Table of Contents
What are food additives?
According to the Food Safety Standard Authority of India (FSSAI). Food additive may be defined as any substance not normally consumed as a food by itself or used as a typical ingredient of the food, whether or not it has nutritive value, the intentional addition of which to food for a technological (including organoleptic) purpose in the manufacture, processing, preparation, treatment, packaging, transport or holding of such food results, or may be reasonably expected to result (directly or indirectly), in it or its by-products becoming a component of or otherwise affecting the characteristics of such food but does not include – contaminants or substances added to food for maintaining or improving nutritional qualities.
Food additives, in simpler terms, are substances that are added to food by manufacturers to make it easier to process or improve its appearance, texture, and quality. This does not include any potential contaminants that might accidentally enter food or ingredients added to food to maintain or improve nutritional quality. It is only used to describe substances that are intentionally added to food. These substances include flavours, vitamins, propionatesorbate, oxidizing agents, and flavours.
- Chemical preservatives are food additives that are added to food to prevent its deterioration.
- These deteriorations can be caused by microorganisms or food enzymes.
- Chemical preservatives are used to inhibit the growth and activity microorganisms.
- Preservatives can inhibit microorganisms through interfering their cell membranes, enzyme activity, and genetic mechanisms.
- Others preservatives can be used to inhibit the oxidation and unsaturated fat oxidation, as neutralizers and stabilizers, to prevent physical changes, to protect microorganisms from damage, to prevent water loss, or to stop undesirable micro-bial and enzymatic reactions.
Why are Additives Used in Foods?
- Many people take the usefulness of additives for food as a given. Because most people don’t live on farms anymore, additives are essential to keep food fresh and delicious while it travels thousands of miles to reach markets.
- Additives can also increase the nutritional value of some foods. They can also make certain foods more appealing by changing their texture, colour, or taste.
- This is why it’s important to look at the ingredients that go into making good bread.
- To achieve whiteness, flour is treated with a mild oxidizing agent. Vitamins may also be added to enhance nutritional quality. Salt, sugar, and flavours are all added to produce desired taste and flavour. Glycerol monostearate provides soft texture, while propionates and sorbates provide better quality for long-distance transportation and marketing.
- Every additional component in bread manufacturing has a positive effect on the desired quality of the final product, which is crucial for its marketability and acceptance by the customer.
Classification Of Food Additives
There are many types of food additives available, including:
- antioxidants
- preservatives
- food colours
- food flavours
- emulsifiers and stabilizers
- anti-caking agents
- sequestrants
- acid, bases and buffers
- anti-foaming agents
- sweeteners
- enzymes, and
- leavening agents
1. Antioxidants
Unsaturated organic molecules, mostly fats, colors, vitamins, and other nutrients in food, are responsible for the problem. They are extremely unstable to atmospheric oxidation. They undergo many chemical and physical changes, and can cause obnoxious odours in food storage. Products made from meat, fish, eggs, and milk are more susceptible to spoilage. The flavour of stored foods can be affected by auto-oxidation. It also causes a loss in essential fatty acids, vitamins and other nutrients. Second, products of oxidation react to proteins, causing loss of essential amino acid, digestibility, flavour, texture, and a decrease in the food’s nutritional value.
- Antioxidant is a substance that, when added to food, retards or prevents the oxidative deterioration.
- The FSS (Food Product Standard and Food Additive Regulation) 2011 states that this excludes substances such as sugar, cereal, oils and flours.
- The regulation prohibits the addition of any other antioxidant than lecithin, ascorbic Acid, and tocopherol to any food.
- The following antioxidants may be added to oils and fats other than butter and ghee, but not in excess of the concentrations mentioned against them.
- Dry mixtures of rasgollas or vadas can contain butylated hydroanisole (BHA), not exceeding 0.02 percent, calculated on the basis fat content.
- Flavoring agents may also contain permitted antioxidants, but in a concentration not exceeding 0.01 percent.
- BHA may be found in butter and ghee, but not more than 0.02 percent. Fat spread may contain BHA or Tertiary-butyl-hydroquinone (TBHQ) in a concentration not exceeding 0.02 per cent by weight on fat basis.
- Ready-to-eat breakfast cereals can contain BHA up to 0.005 percent (50 ppm). Ready-to-drink infant formula milk substitute may contain lecithin or ascrobyl palmitate up to a maximum of 0.5g/100ml and 1mg/100ml, respectively.
- BHA may be used with antioxidants listed as Nos. The mixture must not exceed 0.02 percent in any of the cases 1 through 4.
2. Preservatives
- Preservatives are substances that, when added to food inhibit, inhibit, or arrest the activity microorganisms like fermentation, acidification, and decomposition.
- India has two types of preservatives.
- Class I
- Preservatives of Class II.
Class I preservatives
Included under Class I preservatives are items of common use such as:
- Common salt
- Sugar
- Dextrose
- Glucose Syrup
- Spices
- Vinegar or acetic acid
- Honey
- Edible vegetable oils
These preservatives are found in foods such as pickles, relishes, chutneys, and pastes that we make at home. These preservatives can be added to any food item without restriction, unless otherwise stated in the FSSAI Rules.
Class II preservatives
These are some examples of Class II food preservation additives:
- Benzoic acid, including its salts
- Sulphurous acid, including its salts
- Nitrates and nitrites of potassium or sodium
- Sorbic acid, including its sodium and potassium salts
- Propionic acid, including its calcium and sodium salts as well as its esters
- The sodium, potassium and calcium salts of lactic acid are all included
- Acid calcium phosphate
- Nisin
- Sodium diacetate
- Methyl or propyl parahydroxy-benzoate
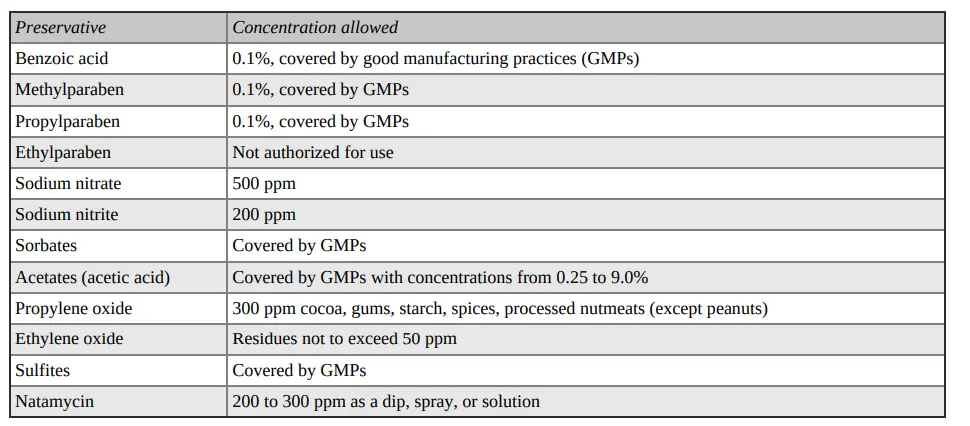
Class II preservatives are only allowed to be used in certain foods. The FSS Rules also specify the maximum amount of preservative that can be added to those foods. As illustrated in Figure 7.5, the presence of any Class II preservatives in food must be disclosed on the food’s packaging. Except as specified in the Rules, food cannot contain more than one Class 2 preservative. In some foods, such as jams, marmalades, and preserves, benzoic acid and sulphur dioxide can be combined in the following amounts: 40 parts per Million and 200 parts per millions, respectively. When both preservatives can be used together, and the amount of sulphur dioxide in the mixture is 20 parts per Million, the percentage of benzoic acid should not exceed 100 parts for every million.
3. Food Colours
- Many food processing operations, such as drying, canning and roasting, frying, etc., result in loss of natural colour. This can cause food to lose their natural colours. This makes it necessary to add synthetic colour to processed foods.
- Foods can also contain colour additives to correct natural variations in colour.
- There are a few other reasons to add colour to food:
- To enhance natural colours but at a lower level than the ones usually associated with food.
- To give colour to food that otherwise would be almost indistinguishable.
- To protect vitamins and other nutrients that could be damaged by the sun.
- To offer a wide range of food options to customers.
- To compensate for seasonal or natural variations in food, raw materials or processing effects to meet consumer expectations
- However, certain foods are not allowed to be colored.
Classification of Food coloring additives
There are two main categories of food colouring:
- Natural and
- Synthetic colours
Natural food colours
- Since prehistoric times, natural food colours have been used. Caramel may be used in place of labelling as one of the natural colours.
Examples
- Anthocyanins comprise a diverse group of glycosidic derivatives of the 2-phenylbenzophyrylium structure, imparting blue, violet and red color to many edible fruits and vegetables.
- Carotenoids, which are oil-soluble colors found in plants and animals and consist of aliphatic and bicyclic unsaturated Terpenes made up of eight isoprene units. Xanthophylls, a group of yellow carotenoid colors closely related to the carotenes, but with keto or hydroxyl substitutes. Examples of carotenoids or xanthophylls extracted from natural extracts include Bixaorellana which contains bixin, Betalaines from Beta vulgaris, Cucurmin derived form the rhizome from Curcuma longa and L. annatto, which is derived from Bixa corellana seeds; and saffron which is a dicarboxylic carotenoid that comes from the stigmas of Crocus species flowers of Crocus s sativus.
- The dried bodies of D. coccus females are crushed to extract cochineal and other pigments. This is just before egg-laying. Carmine can be used in powder form in many foods. Alkannet is an alcohol-extracted extract from the roots Alkanna Tinctoria Tansch’s Alkanna tinctoria Tansch. It is used in ice creams.
- Monoascus is a Monoascus species that produces microbial colorants such as Monascus. They are used to give wine a reddish color. It is heat and oil-soluble.
- The biliproteins of algae are soluble and can be used to make chewing gum. They are known as the red phycoerythrins or the blue phycocyanins.
Synthetic food colours
- Except for titanium dioxide (food-grade), inorganic colouring matter or pigments are not permitted to be added any food. However, it is allowed to be added as a chewing gum ingredient to powered soft drink concentrates mixed fruit beverage drink to a maximum of 1% and 0.01 percent respectively.
These synthetic food colors are only allowed in certain foods.
- Frozen desserts, milk lollies, ice cream, flavoured milk, yogurt, ice-cream powder
- Biscuits include biscuit wafers, pastries and cakes, as well as confectionery, cakes, confectionery and thread candies.
- Peas, strawberries, and cherries in hermatically sealed container, preserved or processed papaya juice, canned tomato juice or fruit syrup, fruit cordial, fruit jellies or jam, marmalade or candied, crystallized, or glazed fruits
- Non-alcoholic carbonated and ready-to-serve non-carbonated synthetic beverages include sherbets.
- Custard powder
- Ice candy and jelly crystal,
- Flavor emulsions and flavour pastes are only allowed for carbonated and non-carbonated beverages.
4. Flavouring Agents
- Flavouring agents can be described as flavour substances, flavour extracts, or flavour preparations that are capable of imparting flavouring qualities, such as taste and odour, to food.
- There are three types of flavouring agents –
- Natural flavour: These flavours can only be obtained through physical processes using vegetable or animal raw materials.
- Nature identical flavourings substances: These are chemically extracted from aromatic raw materials, or synthesized. They are chemically identical with natural products.
- Artificial flavourings substances: These are synthetic flavourings that have not been found in natural products.
- The use of the following flavouring agents are prohibited in any article of food in India, namely: Coumarin and dihydrocoumarin, Tonkabean (Dipteryl Odorat) and, B asarone and cinamyl anthracilate, Estragole, Ethyl Methyl Ketone, Ethyl-3-Phenylglycidate, Eugenyl methyl ether, Methyl napthyl Ketone, P.Propyl anisole, Saffrole and Isosaffrole, Thujone and Isothujone and thujone
5. Emulsifying and Stabilizing Agents
- Emulsifying or stabilizing agents are substances that can facilitate uniform dispersion of oils or fats in aqueous media or vice versa.
- These substances are used extensively in the production of bread, confectionery and ice cream.
- India allows a long list of these substances to be added to food products. These substances include: Agar, alginic acid, calcium and sodium alginates, carrageenan, edible gums (such as guar, karaya, arabic, carobean, furcellaran, tragacanth, gum ghatti), dextrin, sorbitol, pectin, sodium and calcium pectate, sodium citrate, sodium phosphates, sodium tartrate, calcium lactate, lecithin, albumin, gelatin, etc.
- Food Additives are the only exception to this ban.
- The polyglycerol esters and polyglycerol ester of fatty acids, and the polyglycerol ester of interesterified ricinoleic Acid may be used in baking products and chocolate up to 0.2% by weight. Bread and cakes may include diacetyl tartaric Acid, mono and diglyceride esters and sucroglycerides.
Example of commonly used stabilizers
- Modified starches: The food processing industry uses modified starches all over the world as thickeners. Binders, stabilizers and thickeners. These starches are used to make sauces thicker, crisp potato chips crisper and give puddings a smooth texture.
- Gums: Gums made from plants and seaweeds are used for thousands of years. Gums are traditionally used in India to make ladoos (sweet preparation). Gums can be obtained from many sources, as you may have read. Gum arabica is a tree exudate, karaya, ghatti and konjac mannan, are seed and root gums. Pectin comes from fruits peels. Gellan gum and xanthan are microbial gums. Alginate and agar are seaweed extracts. Due to their unique properties, gums are used widely in many food products. Gums are used in jams, gravies, sauces and gelling agents in desserts. They also act as an encapsulating agent to stabilize flavours. As additives to fruit products, pectin is allowed.
6. Anti-caking Agents
- Anti-caking substances, anhydrous substances, can absorb moisture and not become wet. These substances are added to foods such as table salt, garlic powder, onion powder, and fruit powder up to a maximum of 2 percent.
- Anti-caking agents are added to free flowing salt to maintain this property.
- Here are some examples of Anti-caking Agents.
- Calcium and magnesium carbonates
- Phosphates of calcium, magnesium
- silicates of calcium and magnesium, aluminum, sodium, or silicon dioxide
- Myristates, palmitates, or stearates made of aluminium, ammonium and stearates thereof
- Calcium, potassium and sodium
- Calcium, potassium, and sodium ferrocyanide can also be used in anticaking agents in common, ioized, and iron fortified salts in quantities not exceeding 10 mg/kg either alone or in combination as ferrocyanide.
7. Sequestrants
- Sequestrants are substances which have been made to be complex with transition metal ions such as copper, iron and cobalt.
- These metals act as powerful catalysts in auto-oxidation processes. Their binding aids in eliminating/ retarding oxidative degradation of foods that could otherwise lead to decolourisation, rancidity, or an unpleasant taste.
- Only a limited number of foods allow the addition of sequestering agent. Citric acid and phosphoric acids, tartaric Acid, ethylene diamine tetra acetate, EDTA, etc. are some examples of common sequestering agent.
8. Buffering Agents (Acids, Bases and Salts)
- Buffering agents are substances that counter acidic or alkaline changes during storage and processing of food. They improve the taste and stability of food.
- Examples of buffering agents are: acetic acid in beverages and soft drinks; calcium oxide in certain dairy products; ammonium phosphate monbasic as a bread enhancer in flour; ammonium carbonate added as a leavening ingredient for baked foods and confectioneries; citric acid, malic Acid, DL lactic Acid and L (+] tartaric Acid as acidulants.
9. Anti-foaming Agents
- Deep fat frying can cause foamy results, particularly for oils such as mustard oil.
- To prevent deterioration and to reduce foaming heights during heating edible oils and fats, the anti-foaming agent is added.
- Dimethyl polysiloxane can be used in India as an anti-foaming agent for edible oils and fats that are deep fried up to a maximum of 10 ppm.
10. Sweetening Agents
- Sweeteners are a common ingredient in many dishes and food items, so it may surprise you to see them listed under food additives.
- Based on their calorific value, which is the number of calories per gram of sweetener, there are three types.
- They can be classified in
- calorie sweeteners,
- Low-calorie sweeteners
- Non-calorie sweeteners (which have little to no calories)
Calorie sweeteners
- These sweeteners provide 4 calories per gram and a sweet taste.
- Cane sugar, glucose syrups, jaggery and honey are all common natural sweeteners used in food.
- We should be cautious about excessive intake of caloric sweeteners, given the rising incidence of obesity and diabetes in the population.
- These sweeteners can also cause dental problems such as gum disease and caries.
Low-calorie sweeteners
- These substances are less sweet than sucrose (sugar), and they provide energy of between 1 to 3 calories per Gram.
- These sweeteners include sugar alcohols also known as polyols (xylitols, sorbitols, mannitols, etc.).
- They are found in many fruits and vegetables, but they are often made for commercial use.
- Polyols can be used to help with diet control and reduce calories. They also do not cause dental cavities. They are used in food processing to impart unique properties that improve the product’s texture and stability.
Non-calorie sweeteners
- They can be either natural or artificially prepared.
- Non-calorie natural sweeteners include a range of proteins found in tropical fruits and plants, such as monellin. miraculin, monellin, and thaumatin are some examples.
- They have not been thoroughly tested for safety. These sweeteners must also be economically feasible to produce commercially.
- More people are using synthetic high-intensity sweeteners.
- They are also allowed to be used in India. They are very rare and are only used in small amounts.
- These sweeteners do not cause tooth decay. The most commonly used artificial/intense sweetnesseners in India are saccharin, sucralose, acesulfame pot, sucralose, and Neotame. These sweeteners can also be used as table-top sweeteners to flavor your tea, coffee, and milk. Instead of sugar.
11. Other Additives
A. Enzymes
Food processing is a complex process that involves enzymes. They are used primarily in the food processing industry to separate carbohydrates, proteins, and lipids. Enzymes are used in many food processing industries, including for making cheese, bread, crackers and chocolates, as well as tenderizing meat.
Leavening agents
These leavening agents make it possible to bake fluffy pastries, soft breads, and delicious cakes as well as crisp biscuits. The process of leavening is when a gas (usually carbon dioxide) is introduced to the batter or dough, causing it to expand. There are many chemical leavening ingredients that can be used to enhance the texture, appearance and taste of food. Traditionally, yeast was used as a leavening ingredient. Its main disadvantage is the fact that it is difficult to control and can sometimes produce undesirable flavours. This problem is not present in chemical leavening agents such as baking soda (sodium carbonate). Most chemical leavening systems rely on the reaction between an acid and sodium bicarbonate to produce carbon dioxide. There are many acids that can be used, and each one releases the leavening gas at a different rate. Ammonium bicarbonate, ammonium carbonate are two examples.
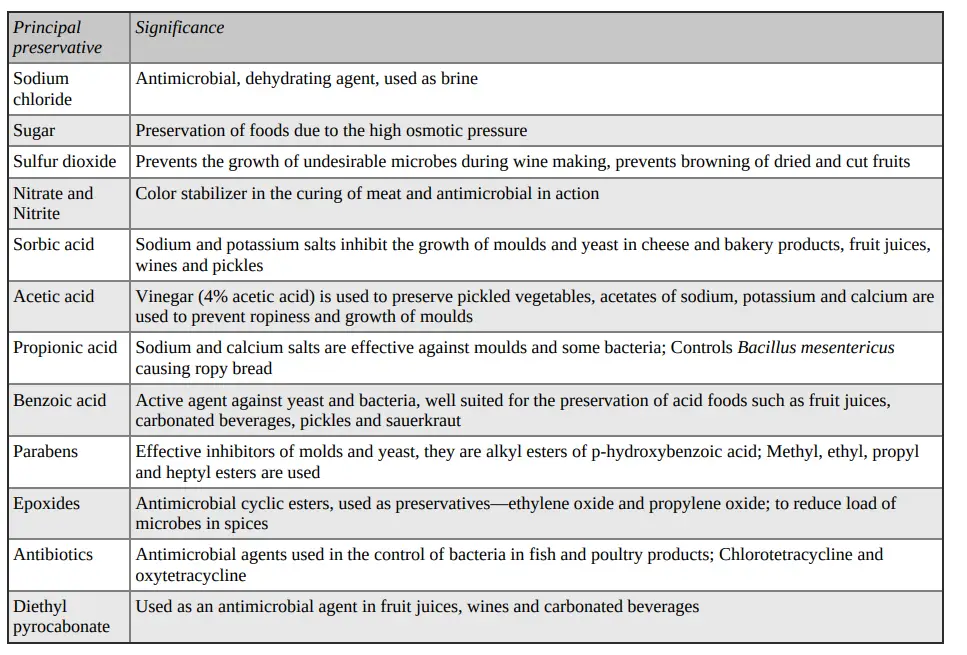
Examples of commonly used Preservatives
1. Organic Acids and Their Salts
Foods can be modified with acetic, acetic and propionic acids, or their salts. In the next section, we will discuss their development in foods during fermentation. As a replacement for fruit flavors, citric acid is used in jams, jellies, sirups and drinks. To brines of different types, green olives, and so on, acetic and lactic acids are added.
Propionates:
- Calcium propionate or sodium is most commonly used to prevent mold growth in baked goods. It also serves as a mold inhibitor in many cheese foods.
- They have been used experimentally or on a small scale in butter, jelly, jams and jellies, figs and apple slices, as well as malt extract.
- They are effective against molds and inhibit most yeast and bacteria.
- They lose effectiveness as pH increases, with pH 5-6 being the optimal pH depending on the food item.
- These are excellent preservatives for bread, and other baked goods. While baking kills most molds and the oven heat can cause them to become contaminated, there are times when the loaves may be sliced or wrapped in crumbs, which is why the propionates are necessary.
- They have little to no inhibitory effect on yeasts so they can be used in yeast-raised bread dough without interfering or affecting leavening.
- Propionic acid (CH3CH2COOH), is a short-chain, fatty acid. It may affect cell-membrane permeability, but its exact mechanism of fungistatic activity is unknown.
- As a preservative developed for cheeses, propionic acid can be found in Swiss cheese at levels of up to 1%.
Benzoates:
- As an antimicrobial agent in food, the sodium salt of benzoic acids has been extensively used.
- It can be found in jams, jellies and margarine as well as fruit salads, pickles and relishes.
- At pH values close to neutral, sodium benzoate is not very effective. The effectiveness of the undissociated acids is more effective.
- The pH range at which sodium benzoate is most effective is between 2.5 and 4.0. However, some yeasts or molds can be inhibited at pH levels that would allow them to grow.
- Foods are also awash in two esters of p-hydroxybenzoic acids, methylparaben and propylparaben. The butyl and the ethyl esters are used less frequently. They are very similar to benzoic acids in their effectiveness.
- They have the distinct advantage of being more effective at higher pH levels than other benzoates. This is because they are esterified with the carboxyl group, which means that the undissociated mole is retained over a greater pH range. Since it is the undissociated mole that inhibits, the esters work at higher pH values.
- It is unclear what the mechanism of action of benzoates is. However, it is known that the effectiveness benzoic acid ester increases with increasing chain lengths.
Sorbates:
- As the calcium, sodium or potassium salt, sorbic acid is used in food as an antimicrobial additive and spray, dip, coating or coating on packaging materials.
- It’s used in many foods, including cheeses, butter, cakes, drinks, jelly, jams and fruit cocktails.
- While sorbic acid and its derivatives have been shown to inhibit yeast and mold growth, they are less effective against bacteria.
- They work best at low pH levels with a maximum level of use around pH 6.5.
- At pH levels above 4.0, these compounds are more efficient than sodium benzoate.
Acetates
- Although some derivatives of acetic acids, such as monochloroacetic, peracetic, dehydroacetic, and sodium diacetate have been suggested for use as preservatives by experts, not all have been approved by the Food and Drug Administration.
- To inhibit mold growth and temporarily preserve squash, dehydroacetic acid was used to impregnate cheese wrappers.
- Acetic acid, which is also known as vinegar, can be found in pickles, catsup and pickled sausages. It’s also used to make mayonnaise, pickles and pickled sausages.
- Acetic acid is more effective than molds against yeasts and bacteria. Its effectiveness also increases with a decrease of pH. This would favor the addition of the undissociated acid.
- Sodium diacetate was used as a treatment for butter wrappers and cheese spreads.
Nitrites, Nitrates
- These salts can be combined to make curing solutions or curing mixtures for meats.
- When nitrites are decomposed to nitric acids, it forms nitrosomyoglobin. This is formed when it reacts against heme pigments found in meats. It then forms a stable color called’red’.
- The use of nitrates is limited as they are likely to act only as a reservoir for the nitrite. The reaction of nitrates with secondary or tertiary aminos can lead to nitrosamines. These nitrosamines are carcinogenic.
- Bacon may pose the greatest danger from possible carcinogenic effects. However, it is unlikely that nitrite will be used in food for a long time. They are added as sodium nitrite (or potassium nitrite), sodium nitrate (or sodium nitrate) and potassium nitrate (or both).
2. Sulfur Dioxide and Sulfites
- As a way to clean their wine-making equipment, storage vessels and other items, the Romans and Egyptians used sulfur to make sulfur dioxide.
- Sulfites and sulfur dioxide are used today in the wine industry to clean equipment and reduce the natural flora of grape must.
- Aqueous solutions contain sulfur dioxide and sulfites such as sodium sulfite and potassium sulfite. These sulfites include sodium metabisulfite and potassium sulfite.
- Low pH values increase the effectiveness of sulfurous acids.
- There are many mechanisms that sulfurous acid can act on microorganisms, including reduction of disulfide links, formation of carbonyl compounds and reaction with ketone group.
- To treat light-colored dehydrated fruits, the fumes from burning sulfur are used. Dehydrated vegetables are then exposed to neutral bisulfites and other sulfites to dry them.
- Also, sirups, fruit juices, and wine-making have all used sulfur dioxide.
- Certain countries allow the use of sulfites in meats and fish.
- They are used in addition to their antimicrobial properties, and to prevent certain foods from becoming enzymatic or non-enzymatically altered.
3. Ethylene and Propylene Oxide
- These two gases, unlike the chemical preservatives mentioned above, are sterilants.
- Propylene oxide is more effective than ethylene oxide in killing all microorganisms.
- They act as strong alkylating agent against labile hydrogens. They are used primarily as sterilants in packaging materials, fumigation and cold sterilization of many plastics, chemicals and pharmaceuticals.
- They can also be used in dried fruits, dried eggs and cereals, as well as dried yeast and spices.
- The FDA prohibits the use ethylene oxide in spices and other natural seasonings, except for mixtures containing salt.
- Propylene oxide can be used as a package fumigant to dry prunes and glace fruits, as well as as a fumigant to cocoa, gums and spices.
4. Sugar and Salt
- These compounds reduce the aw, which can have an adverse effect microorganisms.
- Sodium chloride can be used in brines or curing solutions, or applied directly to food.
- You can add enough to stop or slow down the growth of microorganisms, or just enough to allow an acid fermentation to occur.
- The following effects have been observed in salt:
- It can cause high osmotic pressure, and thus plasmolysis of cell. The percentage of salt required to inhibit growth or harm a cell will vary depending on the microorganism.
- It dehydrates food by drawing out and binding up water as well as microbial cells.
- It ionizes to produce the chlorine ion which is toxic to organisms.
- It reduces the solubility oxygen in the water.
- It sensitizes cells to carbon dioxide.
- It interferes with the actions of proteolytic enzymes.
- NaCl’s effectiveness is directly affected by its concentration and temperature.
- Sugars such as sucrose or glucose are effective preservatives because they can make water inaccessible to organisms. Sweetened condensed milk and fruits in sirups, jelly, and candies are just a few examples of foods that can be preserved with high sugar levels.
5. Alcohol
- Concentrations between 70 and 95 percent of ethanol, which is a coagulant, and denaturizer, of cell proteins, are most germicidal.
- Flavoring extracts such as lemon and vanilla extracts are preserved by alcohol.
- Beer, beer, and unfortified wines have an alcoholic level that isn’t high enough to prevent spoilage by microorganisms, but it limits the types of wine that can be grown.
- Most liqueurs and distilled liquors contain enough alcohol to protect against microbial attack.
- Methanol is poisonous, and should not be added in to food. However, the traces of Methanol that are added to food by smoking are not sufficient to cause harm.
- Glycerol, which has a dehydrating effect, is an antiseptic in high amounts but is not important for food preservation.
- Propylene glycol can be used to kill microorganisms in the air and has been used as an inhibitor of mold growth.
6. Formaldehyde
- Except for a small component of woodsmoke and formaldehyde, it is forbidden to add formaldehyde into foods. However, this compound is effective against bacteria, viruses, and molds and can be used in areas where its irritating and poisonous properties are not objectionable.
- It is therefore useful for treating walls, floors, and shelves to remove mold spores.
- Paraformaldehyde is a treatment that can be used to stop fungal and bacterial growth in maple tree tapholes.
- Formaldehyde is likely to combine with the free amino groups of proteins in cell protoplasm and injures nuclei. It also coagulates proteins.
7. Woodsmoke
- Smoking food serves two primary purposes: adding desired flavors to preserve them.
- However, other desirable effects can be achieved, such as an improvement in the color and finish of meats, or “gloss” of their outsides, and tenderizing of meats.
- Smoking helps preserve food by immunizing it with chemical preservatives. This is done by the combination of heat and preservatives while smoking and the drying effect, particularly at the surface.
- Smoke is most commonly obtained from burning wood, or preferably hardwoods like hickory. However, it can also be made from burning corncobs and other materials.
- Other woods include apple, maple, maple, beech and walnut.
- To give the fire a thick smudge, you can add sawdust. The temperature and humidity levels are set at levels that are comfortable for the product being smoked. The duration of smoking will vary depending on the type of food.
- The smoking temperatures of meat range from 43 to 71.1 C and the smoking time can last from several hours to several days.
- Woodsmoke may contain a number of volatile compounds which could have bacteriostatic or bactericidal effects.
- Formaldehyde, phenols, and cresols are the next most important of these compounds.
- Other compounds found in smoke include aliphatic acid from formic to caproic, primary and secondary alcohols and ketones, acetaldehyde, acetaldehyde, and other aldehydes, waxes, resins, guaiacol, its methyl, and propyl isomers, as well as catechol, methlcatechol and pyrogallol with its methyl ester.
- Sometimes, these compounds are called pyroligneous acids. Woodsmoke has a greater effect on vegetative cells than bacterial spores. The rate of germicidal activity of smoke increases with its temperature and concentration, and also varies depending on the type of wood used.
- It has been shown that the residual effects of smoke in food are more effective against bacteria than those from molds.
- With an increase in humidity, the concentration of woodsmoke mycostatic material necessary to stop mold growth rises.
- Although it adds flavor, liquid smoke is a mixture of chemicals similar to woodsmoke.
8. Spices and Other Condiments
- Although spices and condiments don’t have a marked bacteriostatic effect at the usual concentrations, they may assist other agents in preventing foodborne pathogens.
- Different spice lots have different effectiveness depending on their source, freshness and whether they were ground or whole.
- For example, mustard flour and the volatile oils of mustard are effective against Saccharomyces cerevisiae, but not as potent against all bacteria as cinnamon or cloves.
- Essential oils of spices can be more inhibitive than ground spices.
- Cloves and cinnamon, which contain cinnamic aciddehyde or eugenol respectively, are usually more bacteriostatic than other spices.
- Ground peppercorn, allspice, and mustard are less inhibitive than nutmeg and ginger.
- Thyme bay leaves, marjorams, marjorams, rosemary, black pepper, and other herbs have weak inhibitory powers against most organisms. They may also stimulate some, such as yeasts or molds.
- Concentrations of the most effective spices in high amounts allow mycelial growth of certain molds, but they also inhibit the formation asexualspores. The volatile oil of mustard was the most effective in fighting yeasts, while oils of cinnamon, cloves, and bay leaves were less effective.
- If spices are not treated to reduce their microbial contents, they can add undesirable microorganisms and high numbers to food items they are part of.
- Other plant materials that are used in seasoning foods such as horseradish and garlic may also be bacteriostatic, or germicidal. These plants as well as those of cabbage and turnip have been shown to inhibit Bacillus subtilis as well as Escherichiacoli.
- Acrolein is the active principle in garlic and onions, and horseradish butyl thiocyanate. These volatile compounds and bacteriostatic properties are also lost when the condiment is exposed to air.
9. Other additives
Water can be added to halogens to wash foods and equipment. They are also used to cool water, add some products to the water, such as butter, or to make it more palatable. To extend the shelf life of fruits, Iodine-impregnated wrappings have been used. Iodophors are combinations of nonionic wetting agent and acid with iodine. They are used to clean dairy utensils. Halogens are used to kill organisms through oxidation, injury of cell membranes or direct combination with cells proteins.
The hypochlorites are usually made of calcium or sodium and produce hypochlorous acid. This powerful oxidizing agent is effective in germicidal agents. However, their effectiveness is decreased if there is any organic matter. The treatment of water used for food plants, cooling, processing, cooling, and other purposes is done by the hypochlorites. They are used in ice to icing fish during transit, and in water to wash the outside of fruits and vegetables. The oxidation and direct chlorination (of cell proteins) can cause microorganisms to be damaged.
Some soft drinks contain phosphatic acid, such as colas.
As a preservative, hydrogen peroxide (an oxidizing agent) has been used. It is usually combined with heat. Pasteurization of milk for cheese can be done by adding H2O2 to the milk and heating it at a low temperature. Catalase is used to decompose excess peroxide. Heat and H2O2 combine to destroy the thermophiles in sugar processing. Other peroxides can be used in food, but not to prevent microbial growth.
We will discuss the gas storage connection to preservation by chilling. The most common combination of chilling and carbon dioxide is used. Combining oxygen or air under pressure with chilling is called Hofius. When foods should not be exposed, nitrogen is used as an inert gaz.
Some countries still use boric acid and/or borates as food preservatives. However, their use in the United States is prohibited. Although powdered boric acid can be sprinkled on foods (e.g. meats), it is not considered to be a strong antiseptic and therefore is not recommended for use. Borax (sodium triborate) can be used to wash vegetables as well as whole fruits like oranges.
Functions of Food Additives
After looking at the role of additives when making high-quality bread, we can summarize the various uses of additives as follows:
- To maintain product consistency — Emulsifiers are used to maintain consistency in product texture. Stabilizers, thickeners and other additives give products a uniform texture. Salt, for example, can flow freely through anti-caking agents.
- To improve or maintain nutritional value — Vitamins or minerals are added to common foods like milk, flour, and cereal to replace those that have been lost or become unavailable.
- To maintain palatability and wholesomeness — Preservatives prevent product spoilage from mould, bacteria, yeast, or air. Food borne illness can be deadly if bacteria is present. Antioxidants prevent oils and fats in baked goods from going rancid and causing off-flavours. They prevent fresh cut fruits like apples from becoming brown when exposed to the air.
- To provide leavening or control acidity/alkalinity — Baking soda can react with heat to release acids. This will allow cakes, biscuits, and other baked goods to rise during baking. For proper flavour, taste, and colour, other additives can be used to modify the acidity or alkalinity in foods.
- To enhance flavour or impart desired colour — Spices and natural and artificial flavours can enhance the flavour of food. To meet consumer expectations, colours can also enhance the appearance of certain foods.
- To enhance the keeping quality or stability of a food — Certain preservatives, stabilizers, and anti-caking agents are used to improve the food’s stability. Increases shelf-life of food products.
What are indirect and direct food additives?
Direct additives are substances that are added to food to fulfill a specific purpose. Direct additives include, for example, aspartame (a low-calorie sweetener) which is used to sweeten beverages. On the label of food ingredients, many direct additives can be identified.
Indirect food additives refer to those substances that are added to food in trace quantities due the food’s packaging, storage, or other handling. Indirect food additives can be found in foods by absorbing tiny amounts of the packaging materials. It is important to ensure that all materials in direct contact with food are safe before they can be used. Additives used in raw materials and ingredients can also make their way into the final food product. Chips and other food items made with edible oil could also contain antioxidants. This principle is called the “Carry over” principle.
Reference
- Morya, Sonia & Sharma, Ankit. (2019). Food Additives and Preservatives.
- https://www.who.int/news-room/fact-sheets/detail/food-additives#:~:text=Substances%20that%20are%20added%20to,sulfur%20dioxide%20(in%20wine).
- https://www.fda.gov/food/food-ingredients-packaging/overview-food-ingredients-additives-colors
- https://egyankosh.ac.in/bitstream/123456789/73121/1/Unit-7.pdf
