Table of Contents
What is Cell Cycle? – Cell Cycle Definition
- The process by which a cell divides into two daughter cells by duplicating its DNA and dividing its cytoplasm and organelles is known as the cell cycle.
- Prevost and Dumas (1824) made the discovery of the cell cycle while researching the frog zygote’s cleavage. To divide and create new cells, a cell must go through a number of steps.
- The cell cycle is the full process through which a new cell population grows and develops with the assistance of a single parent cell.
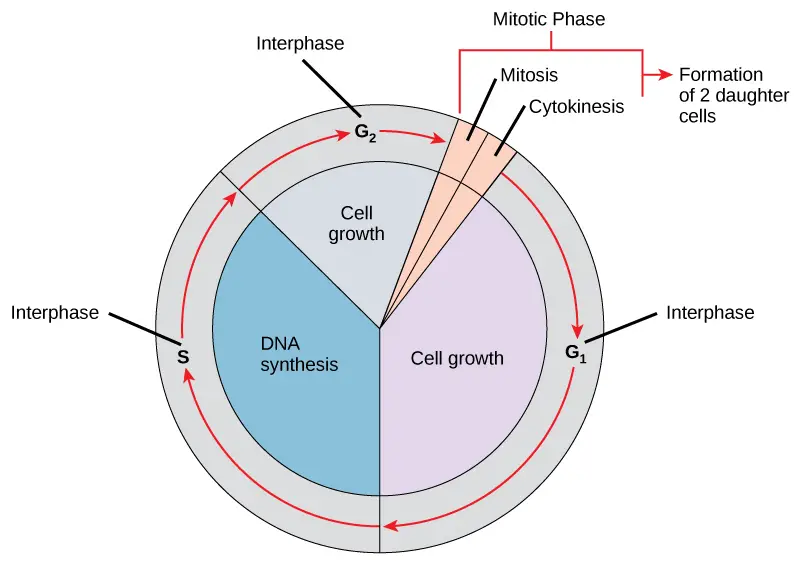
Phases of Cell Cycle
Cell maturation and subsequent division are the results of a cell’s life cycle, also known as cell division. These processes include the genome’s duplication, the production of the cell organelles, and the division of the cytoplasm.
Human cells have a standard eukaryotic cell cycle and go through one round of growth and division in about 24 hours. However, the length of the cycle varies from organism to organism and from cell to cell. The two main phases of a normal eukaryotic cell cycle are as follows:
1. Interphase
- The cell prepares for cell division during interphase while also going through regular activities.
- Interphase is the time when the cell prepares for division by going through both cell growth and DNA replication. It is often referred to as the resting phase of the cell cycle.
- It takes up roughly 95% of the total cycle’s time.
- Numerous internal and external requirements must be satisfied for a cell to transition from the interphase to the mitotic phase. The three interphase phases are designated as G1, S, and G2.
a. G1 Phase
- Because there is minimal change observable, the initial interphase stage is known as the G1 phase, or first gap.
- However, the cell is extremely active at the biochemical level during the G1 stage.
- Between mitosis and the start of the cell’s genetic material replicating, a cell is in the G1 phase.
- The cell is metabolically active and continues to expand throughout this stage without repeating its DNA.
b. S Phase
- Nuclear DNA maintains its semi-condensed chromatin structure throughout interphase.
- Sister chromatids, which are firmly connected at the centromere region of each chromosome, are created as a result of DNA replication during the S phase (synthesis phase).
- Each chromosome is a duplicate at this point and is made up of two sister chromatids. In the S phase, the centrosome is duplicated.
- The mitotic spindle, which directs the movement of chromosomes during mitosis, will develop from the two centrosomes.
- A pair of rod-like centrioles that are at right angles to one another make up the centrosome. Cell division is organised by centroles.
- Many eukaryotic species, including most fungus and plant species, lack centrioles in their centrosomes.
c. G2 Phase
- The cell restores its energy reserves and produces the proteins required for chromosomal manipulation during the G2 phase, also known as the second gap.
- To give the mitotic spindle resources, some cell organelles are replicated, while the cytoskeleton is disassembled.
- During G2, more cell proliferation could occur.
- Before the cell may start the first stage of mitosis, the last mitotic preparations must be finished.
Significance of G1, S and G2 phases of the interphase
- The cell grows while in the G1 phase but does not replicate.
- The replication of the cell’s DNA occurs in the S phase.
- The cell produces the RNA, proteins, and other macromolecules necessary for mitotic division during the G2 phase.
2. The Mitotic Phase or M phase
- The cytoplasm and nucleus’ contents must be split in order to create two daughter cells.
- The duplicated chromosomes are aligned, separated, and transferred to the cell’s opposite poles during the multi-step mitotic phase, after which the cell divides into two brand-new, identical daughter cells.
- Nuclear division is accomplished during the first of the mitotic phase’s five stages, called mitosis.
- The physical division of the cytoplasmic components into two daughter cells occurs during the second stage of the mitotic phase, known as cytokinesis.
Mitosis
- Prophase, prometaphase, metaphase, anaphase, and telophase are the phases of mitosis that lead to the division of the cell nucleus.
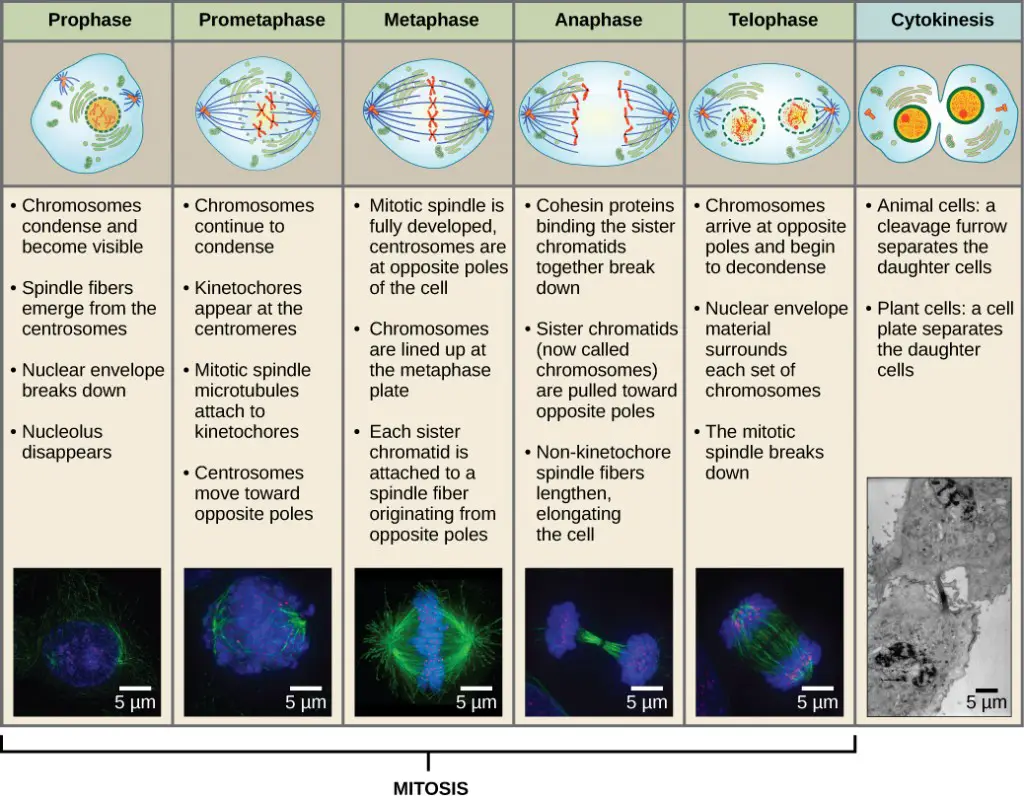
- Several activities must take place during prophase, the “first phase,” to allow access to the chromosomes in the nucleus. In addition to the Golgi apparatus and endoplasmic reticulum fragmenting and spreading to the cell’s periphery, the nuclear envelope begins to split into tiny vesicles. The nucleolus goes away. The centrosomes start to travel to the cell’s opposing poles. The centrosomes are pushed apart by the microtubules that make up the mitotic spindle’s foundation as the microtubule fibres lengthen. Under a light microscope, the sister chromatids start to coil more tightly and become apparent.
- Many of the processes that started in prophase continue to develop during prometaphase, which ends with the creation of a connection between the chromosomes and cytoskeleton. The nuclear envelope’s remains vanish. As more microtubules organise and extend over the length of the previous nuclear region, the mitotic spindle continues to grow. Chromosomes get smaller and appear more distinct. Each sister chromatid uses a protein complex termed the kinetochore to connect to spindle microtubules at the centromere.
- All of the chromosomes are aligned in the metaphase plate, also known as the equatorial plane, which is situated halfway between the cell’s two poles. Sister chromatids remain firmly fused to one another. The chromosomes are currently at their highest level of condensing.
- The centromeres of the sister chromatids at the equatorial plane separate apart during anaphase. Each chromatid, which is now referred to as a chromosome, is quickly drawn towards the direction of the centrosome where its microtubule was connected. The non-kinetochore microtubules slide against one another at the metaphase plate where they overlap, causing the cell to seem elongated.
- All of the processes that occurred during the previous three phases to prepare the duplicated chromosomes for mitosis are reversed during telophase. Chromosomes reach their antipodal positions and start to decondense (unravel). Each daughter cell’s cytoskeleton will be assembled from the monomers that are separated from the mitotic spindles during this process. Chromosomes develop nuclear envelops around them.
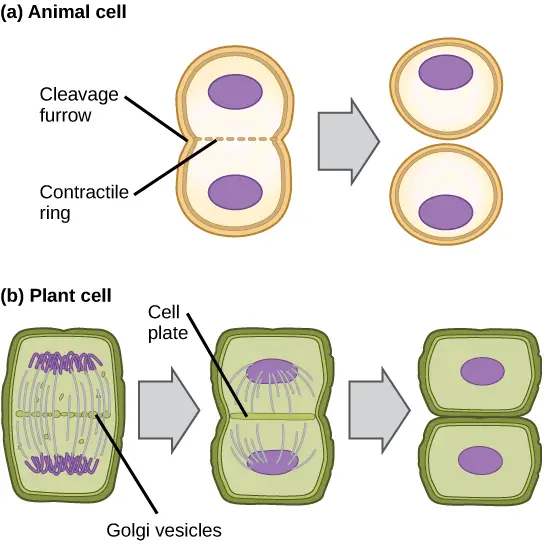
Cytokinesis
- The second stage of the mitotic phase, known as cytokinesis, is when a cell divides into two daughter cells by physically separating its cytoplasm.
- Although most eukaryotes go through the same steps of mitosis, plant cells and other eukaryotes with cell walls go through cytokinesis in a very different way.
- Anaphase starts after the initiation of cytokinesis in cells lacking cell walls, such as animal cells.
- At the former metaphase plate, a contractile ring made of actin filaments develops just inside the plasma membrane. The actin filaments cause a fissure by pulling the cell’s equator inward.
- The term “crack” for this split is the “cleavage furrow.” As the actin ring contracts, the furrow deepens, and eventually the membrane and cell are severed from one another.
- The stiff cell walls that surround the plasma membrane prevent a cleavage furrow from forming in plant cells.
- The daughter cells must create a new cell wall. Enzymes, structural proteins, and glucose molecules are gathered by the Golgi apparatus during interphase before it fragments into vesicles and spreads throughout the dividing cell.
- These Golgi vesicles travel along microtubules during telophase to assemble at the metaphase plate.
- A cell plate is the structure created when the vesicles fuse there, moving from the centre toward the cell walls. The cell plate grows as more vesicles fuse, merging with the cell wall at the cell’s edge.
- The glucose that has collected between the membrane layers is used by enzymes to create a new cellulose cell wall. On either side of the new cell wall, the Golgi membranes change into the plasma membrane.
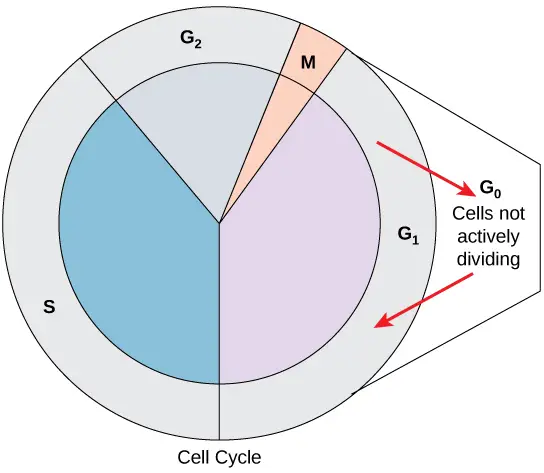
What is G0 Phase?
- Not all cells follow the conventional cell-cycle pattern, which calls for a freshly produced daughter cell to enter interphase right away and then quickly enter the mitotic phase.
- The G0 phase is when a cell is not actively preparing to divide.
- The cell has completed its cell cycle and is now in a quiescent (inactive) stage. Some cells briefly enter G0 before the start of G1 is signalled by an outside source.
- Other cells, such as mature heart muscle and nerve cells, that never or infrequently divide always remain in the G0 state.
Cell Cycles in Vivo
Within a multicellular plant or animal, the ability of different cell types to grow and divide is one of the characteristics that sets them apart from one another. Cells can be divided into three general categories:
- neurons, muscle cells, or red blood cells are examples of highly specialised cells that cannot divide. These cells stay in their differentiated condition till they pass away.
- cells that ordinarily do not divide, but when given the right stimuli, can be made to start synthesising DNA and divide. This group includes lymphocytes and liver cells, which can both be stimulated to multiply by interacting with the right antigen or by surgically removing a portion of the liver.
- cells with a typically moderately high mitotic activity level. This group includes stem cells from several adult tissues, including hematopoietic stem cells, which develop into red and white blood cells, and stem cells at the base of multiple epithelia, which cover the surface and cavities of the body. Rapid and ongoing cell division is also seen in the comparatively unspecialized cells of apical meristems found close to the terminals of plant roots and stems. The ability to divide asymmetrically is a crucial trait that stem cells possess that is not shared by most other cells. When a cell divides asymmetrically, the two daughter cells have different shapes, makeups, or destiny. One daughter cell that results from the asymmetric division of a stem cell is still an uncommitted stem cell like its parent, and the other daughter cell has begun the process of differentiating into a cell of that tissue. In other words, stem cells can engage in both self-renewal and the development of differentiated tissue cells through asymmetric divisions.
Cell cycles in cleaving frog embryos, which lack both G 1 and G 2 phases, can last only 30 minutes, whereas those in slowly developing tissues, such the mammalian liver, can last many months.
Many cells in the body are considered to be quiescent, which means they are not currently undergoing cell division but yet have the capacity to do so if the situation warrants it. Cells that have ceased dividing are, with a few notable exceptions, stalled in a phase before DNA synthesis begins.
Control of the Cell Cycle
- Even within a single organism’s cells, the length of the cell cycle varies greatly.
- In humans, cell turnover varies from a few hours during early embryonic development to an average of two to five days for epithelial cells, or to a full human lifetime for specialised cells like cortical neurons or cardiac muscle cells, which spend their entire existence in the G0 state.
- The amount of time a cell spends in each stage of the cell cycle varies as well. The cycle lasts roughly 24 hours when fast-diverging mammalian cells are cultured in culture (outside the body under ideal growing circumstances).
- The G1 phase of rapidly dividing human cells with a 24-hour cell cycle lasts roughly 11 hours.
- Events in the cell cycle are timed according to both internal and exterior to the cell mechanisms.
Regulation at Internal Checkpoints
- The daughter cells must be identical replicas of the parent cells.
- Chromosome duplication or distribution errors result in mutations that could spread to every subsequent cell that develops from the aberrant cell.
- There are internal control systems that function at three primary cell cycle checkpoints where the cell cycle can be interrupted until circumstances are favourable, preventing a damaged cell from continuing to divide.
- These checkpoints take place during metaphase, at the G2-M transition, and near the conclusion of G1.
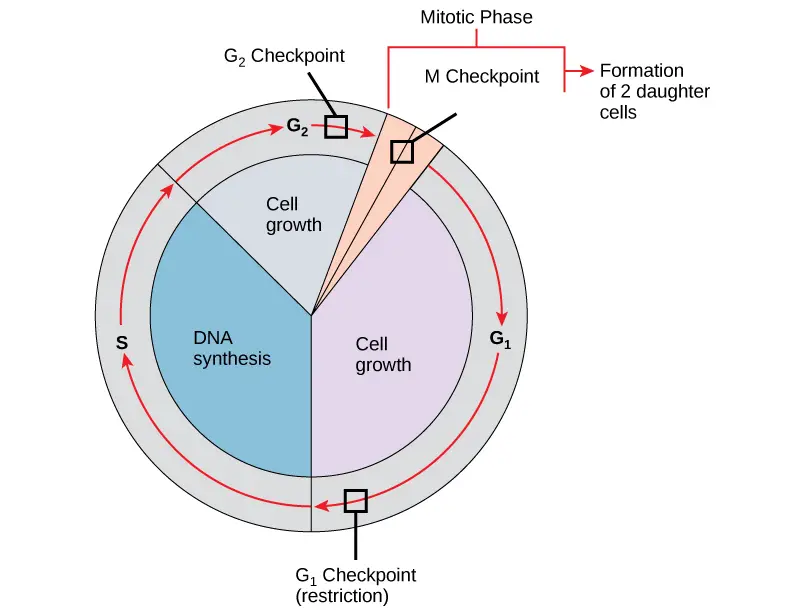
The G1 Checkpoint
- The G1 checkpoint ascertains if all circumstances are ideal for cell division to continue.
- The cell commits irrevocably to the process of cell division at the G1 checkpoint, also known as the restriction point.
- The genomic DNA is examined for damage at the G1 checkpoint in addition to sufficient reserves and cell size.
- A cell won’t enter the S phase if it doesn’t fulfil all the prerequisites.
The G2 Checkpoint
- If specific requirements are not met, the G2 checkpoint prevents entry into the mitotic phase. Protein stores and cell size are evaluated, same like at the G1 checkpoint.
- The G2 checkpoint’s primary responsibility is to make sure that all of the chromosomes have been duplicated and that the DNA that has been replicated has not been damaged.
The M Checkpoint
- Near the conclusion of the metaphase phase of mitosis, the M checkpoint takes place.
- Because it checks to see if all of the sister chromatids are appropriately connected to the spindle microtubules, the M checkpoint is also known as the spindle checkpoint.
- The cycle won’t continue until the kinetochores of each pair of sister chromatids are securely fixed to spindle fibres arising from opposite poles of the cell since the separation of the sister chromatids during anaphase is an irreversible process.
References
- Yang, N., & Sheridan, A. M. (2014). Cell Cycle. Encyclopedia of Toxicology, 753–758. doi:10.1016/b978-0-12-386454-3.00273-6
- Lew, D. J. (2013). Cell Cycle. Brenner’s Encyclopedia of Genetics, 456–464. doi:10.1016/b978-0-12-374984-0.00206-0
- Lew, D. (2001). Cell Cycle. Encyclopedia of Genetics, 286–296. doi:10.1006/rwgn.2001.1557
- Ventura, E., & Giordano, A. (2019). Cell Cycle. Reference Module in Life Sciences. doi:10.1016/b978-0-12-809633-8.90189-4
- Poon, R. Y. C. (2015). Cell Cycle Control☆. Reference Module in Biomedical Sciences. doi:10.1016/b978-0-12-801238-3.98748-8
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. An Overview of the Cell Cycle. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26869/
- https://opentextbc.ca/biology/chapter/6-2-the-cell-cycle/
- https://www.khanacademy.org/science/ap-biology/cell-communication-and-cell-cycle/cell-cycle/a/cell-cycle-phases
