Table of Contents
What is Bile Esculin Agar (BEA)?
- Bile Esculin Agar (BEA) is a selective and differential medium used for the isolation and identification of members of the genus Enterococcus, formerly known as group D streptococci. The medium was first introduced by Swan in 1954 as a way to identify bacteria based on their ability to hydrolyze esculin in the presence of bile.
- BEA agar contains bile, which inhibits the growth of both gram-positive and many gram-negative bacteria. It also contains esculin, which serves as the differential component of the medium. Some formulations of BEA agar may include sodium azide to increase selectivity.
- The primary purpose of BEA agar is to identify enterococci and group D streptococci, as they possess the ability to hydrolyze esculin in the presence of bile. The hydrolysis of esculin produces esculetin and dextrose. Esculetin reacts with ferric ammonium citrate in the medium, forming a brown-black colored complex that is deposited around the colonies as a black halo.
- The medium is formulated with highly nutritious tryptone and soya peptone, which provide nitrogen, carbon sources, long-chain amino acids, vitamins, and other essential nutrients. Hemin is included to support the growth of fastidious anaerobic bacteria like Bacteroides species. Bile inhibits most anaerobic gram-negative bacilli, except for Bacteroides fragilis, while the addition of gentamicin inhibits most organisms other than esculin-positive Bacteroides that can tolerate bile.
- To use BEA agar, the specimen can be directly inoculated onto the medium. However, since the medium is highly selective, it is recommended to also inoculate a non-selective medium alongside it. Bacteroides fragilis group organisms grow well on this medium, and their colonies are surrounded by a black halo due to the esculin hydrolysis.
- It is important to note that BEA agar is primarily used for the selective and presumptive identification of Bacteroides fragilis group and is not suitable for the identification of other bacteria or for complete identification. Further biochemical, immunological, molecular, or mass spectrometry testing should be performed on colonies from pure culture for complete identification of bacterial species.
Bile Esculin Agar (BEA) Composition
Bile Esculin Agar (BEA) contains the following ingredients per liter of the medium:
- Peptic digest of animal tissue: 5.00 grams
- Beef extract: 3.00 grams
- Esculin: 1.00 gram
- Bile salts: 40.0 grams
- Ferric citrate: 0.50 gram
- Agar: 15.00 grams
The final pH of the medium, when measured at 25°C, is approximately 6.6±0.2. These specific components and their concentrations are carefully selected to create a medium that is selective and differential for the identification of enterococci and group D streptococci based on their ability to hydrolyze esculin in the presence of bile.
| Ingredients | Grams/Liter |
|---|---|
| Peptic digest of animal tissue | 5.00 |
| Beef extract | 3.00 |
| Esculin | 1.00 |
| Bile salts | 40.0 |
| Ferric citrate | 0.50 |
| Agar | 15.00 |
| Final pH (at 25°C) | 6.6±0.2 |
Principle of Bile Esculin Agar (BEA)
The principle of Bile Esculin Agar (BEA) is based on its selective and differential properties for the isolation and identification of group D streptococci and enterococci. The key components of the medium contribute to its functionality:
- Esculin: The presence of esculin in the medium allows for the detection of esculin hydrolysis. Group D streptococci and other esculin-positive organisms can hydrolyze esculin to produce dextrose and esculetin.
- Ferric citrate: Ferric ions provided by ferric citrate in the medium react with the esculetin produced from esculin hydrolysis. This reaction forms a dark brown to black complex, indicating a positive result for esculin hydrolysis.
- Bile salts: The inclusion of 4% oxbile in the medium inhibits the growth of most non-group D streptococci and other gram-positive cocci. Bile salts act as a selective agent by suppressing the growth of unwanted organisms while allowing the growth of group D streptococci and enterococci.
- Peptic digest of animal tissue and beef extract: These ingredients provide the necessary carbon, nitrogen, and essential growth factors for the cultivation of bacteria.
Overall, the combination of selective bile salts and the differential reaction of esculetin with ferric ions enables the identification of group D streptococci and enterococci based on their ability to hydrolyze esculin in the presence of bile. The dark brown to black color change in the medium indicates a positive result for esculin hydrolysis, aiding in the presumptive identification of specific organisms.
Preparation of Bile Esculin Agar (BEA)
To prepare and use Bile Esculin Agar (BEA), follow these steps:
- Suspend 64.5 grams of the agar in 1000 ml of distilled water.
- Heat the mixture to boiling to ensure complete dissolution of the medium.
- Dispense the medium into tubes or flasks, taking care to maintain sterility.
- Autoclave the tubes or flasks at 15 lbs pressure (121°C) for 15 minutes to sterilize the medium.
- If using tubes, allow the medium to solidify in a slanted position with a butt of 2.5 cm deep. If using Petri plates, pour the medium into sterile plates.
- Allow the BEA medium to cool down to room temperature before use.
- Inoculate and streak the medium with a single isolated pure colony of the desired organism.
- Incubate the inoculated plates or tubes in an aerobic atmosphere at 35ºC for 24-48 hours.
- Observe the plates or tubes for the presence of growth and blackening of the medium.
- Interpret the results based on the growth and blackening of the medium, which indicates a positive result for esculin hydrolysis by the tested organism.
Quality Control
Quality Control of Bile Esculin Agar involves assessing various aspects of the medium to ensure its effectiveness and reliability. Here are the key parameters and observations:
- Appearance: The Bile Esculin Agar should appear as a cream to yellow homogeneous free-flowing powder. This indicates that the medium is consistent in texture and color.
- Gelling: When prepared, the medium should form a firm gel that is comparable to a 1.5% Agar gel. This ensures that the medium solidifies properly and provides an appropriate surface for bacterial growth.
- Color and Clarity: The prepared medium should have a medium amber color and appear clear to slightly opalescent with a bluish tinge when poured into Petri plates. This indicates that the medium has been properly prepared and allows for easy visual observation of bacterial growth.
- Reaction: A 6.15% w/v aqueous solution of the medium should exhibit a pH of 7.0±0.2 when measured at 25°C. This ensures that the pH of the medium is within the specified range, which is essential for its proper functioning.
- pH Range: The pH of the medium should be maintained between 6.80 and 7.20. This ensures that the medium’s acidity or alkalinity is suitable for supporting the growth of target organisms.
- Cultural Response: The cultural response of the medium should be evaluated by observing the growth and specific characteristics of known organisms when the Bacteroides Selective Supplement (FD062) is added. Incubation should be done anaerobically at 35-37°C for 40-48 hours.
- Organism Inoculum and Growth Recovery: Known strains of organisms, such as Bacteroides fragilis ATCC 23745 and Bacteroides vulgatus ATCC 8482, should be inoculated onto the medium with a specific colony-forming unit (CFU) range. The medium should support good-luxuriant growth of the organisms within the specified CFU range.
- Esculin Hydrolysis: The growth of Bacteroides fragilis ATCC 23745 should exhibit a positive reaction, indicated by blackening of the medium due to esculin hydrolysis. In contrast, Bacteroides vulgatus ATCC 8482 should show a negative reaction, with no blackening observed.
- Inhibition and Negative Reactions: Organisms like Clostridium perfringens ATCC 13124 and Proteus mirabilis ATCC 12453 should display inhibition or poor growth on the medium. Clostridium perfringens ATCC 13124 should show no growth, while Proteus mirabilis ATCC 12453 should exhibit a negative reaction with less than or equal to 10% esculin hydrolysis.
By conducting these quality control assessments, the reliability and consistency of the Bile Esculin Agar can be verified, ensuring accurate results in subsequent bacterial testing and identification procedures.
Results on Bile Esculin Agar (BEA)
On Bile Esculin Agar, the interpretation of results is as follows:
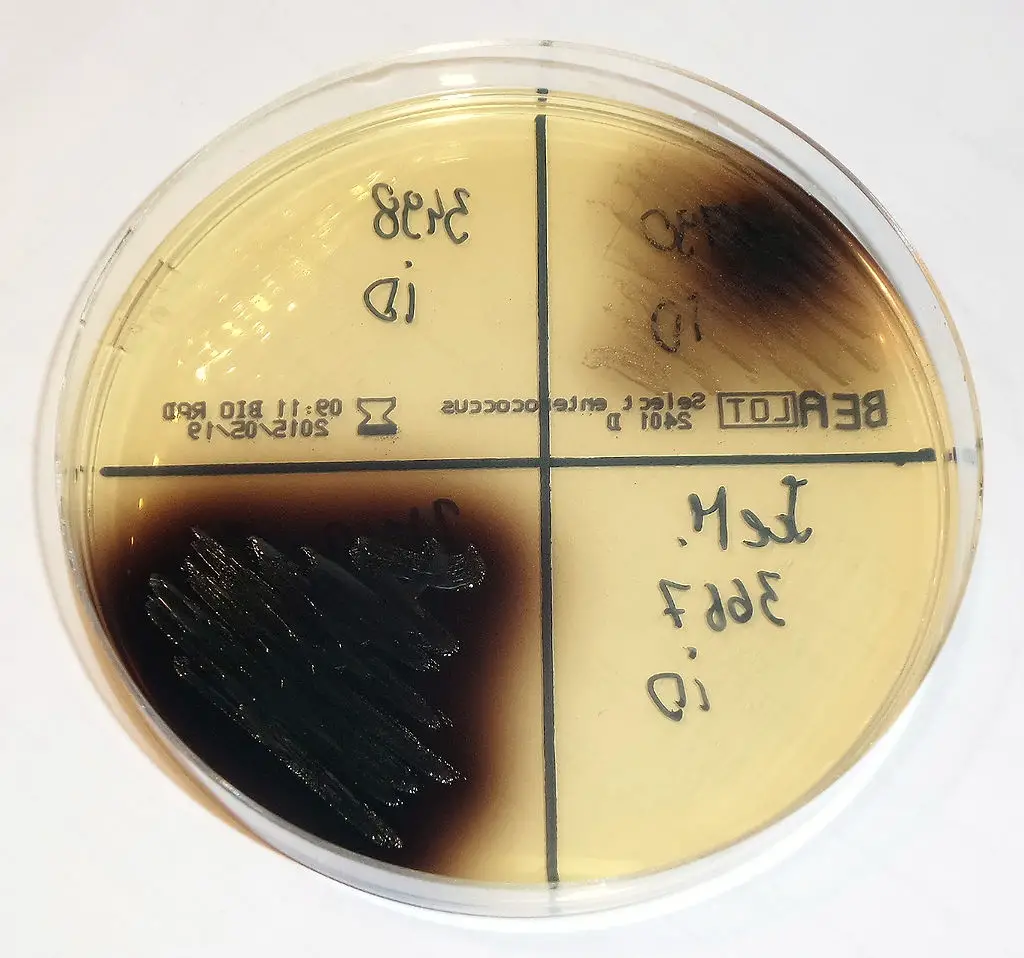
- Enterococcus faecalis: Good-luxuriant growth with positive esculin hydrolysis, indicated by blackening of the medium around the colonies.
- Escherichia coli: Good growth but no blackening of the media around the colonies, indicating a negative esculin hydrolysis result.
- Enterococcus faecium: Good-luxuriant growth with positive esculin hydrolysis, resulting in blackening of the medium around the colonies.
- Yersinia enterocolitica: Good-luxuriant growth with positive esculin hydrolysis, leading to blackening of the medium around the colonies.
- Streptococcus pyogenes: None to poor growth observed, and no blackening of the media around the colonies, indicating a negative result for esculin hydrolysis.
Interpreting the presence or absence of blackening around the colonies helps in the differentiation of organisms based on their ability to hydrolyze esculin in the presence of bile. Positive results with blackening indicate esculin hydrolysis, while negative results without blackening suggest the absence of esculin hydrolysis.
Uses of Bile Esculin Agar (BEA)
Bile Esculin Agar (BEA) has several uses in microbiology:
- It is recommended for the isolation and presumptive identification of enterococci/group D streptococci. The ability of these organisms to hydrolyze esculin in the presence of bile is a characteristic used for their identification.
- BEA is used in conjunction with other biochemical tests to identify cultures of isolated organisms. It provides additional information for the differentiation of various bacterial species.
- It is employed for the presumptive identification of Enterobacter, Klebsiella spp., and Serratia spp. among the Enterobacteriaceae family. The bile esculin test helps distinguish these organisms from others within the same family.
- The use of the bile esculin test on this medium is a reliable way of identifying group D streptococci from non-group D streptococci. The presence of blackening around colonies indicates the hydrolysis of esculin by group D streptococci.
- Bile Esculin Agar is recommended for the isolation and identification of Yersinia enterocolitica from food and animal feeding stuffs. The selective properties of the medium inhibit the growth of other bacteria, allowing for the specific isolation of Yersinia enterocolitica.
Overall, Bile Esculin Agar is a valuable tool in the laboratory for differentiating and identifying specific bacterial groups and species based on their ability to hydrolyze esculin in the presence of bile.
Limitations of Bile Esculin Agar (BEA)
Bile Esculin Agar (BEA) is a specific type of agar used in microbiology. However, it has certain limitations that need to be considered:
- Not for Primary Isolation: BEA is not designed for the initial isolation of patient specimens. It should be used only when cultures of isolated organisms are available. This means it is not suitable for direct testing of patient samples.
- Not for Disease Diagnosis: BEA is not intended for diagnosing diseases or other conditions in humans. It serves as a selective and differential medium rather than a diagnostic tool.
- Additional Testing Required: For complete identification, it is recommended to perform further tests such as biochemical, immunological, molecular, or mass spectrometry testing on colonies obtained from pure culture. BEA alone cannot provide comprehensive identification of microorganisms.
- Certain Organisms may Grow: Some strains of Staphylococcus, Aerococcus, and Listeria monocytogenes can grow in the presence of bile and hydrolyze esculin. In the case of L. monocytogenes, it may form small black colonies. Therefore, reliance solely on BEA may lead to false interpretations or incomplete identification.
- Heavy Inoculum Effect: If a heavy inoculum is applied to BEA, it can make interpretation of the bile esculin test difficult to read. Excess inoculum reduces the bile’s ability to inhibit the growth of other gram-positive organisms that may also hydrolyze esculin.
- False Negative Results: There are certain streptococci that can grow in the presence of bile without hydrolyzing esculin. Therefore, if growth occurs without blackening of the medium, it does not necessarily indicate a positive test result.
- Gram-Negative Organism Growth: BEA does not contain azide, which is commonly used to inhibit the growth of gram-negative rods. Consequently, gram-negative rods can grow on BEA, and many of these organisms can hydrolyze esculin.
It is essential to consider these limitations while working with Bile Esculin Agar to ensure accurate interpretations and comprehensive identification of microorganisms. Supplemental tests and proper culture techniques should be employed for accurate results and diagnosis.
FAQ
What is Bile Esculin Agar (BEA)?
Bile Esculin Agar (BEA) is a selective and differential culture medium used in microbiology to isolate and identify certain bacteria, particularly members of the Enterococcus genus.
What is the composition of Bile Esculin Agar?
Bile Esculin Agar typically contains ingredients such as bile salts, esculin, ferric citrate, agar, and a pH indicator, usually bromocresol purple.
What is the purpose of using Bile Esculin Agar?
The primary purpose of using Bile Esculin Agar is to selectively inhibit the growth of certain bacteria while promoting the growth of enterococci. It also allows the differentiation of enterococci based on their ability to hydrolyze esculin.
How does Bile Esculin Agar work?
Bile salts present in Bile Esculin Agar inhibit the growth of many bacteria, except for enterococci, which are resistant to bile. The esculin in the medium is hydrolyzed by some enterococci, leading to the production of esculetin. The presence of ferric citrate in the medium reacts with esculetin, forming a dark brown to black precipitate, indicating a positive esculin hydrolysis result.
What are the typical colony characteristics on Bile Esculin Agar?
Enterococcus species typically produce small, dark brown to black colonies on Bile Esculin Agar due to the formation of the esculetin-ferric citrate complex. Other non-enterococcal bacteria are usually inhibited and do not grow or produce different colony characteristics.
Which organisms are commonly identified using Bile Esculin Agar?
Bile Esculin Agar is primarily used to identify enterococci, such as Enterococcus faecalis and Enterococcus faecium, which are important pathogens associated with various infections in humans.
How is Bile Esculin Agar used in clinical microbiology?
In clinical microbiology, Bile Esculin Agar is used to isolate and identify enterococci from clinical specimens, such as urine, blood, wound swabs, and feces. It helps in distinguishing enterococci from other bacteria that may be present in the sample.
Are there any limitations or potential issues when using Bile Esculin Agar?
Some non-enterococcal bacteria, particularly certain species of Streptococcus and Lactobacillus, can also hydrolyze esculin and produce false-positive results. Additionally, prolonged incubation or storage of the medium can lead to false-positive reactions.
Can Bile Esculin Agar be used for other purposes besides enterococcal identification?
While Bile Esculin Agar is primarily used for enterococcal identification, it can also be used to screen for group D streptococci and to differentiate between Streptococcus bovis, a potential human pathogen, and other non-pathogenic streptococci.
Is Bile Esculin Agar the only method for identifying enterococci?
Bile Esculin Agar is a useful screening tool for the identification of enterococci. However, additional confirmatory tests, such as biochemical testing and molecular methods, may be required for accurate identification of enterococcal species.
References
- Himedia
- https://microbiologie-clinique.com/bea-agar-bile-esculin.html
- https://www.austincc.edu/microbugz/bile_esculin_test.php
- https://assets.fishersci.com/TFS-Assets/LSG/manuals/IFU1190.pdf
- https://www.dalynn.com/dyn/ck_assets/files/tech/PB65.pdf
