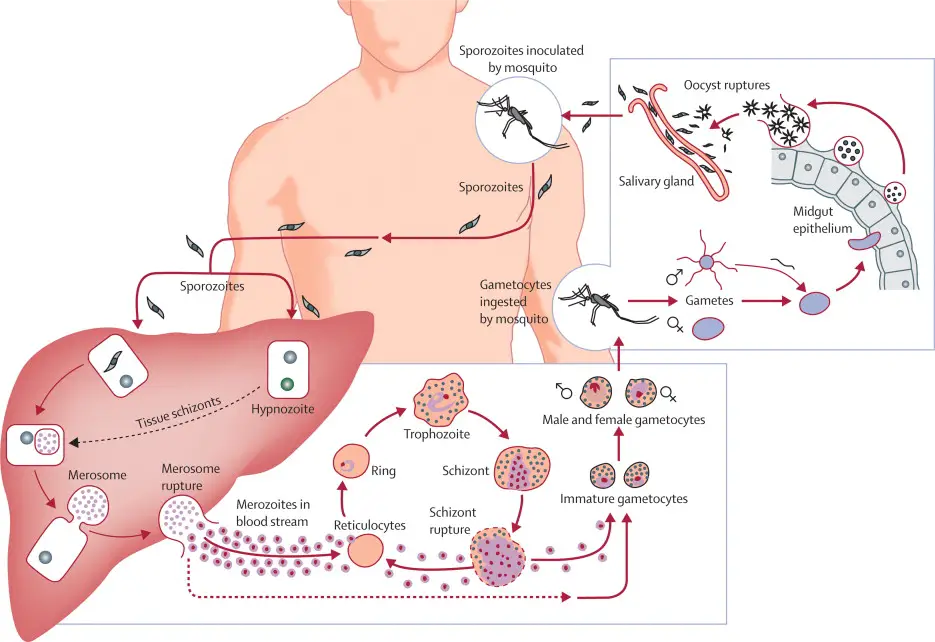
A. Asexual cycle or Schizogony in man
1. Infection
- Schizogony is the asexual reproduction method through which Plasmodium multiplies asexually in human liver cells and RBCs.
- It occurs in liver cells (liver schizogony) and red blood cells (RBC) (erythrocytic schizogony).
- A healthy person becomes infected when bitten by an infected female Anopheles carrying the infectious stage of parasites (sporozoites) in its salivary glands.
- The mosquito punctures the skin of the host with its proboscis and then injects saliva into the bloodstream.
- Together with saliva, it inoculates the bloodstream with thousands of sporozoites.
2. Sporozoites
- These are parasites with infectious forms.
- It is approximately 11 to 12 µ in length and 0.5 to 1 µ in width.
- The organism is spindle-shaped, slightly bent or sickle-shaped, and nucleusless.
- Electron microscopy reveals that the sporozoites are externally covered by an elastic, rigid pellicle with longitudinally oriented contractile microtubules.
- The microtubules facilitate their writhing motions.
- Its front end is the apical cap, which consists of three or more concentric rings into which the paired secretory organelles open.
- These secretory organelles are expected to release a substance that facilitates the drug’s entry into liver cells.
- It has a single, vesicular nucleus that is centred by a nucleolus.
- One mitochondrion with tubular cristae is present.
3. Liver schizogony
The sporozoites are able to glide slightly. Approximately thirty minutes later, sporozoites leave the bloodstream and enter the parenchymal cells of the liver, where they reproduce asexually by schizogony. Within the liver and red blood cells, various types of sporozoite induce infection. The two phases of liver schizogony are pre-erythrocytic and exo-erythrocytic.
a. Pre-erythrocytic phase
In this section, we will delve into the process of pre-erythrocytic schizogony, including the formation of schizonts, merozoites, and cryptozoites.
- The Formation of Schizonts in Liver Cells: After entering the host’s bloodstream through a mosquito bite, sporozoites migrate to the liver and infect hepatocytes, or liver cells. Once inside the hepatocyte, the sporozoites transform into schizonts, which are large, spherical structures that grow in size as they develop. The schizonts undergo asexual reproduction through multiple fission, where the nucleus divides repeatedly to form thousands of merozoites. These merozoites are smaller in size than the schizonts and are enclosed in individual membranes.
- The Release of Cryptozoites into the Bloodstream: The schizonts eventually rupture, releasing the merozoites into the liver sinusoids or venous passages. These merozoites are in the form of cryptozoites or cryptomerozoites, which are immune to medicines and the host’s immune system. These cryptozoites continue to develop in the liver and multiply asexually, forming more merozoites. This stage of development is crucial in the Plasmodium life cycle as it contributes to the onset of clinical symptoms in malaria. The resistance of the cryptozoites to medicines and the host’s immune system makes it challenging to treat and control the disease.
- The Completion of Pre-Erythrocytic Schizogony: The entire process of pre-erythrocytic schizogony in the liver cells takes around 8-10 days to complete. During this period, the blood remains sterile, and inoculation does not produce an infection. However, after the completion of pre-erythrocytic schizogony, the merozoites are released into the bloodstream, where they invade erythrocytes or red blood cells, leading to the onset of clinical symptoms.
The pre-erythrocytic schizogony is a critical stage in the Plasmodium life cycle, where sporozoites develop and multiply in the liver cells. The formation of schizonts, merozoites, and cryptozoites is a complex process that is essential for the onset of clinical symptoms in malaria. The resistance of cryptozoites to medicines and the host’s immune system makes it challenging to control and treat the disease. By understanding the pre-erythrocytic schizogony, we can develop more effective strategies to prevent and control malaria.
b. Exo-erythrocytic phase
The Exo-Erythrocytic Phase Explained
During the exo-erythrocytic phase, the Plasmodium parasites enter fresh liver cells and develop into schizonts. The schizonts then divide to form merozoites, which are the infectious forms of the parasite that can enter the red blood cells and start the erythrocytic phase. The merozoites that are produced during the exo-erythrocytic phase are called metacryptozoites or phanerozoites, depending on their stage of development.
The process of asexual multiplication is repeated several times within the liver cells, resulting in the formation of a reservoir of merozoites. Each time new liver cells are infected, leading to an exponential increase in the number of parasites in the host.
Types of Metacryptozoites
Metacryptozoites can be further classified into two types based on their size and number. The smaller and more numerous merozoites are called micro metacryptozoites, while the larger and less numerous ones are called macro metacryptozoites.
The micro metacryptozoites enter the red blood cells and initiate the erythrocytic phase, leading to the symptoms of malaria such as fever, chills, and headaches. The macro metacryptozoites, on the other hand, remain in the liver cells and continue the exo-erythrocytic phase.
Merozoite Targeting and Susceptibility
It is interesting to note that the merozoites produced during the exo-erythrocytic phase target only the young and immature red blood cells. In contrast, the merozoites of P. malariae attack only old red blood cells, while P. falciparum attacks all kinds of red blood cells indiscriminately.
Another notable aspect of the exo-erythrocytic phase is that the parasites are immune to the host’s resistance mechanisms. The parasites are not susceptible to the action of any kind of anti-malarial drug during this phase.
The exo-erythrocytic phase of asexual multiplication in Plasmodium parasites is a critical stage in the life cycle of the parasite. The formation of a reservoir of merozoites in the liver cells ensures the continuous production of infectious forms of the parasite, leading to the onset of malaria symptoms. The differentiation of metacryptozoites into micro and macro forms adds another layer of complexity to the process, highlighting the parasite’s ability to adapt to different host environments. Understanding the exo-erythrocytic phase is essential for the development of effective treatments and control strategies for malaria.
c. pre-patent and incubation periods
- Pre-patent phase refers to the time between first sporozoite infection and the first appearance of parasites in the blood.
- It lasts around 8 days in P. vivax.
- With P. vivax, the incubation period between infection and the onset of the first malarial symptoms is approximately 10-17 days (14 days on average).
4. Erythrocytic schizogony
- The third multiplication phase of schizogony, also known as erythrocytic schizogony, occurs in erythrocytes. This complex cycle commences when micro metacryptozoites penetrate the erythrocytes. Single metacryptozoites invade individual RBCs and progress through various stages including the trophozoite stage, signet ring stage, amoeboid stage, and schizont stage.
- Upon invasion, the RBC becomes rounded with a large nucleus and enlarges by ingesting hemoglobin of corpuscles. This stage is called the trophozoite stage. As the trophozoites grow in size, a large non-contractile vacuole appears, pushing the nucleus towards the periphery, forming a ring-like structure known as the signet ring stage. The signet ring stage is approximately 1/3 to ½ the size of an erythrocyte.
- The signet ring trophozoites ingest a substantial portion of the cytoplasm of RBC, forming a food vacuole into which they secrete digestive enzymes. These enzymes bring about the proteolysis of blood hemoglobin, breaking down the protein component into hematin. The trophozoites use protein as food while depositing hematin in the form of hemozoin, which is a toxic malarial pigment.
- As trophozoites enlarge and vacuole starts disappearing, they develop pseudopodial processes in the cytoplasm and transform into the amoeboid stage. This stage is called the amoeboid stage. Amoeboid trophozoites, after feeding, become rounded, grow in size, and become erythrocytic schizonts.
- Asexual multiplication takes place in the schizont to form 12 to 24 oval-shaped merozoites. This phase is known as erythrocytic schizogony. The much-weakened erythrocyte bursts, and merozoites are liberated into the plasma in the form of erythrocytic merozoites. These merozoites enter new erythrocytes, repeating the erythrocytic schizogony once every 48 hours.
- The merozoites are arranged towards the periphery due to the presence of hemozoin at the center, similar to the arrangement of petals in a rose flower. Hence, this stage is called the rosette stage. Numerous yellowish eosinophilic granules appear in the cytoplasm of the host corpuscles, known as schuffner’s granules. These dots are believed to be the antigen excreted by the parasites.
5. Post-erythrocytic schizogony
- Merozoites formed by erythrocytic schizogony can occasionally reach liver cells and undergo schizogonic development there. This condition is termed pre-erythrocytic schizogony.
6. Formation of gametocytes
- Malaria is a life-threatening disease that affects millions of people worldwide. It is caused by a parasite called Plasmodium that is transmitted through the bites of infected mosquitoes. The parasite goes through several stages in the human body, including the stage where it produces gametocytes.
- After many generations of schizogony in the blood, some of the merozoites invade new red blood cells (RBCs) and do not change into schizonts. Instead, they grow and transform into two types of gametocytes: macrogametocytes and microgametocytes.
- Gametocytes appear in the peripheral blood at various intervals after the onset of fever, and they remain inactive while in the human blood. Macrogametocytes, also known as female gametocytes, are larger (10-12µ) and numerous in number. They have a small, compact peripheral nucleus and contain reserved food materials. The cytoplasm is dark in color due to the presence of hemoglobin pigment.
- On the other hand, microgametocytes, also known as male gametocytes, are smaller (9-10 µ) and motile, and they are few in number. They have large, centrally placed nuclei and lack reserved food. They stain faintly, and the cytoplasm is light in color and clear.
- Both types of gametocytes contain a large amount of hemozoin, which is a byproduct of hemoglobin digestion by the parasite. This hemozoin enlarges the erythrocytes, or red blood cells, in which the gametocytes reside. The gametocytes do not divide but remain in human blood corpuscles for several weeks.
- For the Plasmodium parasite to complete its life cycle, it is necessary for the gametocytes to be taken into the body of Anopheles mosquitoes for further development. If this does not happen, the gametocytes will either degenerate or die.
- In conclusion, the production of gametocytes by the Plasmodium parasite is an essential step in its life cycle. Understanding the different types of gametocytes and their characteristics can help in developing strategies to control and prevent the transmission of malaria. It is crucial to continue researching and studying the various stages of the parasite to find ways to combat this deadly disease.
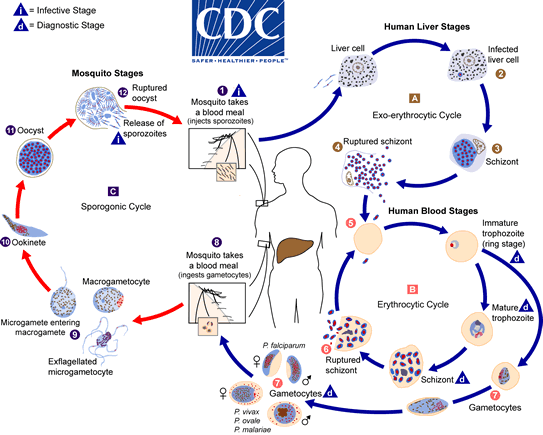
The life cycle of the malaria parasite involves two hosts. During a blood meal, a female Anopheles mosquito infected with malaria injects sporozoites into its human victim. The numeral 1 Sporozoites invade cells of the liver The number 2 and schizonts mature The number 3, which causes merozoites to break and release The numeral four In P. vivax and P. ovale, hypnozoites can survive in the liver and trigger relapses by infiltrating the bloodstream weeks or years later. After this initial liver replication (exo-erythrocytic schizogony) The letter A), the parasites reproduce asexually in the erythrocytes (erythrocytic schizogony The letter B). Infecting red blood cells are The numeral 5 The trophozoites of the ring stage mature into schizonts, which rupture to release merozoites. The value six Certain parasites undergo sexual erythrocytic differentiation (gametocytes) The number seven Blood-stage parasites are responsible for the disease’s clinical signs.
An Anopheles mosquito consumes male (microgametocytes) and female (macrogametocytes) gametocytes during a blood meal. The number eight The process through which parasites multiply in mosquitoes is known as the sporogonic cycle. Capital letter C When in the mosquito’s stomach, microgametes invade macrogametes, resulting in the formation of zygotes. The numeral nine The zygotes then transform into motile and elongated (ookinetes), which enter the midgut wall of the mosquito and grow into oocysts. The number eleven The oocysts multiply, burst, and then produce sporozoites. The number 12, which reach the salivary glands of the insect. By inoculating sporozoites into a new human host, the malaria life cycle is perpetuated.
B. Life cycle in mosquito or Sexual Cycle in mosquito
1. Ingestion by mosquito
- When a female Anopheles mosquito ingests the blood of infected individuals, carrying gametocytes and other phases of the erythrocytic cycle, she transmits the disease (e.g. erythrocytic merozoite). They reach the stomach, where all stages except gametocytes are digested together with RBCs. Now, the life cycle continues towards its conclusion through the subsequent phases.
2. Gametogenesis/ Gametogony (Formation of gametes)
- The creation of gametes from gametocytes is referred to as gametogenesis or gametogony.
- Microgametes and macrogametes are the two forms of gametes, similar to gametocytes.
- In the mid-gut of the mosquito, microgametocytes undergo ex-flagellation.
- It is believed that the mosquito’s chilled blood stimulates the process of ex-flagellation.
- The microgametocyte nucleus divides into six to eight daughter nuclei; the initial division is meiotic.
- These nuclei travel to the periphery with the cytoplasm, generating a structure resembling a flagellum. Consequently, six to eight male gametes with flagella are produced from each microgametocyte. The structure is known as microgametes or sperms.
- The length of these microgametes is around 20 to 25 microns.
- The action of flagella allows the gametes to segregate and actively search for female gametes in the mosquito’s stomach.
- Two polar bodies are then forced out of the maturing macrogametocyte to become female gametes, macrogametes, or megagametes.
- On one side, the non-motile female gamete produces a cytoplasmic protrusion known as the cone of reception or fertilisation cone.
3. Fertilization
- The nucleus of the female gamete positions itself close to the receptive cone.
- If microgametes reach macrogametes, they enter the female gamete at the location of the cytoplasmic cone, resulting in the development of a diploid zygote or synkaryon through the fusing of the nucleus and cytoplasm of two gametes.
- The process of fertilisation is known as syngamy.
- Syngamy is anisogamous because the male and female gametes that it unites are different.
- In the stomachs of mosquitoes, zygotes develop approximately 9 to 10 days following a blood meal.
4. Ookinete
- Approximately 24 hours after fertilisation, the zygote is spherical and stationary, but it soon becomes elongated, worm-like, and motile.
- Nowadays, zygotes are referred to as ookinetes or vermicules.
- It is around 15-22 µ inches long and 3 µ inches wide.
- It penetrates the stomach wall with the assistance of lytic secretion. It settles into the stomach’s inner wall.
- Ookinetes have a central irregular nucleus, dense cytoplasm, brown pigment granules, many mitochondria, and ribosomes, as seen by electron microscopy.
- This shows that extremely fast protein synthesis occurs at this stage of the parasite.
- Due to the existence of ectoplasmic contractile fibrils, ookinetes are motile.
5. Encystment
- Ookinetes invade the midgut wall and settle just under the thin membranes that separate the midgut from the hemocoel.
- The oocyst stage occurs when the ookinete assumes a spherical shape, absorbs nutrients from the stomach wall, and becomes encased in a thin, elastic, and porous cyst wall.
- The expanding oocyst is sometimes referred to as sporont.
- The cyst wall is partly secreted by ookinetes and partly generated from the mosquito’s stomach tissue. Around 500 oocysts are seen on the stomach wall of an infected mosquito. The ookinetes that fail to pierce the stomach wall are expelled with the mosquito’s faeces.
6. Sporogony
- Each oocyst begins sporogony, a phase of asexual proliferation.
- It is the creation of sporozoites by asexual multiple fission from the zygote nucleus.
- Oocysts develop and mature. The oocyst nucleus divides first by meiosis and then by mitosis, creating a large number of haploid nuclei (2-3 days) and sporozoites-making cells called sporoblasts.
- Again, sporoblast nuclei grow, and their cytoplasm becomes restricted.
- Hence, the resulting structures in the sporoblasts elongate to create thin or sickle-shaped sporozoites.
- Each oocyst may contain 10,000 sporozoites, and a cluster of sporozoites forms around the vacuoles.
- The process of sporozoite development is known as sporogony and is finished in 10 to 20 days, depending on the temperature, after the mosquito has ingested gametocytes.
- Thus, several sporozoites fill each oocyst. Now, these exert pressure on the oocyst, causing it to burst or rupture, releasing hundreds of sporozoites into the mosquito’s bodily cavity (hemocoel).
- The very active and mobile sporozoites then reach the mosquito’s salivary glands and enter the hypopharyngeal duct.
- When the mosquito bites a healthy individual, the sporozoites are then ready to infect them. And the life cycle continues to repeat.
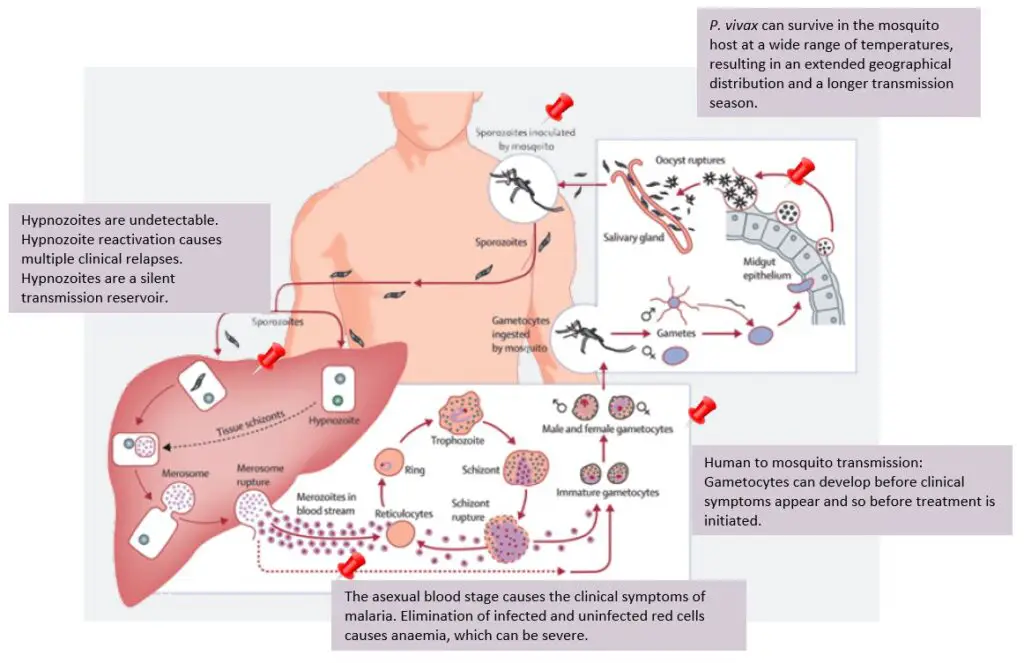
Pathogenicity of Plasmodium Vivax
- Despite its low virulence, Plasmodium vivax is now recognised as one of the leading causes of severe and deadly malaria. Blood cells infected with vivax become malformed despite the apparent rarity of parasite sequestration.
- Severe amenorrhea is observed alongside repeated hemolysis of the majority of healthy erythrocytes with heightened susceptibility. Inflammation of the alveolar-capillary membrane’s permeability causes lung damage. Nevertheless, vivax-related coma is uncommon.
- A double membrane is a plasmodium characteristic of the red blood cell, and the plasmalemma is intimately attached to the cytoplasm. Plasmodium vivax’s cytoplasm is composed of ribonucleoproteins comprising small, dense particles.
- Mitochondria carrying the malaria parasite Plasmodium vivax have a twofold membrane with peripheral cristae and a more rigid, less central area.
- The life cycle of Plasmodium vivax is separated into two stages: asexual life or schizogony in males and sexual life cycle in female Anopheles mosquitoes.
- In schizogony, Plasmodium vivax in humans and plasmodium reproduce asexually in liver and RBC cells. An infected female Anopheles mosquito with sporozoites in its salivary gland infects a man.
- As it punctures the man’s skin, the mosquito injects its infected saliva into the man’s circulation. The sporozoites multiply by the hundreds in the blood of the host. Sporozoites are the parasite’s inflected forms.
FAQ
What is the habitat of Plasmodium vivax?
Plasmodium vivax is a parasite that causes malaria in humans. It is primarily found in tropical and subtropical regions, where the Anopheles mosquito, which transmits the parasite, thrives. The habitat of Plasmodium vivax includes areas with high humidity and rainfall, such as forests, marshes, and areas near stagnant water bodies. These conditions are ideal for the breeding and survival of Anopheles mosquitoes, which require water for their larval development. Plasmodium vivax can also survive in temperate regions, where the climate is suitable for the Anopheles mosquito to breed. It is important to note that the habitat of Plasmodium vivax is not limited to any specific geographical location, and the parasite can be found in various parts of the world. Preventive measures, such as controlling mosquito breeding sites and using insecticide-treated bed nets, can help reduce the spread of the parasite and prevent the transmission of malaria.
Describe the trophozoite stage of Plasmodium vivax.
The trophozoite stage of Plasmodium vivax is one of the several stages of the parasite’s life cycle. During this stage, the parasite is actively growing and multiplying within the red blood cells of the human host.
The trophozoite stage begins after the merozoites, which are the offspring of the parasite, invade and multiply within the red blood cells. Once inside the red blood cell, the merozoites develop into trophozoites.
During the trophozoite stage, the parasite feeds on hemoglobin, which is a protein found in the red blood cells. The parasite breaks down the hemoglobin, releasing heme, which is toxic to the parasite. To avoid the toxic effects of heme, the parasite converts it into a crystalline substance called hemozoin, which accumulates within the parasite’s digestive vacuole.
As the trophozoite stage progresses, the parasite grows in size, and the red blood cell becomes enlarged and distorted. The trophozoite stage lasts for about 24 to 48 hours, depending on the species of Plasmodium.
After the trophozoite stage, the parasite enters the schizont stage, where it undergoes multiple divisions to produce new merozoites. The merozoites are then released from the red blood cells, and the cycle continues.
Understanding the different stages of the Plasmodium vivax life cycle is crucial in developing strategies to control and prevent the spread of malaria. Preventive measures, such as using insecticide-treated bed nets and taking antimalarial medication, can help reduce the transmission of the parasite and prevent the development of severe malaria.
Why is Plasmodium called Sporozoa?
Plasmodium is classified as a type of protozoa known as Sporozoa because of its unique reproductive mechanism. Sporozoa are a group of parasitic protozoans that reproduce through a process known as sporogony.
In the case of Plasmodium, the parasite undergoes a complex life cycle that involves both sexual and asexual reproduction. The asexual stage of the parasite’s life cycle occurs within the red blood cells of the human host, where the parasite multiplies through schizogony, a process where the parasite undergoes multiple divisions to produce new merozoites.
The sexual stage of the parasite’s life cycle occurs within the mosquito vector, where the parasite undergoes sporogony. During sporogony, the parasite divides to produce sporozoites, which are then transmitted to the human host when the mosquito bites.
The unique feature of sporozoans, including Plasmodium, is that they produce spores, which are specialized cells that enable the parasite to survive in harsh environments, such as outside the host’s body. The spores are resistant to heat, cold, and desiccation, allowing them to remain viable for extended periods.
The production of spores during sporogony is why the group is called Sporozoa. This feature distinguishes them from other protozoans that reproduce through binary fission or budding.
Which is known as the infective stage of Plasmodium vivax?
The infective stage of Plasmodium vivax is the sporozoite stage. Sporozoites are the form of the parasite that is transmitted from the mosquito vector to the human host during a mosquito bite.
When a mosquito carrying the sporozoites bites a human, the sporozoites are injected into the human’s bloodstream. The sporozoites then travel to the liver, where they invade and multiply within the liver cells. This is known as the exoerythrocytic stage of the parasite’s life cycle.
After a period of time, the sporozoites develop into merozoites, which are released into the bloodstream and invade the red blood cells. This is known as the erythrocytic stage of the parasite’s life cycle, during which the symptoms of malaria occur.
Understanding the infective stage of Plasmodium vivax is important in developing strategies to prevent the transmission of the parasite. Preventive measures, such as using insecticide-treated bed nets, wearing long-sleeved clothing, and using mosquito repellent, can help reduce the risk of being bitten by an infected mosquito. Additionally, antimalarial medication can be taken to prevent the development of the parasite if bitten.
What is the signet ring stage?
The signet ring stage is a stage in the development of Plasmodium vivax, a parasite that causes malaria. During the signet ring stage, the parasite is in its early trophozoite stage and is characterized by a small, ring-shaped structure within the red blood cell. The parasite appears as a small, pink or purple dot within the center of the red blood cell, giving it a signet ring appearance.
The signet ring stage is an important stage in the life cycle of the parasite because it is the beginning of the parasite’s asexual replication within the human host. During this stage, the parasite feeds on hemoglobin, a protein found in red blood cells, and begins to replicate.
As the parasite replicates, it undergoes multiple stages of development within the red blood cells, causing the cells to rupture and release the newly formed merozoites into the bloodstream. These merozoites can then invade other red blood cells, causing further infection and the development of the symptoms of malaria.
Identification of the signet ring stage is important in the diagnosis of malaria, as it is a characteristic feature of the early stages of infection. Blood smears are typically used to identify the presence of the parasite and the stage of infection. Early diagnosis and treatment of malaria are critical in preventing the development of severe complications associated with the disease.
References and Sources
- https://www.vivaxmalaria.org/p-vivax-malaria-an-introduction/lifecycle-of-plasmodium-vivax-malaria
- https://docs.google.com/file/d/0B0Izh6GcIA_DZ2RsYzRvbFlHRUU/edit?resourcekey=0-rSNhHnnTJzwTMz9HLZRqVA
- https://www.onlinebiologynotes.com/plasmodium-falciparum-morphology-life-cycle-pathogenesis-and-clinical-disease/
- https://www.biologydiscussion.com/protozoa-2/plasmodium-vivax-morphology-and-life-history-zoology/49295
- https://www.biologydiscussion.com/malaria/plasmodium/life-cycle-of-plasmodium-with-diagram-malaria-human-diseases-biology/82276
- https://microbiologynotes.com/life-cycle-of-plasmodium-vivax/
- https://www.vedantu.com/biology/plasmodium-vivax
- https://microbenotes.com/plasmodium-vivax-life-cycle/

good.