Table of Contents
- Current nitrite reduction tests are based on Peter Griess’s 1858 description of the Griess diazotization reaction. Peter Griess, the son of a blacksmith, was raised on a farm in Prussia, but “…tilling the soil was not to his liking, and his father often discovered him in a corner of the field, engrossed in a book while seated on the plough”.
- In his first attempts at higher study, he was anything but a model student, spending time in the institution’s prison before being expelled for a year. In his sixth year of college, he began to study chemistry seriously. Senior chemists found and developed the aniline dye industry at the coal-tar distillery where he was employed.
- Griess had become intrigued with the chemistry of dye manufacturing despite the distillery’s destruction by fire. His first publication on potential diazo compounds, “A Preliminary Notice on the Influence of Nitrous Acid on Aminonitro- and Aminodinitrophenol,” was published on the same day that he was recommended for a seat at the Royal College of Chemistry in Great Britain.
- Griess’ initial attempts at diazotization resulted in explosions, but his commission at the Royal College was to examine his new nitrogen intermediates, resulting in the isolation of diazobenzoic acid and the discovery of an entirely new class of chemicals.
- Griess is revered as the founder of the modern azo dye industry due to the discovery that a number of these compounds were stable and could be used to dye fabric without a mordant.
- Journal of the Society of Dyers & Colourists, February 1930; Journal of the Society of Dyers & Colourists, June 1959; and Journal of Chemical Education, April 1958, contain pieces that provide more colourful insights about Griess’ life.
- Griess developed a reagent for detecting nitrite in solutions in 1879. A solution of sulfanilic acid and alphanaphthylamine undergoes a diazotization reaction with nitrites, resulting in the formation of a red azo dye.
- There are numerous variations of the so-called Griess test in chemistry, medicine, and industry, but they all rely on the diazotization of nitrite to produce an azo dye.
- The study of crime scenes is one fascinating use of the reaction. Using a modified Griess test, the nitrites of spent gunpowder can be detected.
- Adaptations of the Griess test were proposed for many years as a technique of evaluating the urine of asymptomatic patients, particularly pregnant women, for the presence of nitrites as an indicator of bacteriuria.
- Currently, “dipstick” urine chemistry assays for nitrites utilise a similar chemical process.
- Recently, the Griess reaction has been used to detect nitrite and nitrate in human cells and biological systems as products of nitric oxide synthase. A constitutive, low-output, endothelial isoform modulates vascular tone; a constitutive, low-output, neuronal isoform modifies synaptic plasticity; and a cytokineinducible, high-output, immunological inflammatory isoform works as an effector component of the cell-mediated immune response.
- Nitric oxide is difficult to quantify since it is created in small amounts under most situations and has a short half-life; nonetheless, measuring the buildup of nitrite and nitrate is a good method for determining the activity of nitric oxide synthase.
- Although all applications of the Griess reaction, including those involving water analysis and plant physiology, provide interesting background for the student and instructor, the current technique will focus on the reduction of nitrates and nitrites by bacteria in artificial media.
Principle of Nitrite Reduction Test
Nitrites combine with an acidic solution of sulfanilic acid and alpha-naphthylamine to produce a red azo dye. In each test reaction, the appearance of the red dye shows the presence of NO2- in the test tube, either as an unreduced main substrate, a product of the test organism’s reduction of NO3-, or a product of the forced reduction of NO3- with a reducing agent (zinc) for control purposes. Each reaction’s defining characteristic is its ability to detect NO2-. In the presence of NO2-, the colour reaction begins when NO2- is acidified by the acetic acid in reagents A and B to form HNO2. The following reaction depicts the further colour development:
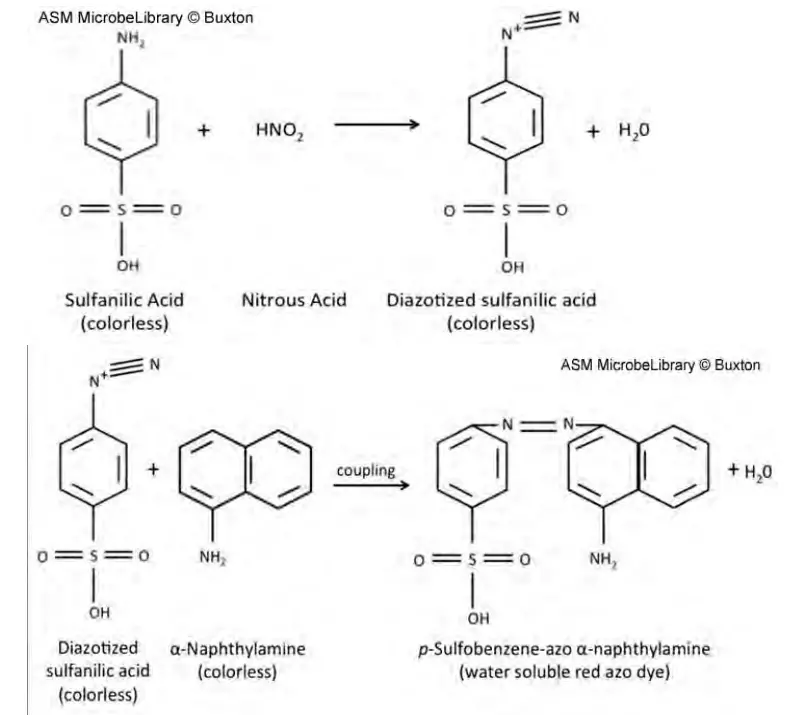
By way of a nitroso reaction, the -N=N-azo linkage produces a colourful molecule. By connecting an aromatic amine through an azo link with a phenolic-type chemical, typically at the paraposition to a hydroxyl (OH) or amino group, diazonium dye compounds are produced (NH2). In this instance, para coupling to an amino group occurs. In his introduction, Richardson provides a general summary of nitrate reduction and the nitrogen cycle. The complexity of nitrate reduction routes is described in detail in an excellent review by Moreno-Vivian.
Required Media and reagents for Nitrite Reduction Test
Nitrite reduction medium
Composition
| Ingredients | Amount |
| Beef (meat) extract | 3.0 g |
| Gelatin peptone | 5.0 g |
| Potassium nitrite (KNO2) | 1.0 g |
| Deionized water | 1,000 ml |
Nitrite reduction medium Preparation
- For either broth substrate, weigh the components carefully and boil them slowly until dissolved.
- Add inverted Durham tubes to dispensed test tubes.
- Autoclave for 15 minutes at 121°C, 15 pressure. The autoclave’s pressure will force the soup into the Durham tube.
- Before usage, the item must be cooled to room temperature.
- Refrigerate for storage at 4°C to 10°C.
- Six months estimated shelf life
When the broth is injected into the Durham tube, there should be no visible bubbles. Before adding reagents, use a heavy inoculum and incubate the sample for one night. Some strains require up to five days to completely degrade their substrates.
Reagent A
Composition
| Ingredients | Amount |
| N,N-Dimethyl-αnaphthylamine | 0.6 ml |
| Acetic acid (5N) | 100 ml |
Multiple formulations of reagent A are reported and commercially accessible. The substance mentioned below is not a known carcinogen and provides a hue that is relatively stable.
Note: fresh reagent has a very slight yellowish color.
Reagent B
| Ingredients | Amount |
| Sulfanilic acid | 0.8g |
| Acetic acid (5N) | 100 ml |
Note: fresh reagent is colorless
Adding 287 ml of glacial acetic acid (17.4N) to 713 ml of deionized water yields 5N acetic acid.
Both Reagents A and B should be refrigerated and shielded from light. If the reagents become discoloured, discard them.
Zinc dust
Zinc dust must be devoid of nitrates and nitrites. When used sparingly, zinc dust will convert nitrate to nitrite, but it will not convert nitrite to nitrogen gas or other nitrogenous byproducts.
Procedure of Nitrite Reduction Test
- For NO2, put a lot of the test organism from colonies that are well-separated into the medium.
- At 35°C, let it sit for 12 to 24 hours. Rarely, it may be necessary to let the egg sit for up to 5 days.
- When there is enough growth in the tube, check the broth to see if the substrate has been used up.
- Watch for gas to come out of the ground and the Durham tube.
- In a small test tube, mix two drops each of reagents A and B. (12mm x 75 mm).
- About 1 ml of the broth culture should be added to the test tube and mixed well.
Result of Nitrite Reduction Test
Nitrite Reduction Positive Test
If the test organism has gotten rid of all of the NO2-, there won’t be any change in colour. This means that all of the NO2- is gone, or “reduced.” N2 gas in the Durham tube or on the surface of the broth is often a sign of reduction. However, other nitrogenous products may also be made. So, the fact that there is no gas does not mean that NO2 – can’t be lowered.
NO2- → NO → N2O → N2
Nitrite Reduction Negative Test
Nitrite is broken down into nitric oxide, which can then be turned into nitrous oxide or nitrogen gas. If a red colour shows up, it means that NO2 is present, which is a bad reaction.
Partial reduction, which means that some of the primary NO2- substrate is still in the tube, can sometimes cause a lighter pink colour. The first tube can be put back in the incubator and tested again the next day. Zinc dust is not needed for this reaction.

Quality Control
- Positive: Proteus mirabilis (ATCC12453)—colorless; gas production
- Negative: Acinetobacter baumannii (ATCC19606)—red; no gas production
Limitations
- Reagents A and B are poisonous. If you swallow them, they could hurt you or kill you. They are also corrosive, which means they can burn or irritate skin, eyes, and the airways. Don’t breathe in the vapours or let them touch your eyes or skin. If it gets in your eyes, rinse them right away with water and see a doctor.
- When zinc dust comes in contact with water, it gives off gases that are very flammable. Keep the lid on tight and the container dry. If there is a fire, you should never use water to put it out. Instead, use sand, carbon dioxide, or a powdered extinguishing agent.
Keynotes on Nitrite Reduction Test
- In the original recipe for reagent B, alpha-naphthylamine was used. Since it is known to cause cancer, N,NDimethyl—naphthylamine has been used instead. Thankfully, this formula is also less likely to cause the colour to fade.
- Some authors say to add zinc to colourless NO2- reactions that don’t produce gas to make sure that the NO2 hasn’t been oxidised to NO3 instead of being reduced to something other than N2 gas. However, this reaction doesn’t happen very often.
- Some fungi and mycobacteria can be identified in the same way, but that is not what this article is about.
- Filter paper disc tests can be bought to find out if anaerobic species that grow on solid-plated media in an anaerobic atmosphere can reduce nitrate.
- The instructor may want to give out the zinc dust to emphasise safety in the lab and with the students. This could be a good time for the teacher to see how well the students understand the exercise.
- Be sure to run a negative control, which is broth that hasn’t been inoculated, to show that the remaining NO2 will be taken away by the zinc dust, turning the broth red.
Uses
- All members of the family Enterobacteriaceae can turn nitrate into nitrite, but only some can turn nitrite into other compounds. It is used to tell the difference between Enterobacteriaceae bacteria that make the enzyme nitrate reductase and Gram-negative bacteria that don’t make this enzyme.
- In some species, getting rid of nitrate may be linked to breathing without oxygen.
- It is used to tell Mycobacterium apart.
- Finding Neisseria species and telling them apart from Moraxella and Kingella The nitrate reduction test is an important way to tell the difference between N. gonorrhoeae and K. denitrificans, especially when stained smears show that K. denitrificans strains look like gram-negative diplococci.
- Making it easier to identify Corynebacterium species
References
- Tille P.M. 2014. Bailey and Scott’s diagnostic microbiology. Thirteen edition. Mosby, Inc., an affiliate of Elsevier Inc. 3251 Riverport Lane. St. Louis. Missouri 63043
- https://asm.org/Protocols/Nitrate-and-Nitrite-Reduction-Test-Protocols
- https://accepta.com/product/water-testing-water-quality-analysis-equipment/incubators-microbiological-incubation-analysis/nitrite-reducing-bacteria-test-tubes-10-pack
- https://www.fda.gov/food/laboratory-methods-food/bam-r48-nitrite-detection-reagents
- https://www.clinmicronow.org/doi/abs/10.1128/9781683670438.CMPH.ch3.17-35
- https://microbiologyinfo.com/nitrate-reduction-test/
- https://microbenotes.com/nitrite-reduction-test/
