Table of Contents
What is Type IV or Delayed-Type Hypersensitivity (DTH)?
- Type IV hypersensitivity, also known as delayed-type hypersensitivity (DTH), is a specific type of immune response that is mediated by T cells rather than antibodies. Unlike other types of hypersensitivity reactions, which occur immediately or within a short period, type IV hypersensitivity takes a longer time, typically a day or more, to develop.
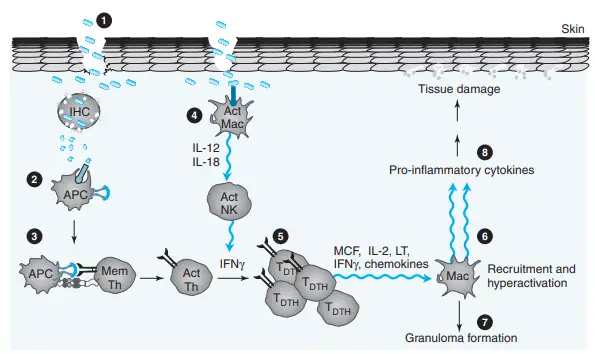
- The underlying mechanism of type IV hypersensitivity involves the interaction of T cells, monocytes, and macrophages. It starts when CD4+ Th1 cells recognize foreign antigens presented by antigen-presenting cells (APCs) through the major histocompatibility complex class II (MHC II) molecules on their surface. Macrophages are a type of APC that can secrete interleukin-12 (IL-12), which promotes the proliferation of CD4+ Th1 cells. These activated CD4+ T cells release cytokines such as IL-2 and interferon gamma (IFNγ), which further stimulate other Th1 cells and orchestrate the immune response. In addition, activated CD8+ T cells directly destroy target cells upon contact, while activated macrophages produce hydrolytic enzymes and can transform into multinucleated giant cells in response to certain intracellular pathogens.
- The exaggerated response of helper T cells and the overproduction of cytokines in type IV hypersensitivity lead to tissue damage, inflammation, and cell death. However, this type of hypersensitivity reaction can generally be resolved by using topical corticosteroids and avoiding triggers.
- Delayed-type hypersensitivity (DTH) occurs when a specific subpopulation of activated T helper cells encounters a particular antigen. These T cells secrete cytokines that induce a localized inflammatory reaction. The inflammatory response is characterized by the influx of non-specific inflammatory cells, particularly macrophages. DTH develops slowly and typically reaches its peak within 48-72 hours after the second encounter with the antigen.
- Unlike antibody-mediated hypersensitivity reactions, tissue damage in DTH is mediated by Th cells and macrophages rather than antibodies. DTH serves as an important defense mechanism against intracellular microorganisms like Mycobacterium tuberculosis, Mycobacterium leprae, and Brucella spp.
- Hypersensitivity refers to an exaggerated immune response or increased reactivity of the immune system to an antigen that the host has previously encountered. According to the Gell and Coombs classification, there are four main types of hypersensitivity reactions: type I, II, III, and IV. Type I, II, and III are immediate hypersensitivity reactions mediated by immunoglobulins (antibodies), whereas type IV is a delayed-type hypersensitivity reaction mediated by lymphoid cells, particularly T cells.
- In type IV hypersensitivity, destruction of cells is mediated by T lymphocytes, along with the involvement of dendritic cells, macrophages, and cytokines. Specific subsets of CD4+ helper T cells, such as Th1 and Th17 cells, or CD8+ cytotoxic T cells mediate the reaction. The onset of type IV hypersensitivity occurs approximately 24 hours after contact with the antigen, usually starting at 2 or 3 days and often lasting for several days. This delayed nature of the reaction is why it is referred to as “delayed hypersensitivity.”
- Type IV hypersensitivity is unique because, unlike the first three types that are antibody-mediated, it is a cell-mediated and delayed response.
Definition of Type IV or Delayed-Type Hypersensitivity (DTH)
Type IV or Delayed-Type Hypersensitivity (DTH) is an immune response mediated by T cells rather than antibodies, characterized by a delayed onset (usually 24-72 hours) after exposure to an antigen. It involves the interaction of CD4+ T cells, monocytes, and macrophages, leading to tissue damage, inflammation, and cell death. DTH is a defense mechanism against intracellular microorganisms and is resolved with topical corticosteroids and trigger avoidance.
Mechanism of DTH
- TH1 subtype CD4 cells are activated during the sensitization phase, which occurs 1–2 weeks after the initial interaction with an antigen.
- Langerhans cells and macrophages, among other antigen-presenting cells (APCs), have been implicated in the activation of a DTH response. It is believed that these cells transfer antigens that enter the body through the epidermis to regional lymph nodes, where they activate T cells.
- Antigen-presenting cells (APCs) display antigens complexed in the groove of major histocompatibility complex (MHC) molecules on their cell surface.
- Antigens coupled to MHC class II alleles, human leukocyte antigen (HLA)-DR, HLA-DP, and HLA-DQ, are presented to CD4 T cells for the majority of protein antigens or haptens related with cutaneous DTH. Certain MHC class II alleles have been identified as causing increased immunological activation in response to antigens.
- Subsequent exposure stimulates the effector phase. The TH1 cells secrete several cytokines that recruit and activate macrophages and other nonspecific inflammatory cells.
- The response is not noticeable until 2–3 days following the second exposure. In most cases, the infection is eliminated swiftly with minimal tissue harm. Nonetheless, in rare instances, particularly if the antigen is not quickly eliminated, a protracted DTH response can be harmful to the host, as the severe inflammatory response evolves into a noticeable granulomatous reaction.
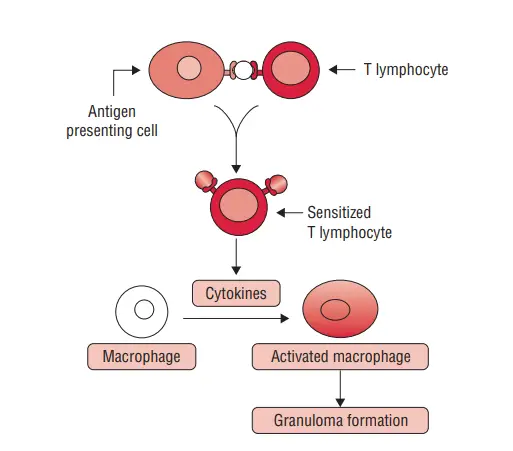
DTH occurs in two phases
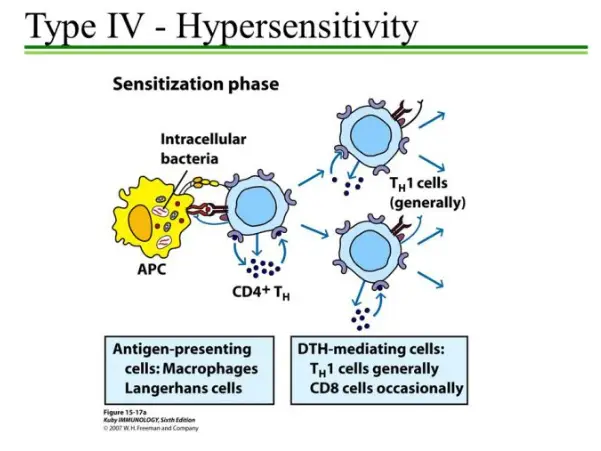
1. Sensitizing phase of DTH
- The sensitizing phase of delayed-type hypersensitivity (DTH) is the initial step in the development of the immune response. It occurs when an individual is exposed to an antigen for the first time.
- During this phase, the antigen is processed by antigen-presenting cells (APCs) such as macrophages and Langerhans cells. These APCs present the processed antigen to CD4+ T cells, which are also known as helper T cells. The interaction between the antigen and the major histocompatibility complex class II (MHC-II) molecule on the surface of the APCs activates the CD4+ T cells, leading to their differentiation into various subsets known as TH cells.
- The TH cells undergo clonal expansion, resulting in an increased population of activated T cells specific to the antigen. The expansion occurs through the binding of the T-cell receptor (TCR) on the TH cells to the antigen-MHC-II complex presented by the APCs. Different types of APCs, including Langerhans cells and macrophages, have been identified as participants in the activation of the DTH response.
- During the sensitizing phase, CD4+ T cells are the primary cells activated in the immune response leading to DTH. However, in some cases, CD8+ T cells, also known as cytotoxic T cells, have been found to induce DTH responses as well. This highlights the flexibility and complexity of the immune response and the involvement of different T-cell subsets in different scenarios.
- Overall, the sensitizing phase of DTH involves the processing and presentation of the antigen by APCs, the activation and clonal expansion of CD4+ T cells, and the potential involvement of CD8+ T cells in certain cases. This phase sets the stage for the subsequent effector phase, where the immune response is further amplified and manifests as the characteristic delayed hypersensitivity reaction.
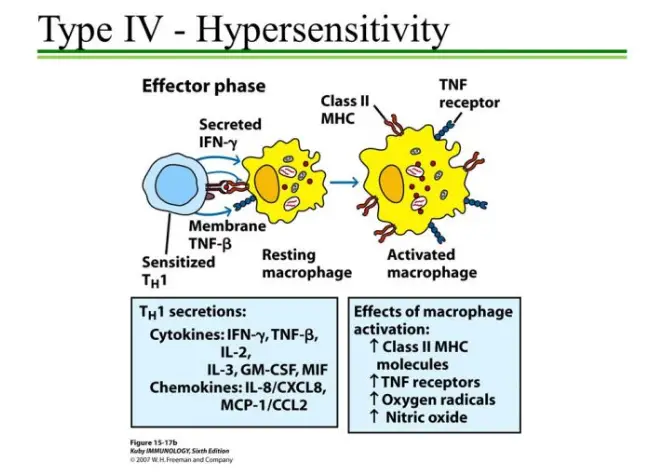
2. Effector phase of DTH
- The effector phase of delayed-type hypersensitivity (DTH) occurs upon subsequent exposure to the antigen after the sensitizing phase. During this phase, the immune response is amplified, leading to the characteristic inflammatory reaction.
- In the effector phase, TH1 cells, a subset of CD4+ T cells, play a crucial role. TH1 cells secrete various cytokines that recruit and activate macrophages and other non-specific inflammatory cells to the site of antigen injection or infection. Macrophages are the principal effectors of the DTH response. Once activated, macrophages exhibit increased phagocytic activity and an enhanced ability to kill the antigen, particularly microorganisms, using cytotoxic lytic enzymes.
- The activated macrophages release lytic enzymes that not only damage the surrounding tissues but also target intracellular bacteria. This influx and activation of macrophages are important for host defense against intracellular bacteria and parasites that are inaccessible to circulating antibodies.
- The increased phagocytic activity and release of lytic enzymes by macrophages in the infected area lead to non-specific destruction of tissues and intracellular pathogens. In most cases, pathogens are rapidly killed with minimal tissue damage. However, in some instances, especially if the antigen is not easily cleared, a prolonged DTH response can become destructive to the host. Intensive and chronic inflammation can develop, leading to a visible granulomatous reaction.
- The formation of a granuloma occurs when continuous activation of macrophages induces them to assume an epithelioid shape and sometimes fuse together to form multinucleated giant cells. These giant cells displace normal cells, forming palpable nodules. They also release high concentrations of lytic enzymes, which further destroy the surrounding tissues, leading to necrosis.
- In summary, the effector phase of DTH involves the secretion of cytokines by TH1 cells, recruitment and activation of macrophages, increased phagocytic activity, and the release of lytic enzymes. This immune response aims to eliminate the antigen and protect the host, but in certain cases, it can lead to chronic inflammation and granuloma formation, causing tissue damage and necrosis.
Types of DTH Reactions
There are two forms of DTH reactions: contact hypersensitivity and tuberculin-type hypersensitivity.
1. Contact hypersensitivity
- Contact hypersensitivity is a sign of DTH resulting from substance-specific sensitization.
- These include medications like sulfamides and neomycin, plant products like poison ivy and poison oak, chemicals like formaldehyde and nickel, as well as cosmetics, soaps, and other compounds.
- This reaction occurs when chemicals acting as haptens penetrate the skin and combine with body proteins to form full antigens to which a person develops a hypersensitivity.
- On second exposure to the same antigen, the immune system responds by launching an onslaught of cytotoxic T cells, which mostly cause skin damage.
- Within 12–48 hours of the second encounter, the syndrome presents as irritation, erythema, vesicles, eczema, or skin necrosis.
2. Tuberculin-type hypersensitivity reaction
Tuberculin reaction is a common instance of delayed hypersensitivity to microbial antigens, which is utilised to diagnose the condition.
Tuberculin skin test
- This test is performed to identify whether or not an individual has previously been exposed to Mycobacterium tuberculosis.
- Tuberculin (PPD), a protein generated from the cell wall of M. tuberculosis, is injected intradermally in this test.
- Positive test results are indicated by the development of a red, slightly enlarged, and firm lesion at the injection site within 48 to 72 hours.
- A positive test result indicates that the individual has been infected with the bacteria, but it does not prove the diagnosis of tuberculosis.
- However, if a patient with a negative tuberculin skin test develops positive, this implies that the patient was infected recently. However, the skin test may also be negative if:
- Infected individuals getting immunosuppressive medicines (such as corticosteroids and anticancer drugs).
- In patients with illnesses linked with reduced cell-mediated immunity, immunosuppression is observed (such as AIDS, sarcoidosis, lymphoma, post measles vaccination, etc.).
- The response to Mycobacterium TB demonstrates that while processes involved in DTH are needed for defence against the infection, they are also responsible for long-term tissue damage. Cytokines (such as TNF and IFN-), which are created to activate macrophages and so control an infection, also initiate additional cascades that ultimately result in substantial tissue damage.
- Other skin tests are also utilised to diagnose DTH. Included are numerous skin tests for bacterial, fungal, viral, and helminthic illnesses. Lepromin is an effective test for leprosy.
- A positive lepromin test implies tuberculosis with intact cell-mediated immunity. A negative lepromin test, on the other hand, shows the presence of lepromatous leprosy with compromised cell-mediated immunity.
- Exposure to the fungi is indicated by positive skin tests in coccidioidomycosis, paracoccidioidomycosis, and other fungal illnesses.
- In both viral and parasite illnesses, skin tests are less specific and less valuable for diagnosis than serological tests.
Chronic DTH Reactions
- DTH reactions are typically triggered by antigens originating from intracellular infections that are resistant to standard immune response strategies. For instance, chronic Mycobacterium tuberculosis is eventually compartmentalised by the formation of a granuloma.
- Granuloma development is dependent on activated Th1 cells and hyperactivated macrophages, as we have observed. The hypersensitivity occurs when these cells do not “retreat” after the virus has been contained.
- Persistent production of pro-inflammatory cytokines and substances causing granuloma development begins to compromise the surrounding healthy tissue. The DTH response then develops into a chronic type IV HS reaction.
- This kind of HS develops more slowly than antibody-mediated varieties due to the time required for T cell activation and differentiation, cytokine and chemokine production, and macrophage accumulation at the site of exposure.
- The site of antigen entrenchment affects the clinical manifestations of a DTH reaction. 2–3 days after exposure, the skin above the location of exposure may seem unnaturally red.
- Calcification, caseation necrosis (tissue deterioration that results in a cheese-like appearance), and cavity formation are frequent clinical findings.
- DTH responses associated with infectious disorders (tuberculosis, leprosy, leishmaniasis), reactions to non-infectious agents (silicosis, berylliosis), and reactions to unknown agents (Crohn’s disease, sarcoidosis) are characterised by infiltrating mononuclear leukocytes.
- When a granuloma is finally encased in a layer of fibroblasts and connective tissue, it can form a lesion that may impair the function of an organ such as the liver or lung.
- Consequences may include chronic liver disease or respiratory failure. Corticosteroids can be utilised to inhibit cytokine production by effector T cells during type IV HS responses.
- If the allergic reaction is localised, topical treatments may be utilised. More severe tissue involvement may necessitate systemic corticosteroid therapy.
- As described in Chapter 22, DTH reactions have been utilised to determine whether an individual has been exposed to a disease in the past.
- An example of such a test is the skin prick test for TB, in which redness and swelling at the injection site of a little quantity of M. tuberculosis antigen indicate that the subject has been infected with the bacteria in the past.
- Comparable tests can be done to determine a previous infection with germs that cause diphtheria or brucellosis. The DTH reaction can also be used to evaluate the functioning of T cells in an individual.
- Candida albicans is so common in our environment that almost everyone has had at least one infection as a youngster.
- Therefore, a DTH test with Candida antigen should induce redness and swelling at the testing site in the vast majority of patients.
- A patient who fails to mount a DTH response during this test generally has impaired T cell function and may have acquired immunodeficiency.
Hypersensitivity Pneumonitis (Hp)
- Hypersensitivity pneumonitis, also known as extrinsic allergic alveolitis, is a type IV HS lung reaction produced by continuous inhalation of an antigen.
- HP can be triggered by a wide variety of antigens, but the resultant lung response often follows the same pattern and occurs in three distinct clinical phases: the acute phase, the subacute phase, and the chronic phase.
- When a sensitised individual inhales the offending antigen, macrophages in the lungs are activated either because these cells ingest the antigenic particles directly or because the complement system is engaged.
- Within 48 hours, macrophage-secreted chemokines (IL-8, MIP-1, and RANTES) induce an influx of neutrophils and T cells. CD4 Th0 cells initiate development into cytokine-secreting Th1 effectors.
- At this stage, the infected individual is likely to develop influenza-like symptoms, including fever, chills, muscle discomfort, a nonproductive cough, and difficulty breathing. Typically, if future exposure to the causal antigen is avoided, symptoms diminish rapidly.
- In the event of a formal diagnosis, a brief course of corticosteroids may be administered to assist reduce the inflammatory reaction. However, if the acute phase of HP is unrecognised and antigen exposure persists or reoccurs, the sub-acute phase ensues, during which hyperactivated macrophages commence granuloma development.
- For several days or weeks, there are few obvious symptoms at this phase. However, weariness and cough then recur and develop gradually but steadily.
- Again, corticosteroids can be of tremendous value to a patient with HP at this stage. In contrast, if no action is taken and exposure persists, the chronic phase of HP develops and leads to fibrosis of lung tissue via the same processes outlined for chronic DTH responses.
- Activated alveolar macrophages produce substantial amounts of TGF, hence promoting fibrosis. HP patients may potentially develop particular antibodies that contribute to the reported disease via type II and type III HS reactions.
- For instance, antibodies may bind to organs in which the inhaled antigen has taken up residence, or they may produce ICs whose deposition promotes lung inflammation.
- In the last stages of chronic HP, patients exhibit progressively difficult breathing and inexorable weight loss. Unfortunately, this phase of the disease is often confused with other lung diseases involving fibrosis, and despite the use of corticosteroids, the damage to the lungs may be irreparable and finally deadly.
- HP is linked to a variety of illnesses that are often designated for their distinguishing antigen. Typically, these antigens are proteins originating from microorganisms, fungi, plants, or animals.
- In some cases, the inducing agent is an inorganic chemical molecule that can activate specific cells only after reacting with lung tissue. In some instances, the induction of type IV HS in HP resembles a lung CHS event.
- Historically, exposure to trigger antigens happened mostly in the workplace, resulting in diseases such as “farmer’s lung” and “cheese washer’s lung.” Nonetheless, when the nature of these illnesses became clearer, work exposure was regulated, so that the majority of HP cases currently result from residential or recreational contact to inhaled antigens.
- Thus, the terms “sauna-disease,” taker’s “hot-tub lung,” and “bird-lung” fancier’s are more common. Studies indicate that only a small percentage of persons exposed to a certain antigen develop HP.
- In farming areas in North America, less than 6% of exposed individuals develop the disease. Disease-free individuals appear to mount a harmless IgG response to inhaled antigens without cellular involvement.
- Similar to IgE-mediated type I HS, the determinants of HP susceptibility are poorly understood. In some cases, persons with HP recall having an acute respiratory infection prior to the onset of symptoms, suggesting that infection may be a risk factor.
- Our explanation of allergy and hypersensitivity concludes here. We will now address autoimmunity, one of the greatest immunological mysteries.
Examples of Type IV (Cell Mediated) Hypersensitivity
- The tuberculin reaction (Mantoux test): This is a “recall” response to pure mycobacterial antigens and serves as the foundation for a diagnostic skin test for a tuberculosis immune response.
- Granuloma formation: Inability of macrophages to eliminate intracellular infections frequently results in persistent activation of pathogen-specific T cells, which leads to granuloma development. Granulomas are generated as a result of macrophages harbouring persistent antigens being ‘walled off’ by the cytokines released.
- Allergic contact dermatitis: Environmental pollutants, metals, or topical drugs that cause epidermal necrosis, inflammation, skin rash, and blisters; allergic contact dermatitis.
- Type-1 diabetes: Insulin insufficiency caused by the destruction of the pancreatic islet cells by cytotoxic T lymphocytes.
Numerous Cytokines Participate in the DTH Reaction
- TH1 cells produce a multitude of cytokines that attract and activate macrophages at the site of an infection.
- IL-3 and GM-CSF cause localised granulocyte-monocyte lineage hematopoiesis. IFN-γ and TNF-β (together with macrophage-derived TNF-α and IL-1) induce a number of alterations in adjacent endothelial cells that promote the extravasation of monocytes and other nonspecific inflammatory cells.
- Neutrophils and monocytes in circulation attach to adhesion molecules expressed by vascular endothelial cells and extravasate into tissue spaces.
- Neutrophils develop early in the reaction, reach a peak at approximately 6 hours, and subsequently decrease in number. After antigen exposure, monocyte infiltration occurs between 24 and 48 hours later.
- As monocytes penetrate tissues to become macrophages, chemokines such as monocyte chemotactic and activating factor attract them chemotactically to the location of the DTH response (MCAF).
- Migration-inhibition factor (MIF), an additional chemokine, prevents macrophages from moving beyond the site of a DTH reaction. As macrophages aggregate at the site of a DTH reaction, they are triggered by cytokines generated by TH1 cells, specifically IFN-γ and membranebound TNF-β.
- Activation increases the efficiency of macrophages as antigen-presenting cells, as mentioned previously. Thus, active macrophages can easily facilitate the activation of other T cells, which then release additional cytokines that attract and activate additional macrophages.
- This self-sustaining reaction, however, is a double-edged sword, with a thin line separating a good, protective response and a harmful response marked by significant tissue damage.
- Experiments with mutant mice incapable of producing IFN-γ revealed the significance of this cytokine in the DTH response.
- When these knockout mice were infected with an attenuated form of Mycobacterium bovis known as BCG (Bacillus Calmette-Guérin), almost all of them perished within 60 days, whereas wild-type mice survived.
- It was discovered that macrophages from IFN—γ deficient animals have lower concentrations of class II MHC molecules and bactericidal metabolites such as nitric oxide and superoxide anion.
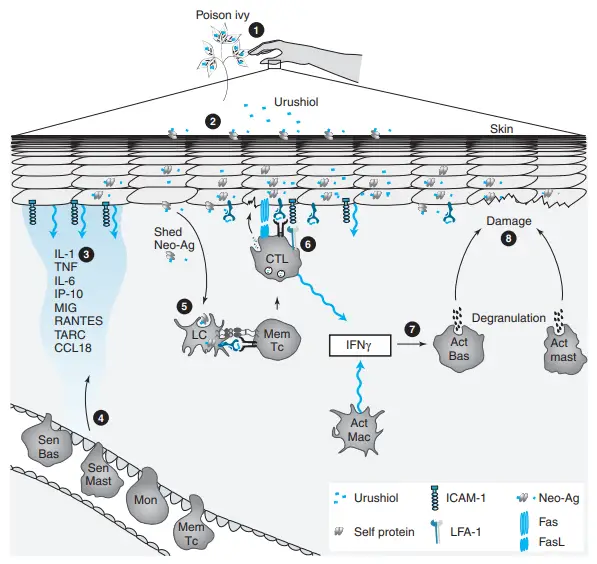
A misled individual has touched poison ivy (1), causing urushiol to attach to self-proteins in the skin (2) and generate a neoantigen. The neoantigen causes skin cells to upregulate the expression of adhesion molecules and produce a large number of chemokines and cytokines (3), which attract immune system cells from the circulation (4). Neo-antigen shed by skin cells is picked up by Langerhans cells (LC), from which peptides are cross-presented to memory Tc cells (5). IFN is produced alongside CTL effectors that directly harm host skin cells by degranulation and/or Fas-mediated apoptosis (6). A high concentration of IFN in the surrounding environment induces the degranulation of mast cells and basophils (7) and the release of lytic mediators that lead to host cell destruction (8).
Examples of Type IV (Cell Mediated) Hypersensitivity
Type IV or cell-mediated hypersensitivity can manifest in various examples. Some notable examples include:
- Tuberculin reaction (Mantoux test): The Mantoux test is a diagnostic skin test used to assess an immune response to tuberculosis. It involves injecting purified mycobacterial antigens under the skin. If an individual has been previously exposed to tuberculosis or received the BCG vaccine, a positive reaction will occur, characterized by the formation of a localized swelling and redness at the injection site.
- Granuloma formation: In situations where macrophages are unable to effectively eliminate intracellular pathogens, a chronic stimulation of pathogen-specific T cells can occur. This persistent immune response leads to the release of cytokines that contribute to the formation of granulomas. Granulomas are organized collections of immune cells that aim to “wall off” the infected macrophages and prevent the spread of the pathogen.
- Allergic contact dermatitis: Certain environmental chemicals, metals, or topical medications can trigger allergic contact dermatitis. This condition occurs when the skin comes into contact with an allergenic substance, leading to an immune response. The immune cells, particularly T cells, recognize the allergen as foreign and mount an inflammatory reaction. This immune response causes epidermal necrosis, skin inflammation, rash, and blister formation.
- Type 1 diabetes: Type 1 diabetes is an autoimmune condition where the immune system mistakenly targets and destroys the insulin-producing cells (islet cells) in the pancreas. In this case, cytotoxic T cells specifically recognize and attack the pancreatic islet cells, resulting in insulin deficiency. Without sufficient insulin, blood sugar regulation becomes impaired, leading to the symptoms and complications associated with diabetes.
These examples highlight different scenarios where a cell-mediated immune response plays a significant role in the development of hypersensitivity reactions.
FAQ
What is Type IV or Delayed-Type Hypersensitivity (DTH)?
Type IV or Delayed-Type Hypersensitivity (DTH) is an immune response that is mediated by T cells rather than antibodies. It is characterized by a delayed onset, typically taking a day or more to develop after exposure to an antigen.
How is DTH different from other types of hypersensitivity reactions?
DTH is distinct from other types of hypersensitivity reactions (Type I, II, and III) because it is cell-mediated rather than antibody-mediated. It involves the interaction of T cells, monocytes, and macrophages, resulting in tissue damage, inflammation, and cell death.
What triggers a Type IV hypersensitivity reaction?
Type IV hypersensitivity can be triggered by various antigens, including certain chemicals, metals, medications, infectious agents, and environmental substances. Examples include allergens causing contact dermatitis or intracellular pathogens like Mycobacterium tuberculosis.
How does the sensitizing phase of DTH occur?
The sensitizing phase of DTH occurs during the initial exposure to an antigen. Antigen-presenting cells (APCs) process and present the antigen to CD4+ T cells. This leads to the activation and clonal expansion of T cells, which sets the stage for the subsequent immune response.
What happens during the effector phase of DTH?
The effector phase of DTH occurs upon re-exposure to the antigen. TH1 cells secrete cytokines that recruit and activate macrophages and other inflammatory cells to the site of antigen exposure. Macrophages become the principal effectors, releasing lytic enzymes that damage tissues and destroy intracellular pathogens.
Can Type IV hypersensitivity be resolved?
Type IV hypersensitivity reactions can often be resolved with appropriate management. Topical corticosteroids are commonly used to alleviate inflammation and symptoms. Additionally, identifying and avoiding triggers or antigens can help prevent future reactions.
Is Type IV hypersensitivity involved in any diseases?
Yes, Type IV hypersensitivity is implicated in various diseases. Examples include contact dermatitis, tuberculosis, granulomatous diseases, autoimmune conditions like type 1 diabetes, and some organ transplant rejection cases.
How long does a Type IV hypersensitivity reaction last?
Type IV hypersensitivity reactions typically have a delayed onset, usually starting at 24-72 hours after exposure to the antigen. The reaction can persist for several days to weeks, depending on the specific immune response and the presence of ongoing antigen exposure.
Can Type IV hypersensitivity lead to tissue damage?
Yes, Type IV hypersensitivity can lead to tissue damage. The overreaction of T cells and the release of inflammatory cytokines can cause damage to the surrounding tissues, resulting in inflammation, cell death, and, in some cases, the formation of granulomas.
How does Type IV hypersensitivity contribute to host defense?
Type IV hypersensitivity plays a critical role in host defense against intracellular pathogens that are not easily eliminated by circulating antibodies. The immune response mobilizes T cells and macrophages to the site of infection, promoting the destruction of infected cells and the containment of pathogens.
