Table of Contents
What is Cell Disruption?
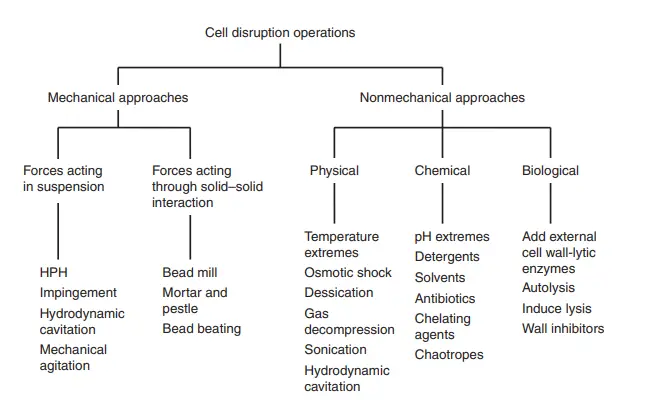
- Cell disruption refers to the process of accessing the intracellular fluid contained within cells by employing techniques that open the cell wall. The ultimate objective of cell disruption is to extract the intracellular fluid while preserving its constituents intact. The specific method employed for cell disruption may vary depending on the type of cell being targeted and the composition of its cell wall.
- Regardless of the chosen method, the primary goal of cell disruption is to ensure its effectiveness while minimizing damage, allowing the recovered product to remain in its active form. Cell disruption methods can generally be classified into two main categories: mechanical methods and non-mechanical methods.
- Mechanical methods involve the application of physical force to break open the cell wall. These methods can be further classified into solid shear methods and liquid shear methods. Solid shear methods involve subjecting the cells to intense mechanical forces, such as grinding or bead beating, to rupture the cell wall and release the intracellular fluid. On the other hand, liquid shear methods utilize high-pressure homogenization or sonication to disrupt the cells and liberate the intracellular content.
- Non-mechanical methods, as the name suggests, do not rely on physical force to disrupt the cell wall. Instead, they utilize alternative means to achieve cell disruption. Non-mechanical methods can be categorized into physical methods, chemical methods, and enzymatic methods.
- Physical methods involve subjecting cells to extreme temperatures (thermal shock), high-frequency sound waves (ultrasonication), or electric pulses (electroporation) to disrupt the cell wall and release the intracellular fluid. Chemical methods utilize various chemical agents, such as detergents or organic solvents, to dissolve or disrupt the cell wall and release the intracellular content. Enzymatic methods employ specific enzymes that can degrade or degrade the components of the cell wall, facilitating cell disruption and intracellular fluid extraction.
- The choice of cell disruption method depends on factors such as the type of cells being targeted, the desired outcome, and the downstream applications of the intracellular fluid. It is crucial to select an appropriate method that effectively disrupts the cell wall while ensuring minimal damage to the intracellular components, ultimately yielding the desired product in its active and functional state.
Cell Disruption Definition
Cell disruption is the process of breaking open cells to access their intracellular fluid while preserving the integrity of its components.
Methods of Cell Disruption
Different cells have different structure and therefore require different methods to disrupt. Cell walls are additional disruptors and yeast cells are especially difficult to destroy due to the fact that the cell’s wall restricts the solvent’s access to the products you want. Other kinds of cells that require disruption are bacterial cells moulds, plant cells mammalian cells, and ground tissue. Bacterial cells could use different methods of disruption, dependent on whether they’re either gram positive or negative, since the amount of peptidoglycan in the cell and the existence of an envelope impact the entire process. Mammalian cells can be the easiest ones to destroy because they do not have walls, as opposed to plants, which tend to be more challenging dislodge.
Drying cells can enhance the methods of disruption and could aid in reducing costs. In certain situations multiple disruption method might be required to ensure full recovery of the product. The factors that determine the choice of the disruption method are
- Size of the cell
- The toughness of the cell determines regardless of whether it’s bacteria or fungi, plants or animal cells.
- The effectiveness of the disruption method
- The stability of the item with respect to the procedure used.
- Simple extraction and purification
- The cell’s biohazard potential is a factor or not
- Time and cost
- Training or expertise required, or not.
Important note: before cell disruption occurs the cells need to be removed from the culture medium. Extracellular substances secreted by cells must be reduced and non-utilized media components should also be eliminated. It is ideal that the method for cell disruption is appropriate for the cells to be affected, uses a known mechanism, and is sterilisable, contained and verified, and has the possibility of automated. Other benefits include a consistent and compact technique that is affordable and effective.
A. Mechanical Methods of Cell Disruption
The principle behind mechanical methods of disruption is that cells are exposed to extreme stress through pressure, abrasion and rapid agitation using beads or ultrasound. There are a variety of methods for disruption, including impingement, shearing, cavitation or a combination of these. The suspension must be cooled extensively after treatment is needed in order to get rid of the heat generated through the dissipation process of the mechanical energy. Certain high-pressure methods are only able to be utilized in laboratory scale, like French presses and Hughes press. For industrial applications bead mills, as well as homogenizer with high pressure are appropriate.
1. Bead mill Method of Cell Disruption
Basic principle of bead mill
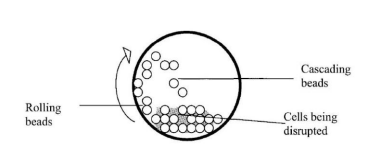
- The basic principle of a bead mill revolves around the destruction of microbial cells, and it has different designs tailored to specific applications. This method is based on an enclosed grinding chamber with a central rotating shaft. The shaft is equipped with agitators that provide an energy source to the tiny beads present inside the chamber. As a result, the beads within the chamber come into contact and collide with each other.
- The dimensions and weight of the beads used in the bead mill are determined by the characteristics of the targeted cells. The nature of the cells dictates the optimal parameters for effective cell disruption. The size of the beads plays a crucial role in the effectiveness of the cell disruption process, as it influences the localization of the desired enzyme within the cell.
- The number of interactions between the beads is directly related to the effectiveness of the bead mill. However, increasing the number of beads can impact power consumption and heating. Ideally, the beads should occupy around 80 to 85 percent of the free volume within the chamber. The rotating discs typically operate at speeds ranging from 1500 to 2500 rpm.
- For yeast cells, glass beads with diameters larger than 0.5 millimeters are considered ideal, while for bacteria, beads with diameters less than 0.5 millimeters are preferred. The key variables in the bead mill process include the speed of the agitator, the percentage of bead volume, the size of the beads, the concentration of the cell suspension, the flow rate of the cell suspension, and the design of the agitator disc.
- The equation In = (Rm/Rm-R) + kt is used to describe the protein released during the bead mill process. The rate constant, k, is determined by the agitation rate (typically 1500-2250 rpm), cell concentration (30-60 percent wet solids), bead diameter (0.2-1.0 millimeters), and temperature. R represents the protein released (in kg biomass/kg protein), while Rm is the maximum protein released, which is dependent on temperature.
Advantages of bead mill Method
The bead mill offers several advantages that make it a valuable tool in various applications. Here are some key advantages of the bead mill:
- Effective for smaller sized materials: The bead mill is particularly useful for processing smaller-sized materials. The tiny beads used in the mill can efficiently break down and disrupt cells or grind materials into fine particles, allowing for enhanced extraction of intracellular components or improved sample preparation.
- Safety: One notable advantage of bead mills is that they do not emit dangerous aerosols. This feature ensures a safer working environment for researchers and minimizes the risk of exposure to potentially harmful substances.
- Versatility in operation: Bead mills can be operated in both batch and continuous modes. This flexibility enables researchers to choose the most suitable mode based on the specific requirements of their experiment or production process. Batch operation is often preferred for smaller-scale experiments, while continuous operation is beneficial for larger-scale processes and higher throughput.
- Disruption of yeast cells: Bead mills are commonly employed for the disruption of yeast cells. The mechanical forces generated by the bead mill effectively break open the yeast cells, releasing their intracellular contents. This allows for the extraction of proteins, enzymes, or other valuable components from yeast cells for various applications in biotechnology, fermentation, or research.
- Grinding of animal tissues: In addition to yeast cells, bead mills are also widely used for the grinding of animal tissues. By subjecting the tissue samples to the grinding action of the beads, the bead mill can efficiently disrupt the cell structure and facilitate the extraction of proteins, nucleic acids, or other target molecules from the tissue samples.
Limitation of bead mill
While bead mills have several advantages, they also come with certain limitations that need to be considered. Here are some of the key limitations associated with bead mills:
- Temperature increase: One limitation of bead mills is that the temperature tends to increase as the volume of beads used in the mill increases. The friction and collision between the beads generate heat, which can lead to an increase in temperature within the milling chamber. This rise in temperature can potentially affect the stability or functionality of sensitive biological molecules or compounds present in the sample.
- Poor scale-up: Another limitation of bead mills is the challenge of scaling up the process. The performance and effectiveness of bead mills may vary when transitioning from small-scale laboratory experiments to large-scale production. Factors such as the efficiency of bead-to-bead interactions, temperature control, and the homogeneity of the disruption process may be more challenging to maintain at larger scales. Proper optimization and validation are required when scaling up bead mill processes to ensure consistent and reliable results.
- Contamination risk: Contamination is a significant concern when using bead mills. The beads themselves can introduce contaminants into the sample, especially if they are not properly cleaned or sterilized before use. Additionally, the wear and tear of the milling chamber, agitators, or seals can lead to the generation of particulates or debris that can contaminate the sample. Careful attention must be given to selecting suitable materials for the beads and ensuring proper cleaning and maintenance protocols to minimize the risk of contamination.
Application
- Bead mills were initially employed in the paint industry and were later adapted to disrupt cells in both large and small-scale production.
2. Ultrasonic disruption/Ultrasound Method of Cell Disruption
Principle of Ultrasonic disruption/Ultrasound
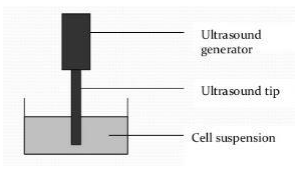
- The principle of ultrasonic disruption, also known as ultrasound, involves the use of high-frequency sound waves to disrupt cells or samples. This disruption is achieved through the action of ultrasonic vibrators, which generate high-frequency sound waves at a density of approximately 20 kHz/s.
- In the process of ultrasonic disruption, a transducer is employed to convert these sound waves into mechanical oscillations. This transducer typically consists of an aluminum probe that is submerged into the cell fluid or the sample suspension. When the ultrasonic waves pass through the fluid, they create alternating high-pressure and low-pressure regions, resulting in the phenomenon known as cavitation.
- Cavitation occurs when the pressure changes cause the formation and rapid collapse of tiny bubbles or voids within the fluid. The collapse of these bubbles produces intense localized forces and shockwaves, leading to the disruption of cells or samples in the vicinity. The mechanical energy released during cavitation can break open cell walls or membranes, facilitating the release of intracellular components.
- The ultrasonic disruption method is particularly effective for disrupting cells and samples that are difficult to disrupt using other techniques. It is commonly employed in various scientific and industrial applications, such as cell lysis, particle size reduction, emulsification, and extraction of intracellular components.
- It is worth noting that the specific parameters of ultrasonic disruption, such as the frequency and intensity of the ultrasound, can be adjusted based on the characteristics of the cells or samples being treated. Optimization of these parameters is crucial to ensure effective disruption while minimizing damage to sensitive components or molecules within the sample.
- Overall, the principle of ultrasonic disruption relies on the generation of high-frequency sound waves and the resulting phenomenon of cavitation to disrupt cells or samples. This method provides a versatile and efficient means of achieving cell lysis and sample disruption in various scientific and industrial applications.
Advanatges of Ultrasonic disruption/Ultrasound
Ultrasonic disruption, also known as ultrasound or sonication, offers several advantages that make it a valuable technique in various applications. Here are some key advantages of ultrasonic disruption:
- Rapid and efficient disruption: Ultrasonic disruption enables rapid and efficient disruption of cells or samples. The high-frequency sound waves create intense mechanical forces through cavitation, leading to the disruption of cell walls or membranes and the release of intracellular components. This efficient disruption process saves time and enables high-throughput sample processing.
- Versatility: Ultrasonic disruption is a versatile technique that can be applied to a wide range of samples and materials. It is effective for the disruption of microbial cells, including bacteria and yeast, as well as animal and plant cells. Additionally, it can be used for the disruption of various sample types, such as tissues, cells, nanoparticles, emulsions, and suspensions.
- Preservation of sample integrity: Ultrasonic disruption is a non-thermal method, which means it does not rely on excessive heat to disrupt cells. This non-thermal nature helps preserve the integrity of heat-sensitive components, such as enzymes, proteins, and delicate cellular structures. As a result, ultrasonic disruption allows for the extraction of bioactive compounds or delicate intracellular components in their native and active forms.
- Compatibility with other techniques: Ultrasonic disruption is often employed in conjunction with other techniques, such as chemical processes or downstream analytical methods. It can enhance the effectiveness of chemical lysis by providing mechanical energy, leading to improved extraction efficiency. Furthermore, ultrasonic disruption prepares samples for subsequent analysis techniques, including DNA extraction, protein analysis, chromatography, or mass spectrometry.
- Ease of use and scalability: Ultrasonic disruptors are relatively easy to use and require minimal setup. They can be operated with user-friendly interfaces and offer adjustable settings for frequency, power, and duration. Additionally, ultrasonic disruption can be scaled up for larger volumes or production processes, making it suitable for both laboratory-scale experiments and industrial applications.
- Cost-effectiveness: Ultrasonic disruptors are generally cost-effective compared to some other disruption methods. They require relatively simple equipment and have lower maintenance costs. Moreover, the high efficiency and shorter processing times of ultrasonic disruption contribute to cost savings in terms of labor, energy consumption, and overall operational expenses.
In summary, ultrasonic disruption or ultrasound offers advantages such as rapid and efficient disruption, versatility in sample types, preservation of sample integrity, compatibility with other techniques, ease of use, scalability, and cost-effectiveness. These advantages make ultrasonic disruption a valuable tool in various scientific, biomedical, and industrial applications for cell disruption, sample preparation, and analysis.
Limitation of Ultrasonic disruption/Ultrasound
While ultrasonic disruption, also known as ultrasound or sonication, has its advantages, it also has certain limitations that should be considered. Here are some of the key limitations associated with ultrasonic disruption:
- Limited scalability: Ultrasonic disruption is highly effective for small-scale projects or laboratory applications. However, when it comes to scaling up the process for larger volumes or industrial production, the efficiency and effectiveness of sonication may decrease. Challenges such as maintaining consistent energy distribution and achieving uniform disruption throughout larger volumes can make scaling up ultrasonic disruption techniques inefficient or impractical.
- High energy demand: Ultrasonic disruption requires a significant amount of energy to generate the high-frequency sound waves and mechanical oscillations needed for disruption. This high energy demand can be a limitation, especially in large-scale or continuous production processes. It may result in increased operational costs and energy consumption, making it less economical compared to other disruption methods in certain scenarios.
- Health and safety risks: Sonication generates high-frequency sound waves that can create excessive noise levels in the laboratory or production environment. Prolonged exposure to high levels of noise can pose risks to the health and safety of operators, potentially causing hearing damage or other health issues. Adequate measures, such as personal protective equipment (PPE) and noise reduction strategies, should be implemented to minimize these risks.
- Lack of continuity: Ultrasonic disruption is typically performed in a batch mode, where samples are sonicated for a specific duration and then stopped. This lack of continuity can be a limitation in applications where continuous processing or uninterrupted disruption is desired. It may require additional efforts and equipment to achieve continuous or semi-continuous sonication processes.
Uses of Ultrasonic disruption/Ultrasound
Ultrasonic disruption, also known as ultrasound or sonication, finds various uses in the field of cell disruption and sample preparation. Here are some common applications of ultrasonic disruption:
- Cell disruption: Ultrasonic disruption is widely employed for the destruction of fungal and bacterial cells. It is a rapid and efficient method to break open cell walls and membranes, releasing intracellular components such as proteins, nucleic acids, enzymes, and metabolites. Bacterial cells can be disrupted within a relatively short time frame of 30 to 60 seconds, while yeast cells may require a slightly longer duration of 2 to 10 minutes.
- Sample lysis: Ultrasonic disruption is often used in conjunction with chemical processes, particularly lysis, for sample preparation. Chemical lysis methods involve the use of detergents, enzymes, or other chemical agents to break down cell structures. Ultrasonic disruption can enhance the effectiveness of chemical lysis by providing additional mechanical energy to aid in the disruption and release of cellular components. The combination of ultrasonic disruption with chemical lysis can result in improved extraction efficiency and yield.
- Homogenization and emulsification: Ultrasonic disruption is employed for the homogenization and emulsification of various samples. It can effectively break down and disperse solid particles or droplets in a liquid medium, resulting in a more uniform and homogeneous sample. This is particularly useful in applications such as emulsion preparation, particle size reduction, and dispersion of insoluble materials.
- Sample preparation for analysis: Ultrasonic disruption is utilized in sample preparation for various analytical techniques, such as DNA extraction, protein extraction, and sample pretreatment for chromatography or mass spectrometry. By disrupting cells and releasing target molecules or analytes, ultrasonic disruption enhances the efficiency and accuracy of downstream analysis.
- Industrial applications: Ultrasonic disruption is also applied in industrial settings for various purposes. It is used in food processing to enhance extraction of flavors, colors, or bioactive compounds from plant materials. In the pharmaceutical and biotechnology industries, ultrasonic disruption is employed for the extraction of pharmaceutical compounds, production of nanoparticles, or formulation development.
3. French press and high pressure homogeniser
Principle of French press and high pressure homogeniser
The principle of a French press and high-pressure homogenizer involves the application of high pressure to disrupt cells or samples. Here’s an explanation of the principles underlying these two techniques:
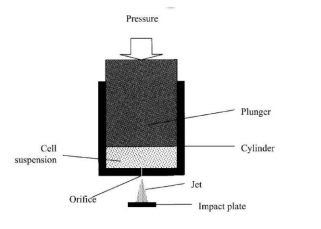
- French press: The French press, also known as a high-pressure homogenization device, operates by pumping a suspension of cells through a valve into a cylinder pump. From there, the suspension is pushed at high pressures, reaching up to 1500 bar, through an annular gap or a small opening. At this point, the pressure abruptly decreases to atmospheric pressure as it passes through discharge valves. This sudden pressure drop causes the cells to explode or undergo disruption. The disruption occurs due to the rapid reduction in pressure, which leads to the release of intracellular components and the disruption of cell walls or membranes.
The French press offers control over the pressure applied, allowing for adjustment to achieve the desired level of disruption. Higher pressures can increase the number of passes through the press, enhancing disruption efficiency. Conversely, pressure can be reduced to achieve the desired level of disruption while minimizing damage to sensitive cellular components.
- High-pressure homogenizer: Similar to the French press, a high-pressure homogenizer operates on the principle of applying high pressure to disrupt cells. In this technique, the sample or cell suspension is passed through a narrow gap or valve at high pressures, typically ranging from several hundred to several thousand bars. The sample is forced through the gap, leading to high shear forces and turbulence. These forces cause disruption of the cells, resulting in the release of intracellular contents.
High-pressure homogenizers can be used to achieve uniform particle size reduction, emulsification, and homogenization of various samples. The disruption efficiency and degree of disruption can be controlled by adjusting the pressure, number of passes, and specific equipment settings.
Both the French press and high-pressure homogenizers are powerful techniques for cell disruption and sample preparation. They are commonly used in research, biotechnology, and industrial applications to release intracellular components, extract proteins, disrupt tough cell walls, and prepare samples for downstream analysis. The ability to control pressure and optimize disruption parameters makes these techniques versatile and adaptable to various sample types and processing requirements.
Limitation of French press and high pressure homogeniser
The French press and high-pressure homogenizer techniques have certain limitations that should be considered. Here are some of the limitations associated with these methods:
- Deactivation of heat-sensitive proteins: The application of high pressure and the associated increase in temperature during the French press and high-pressure homogenization processes can result in the deactivation or denaturation of heat-sensitive proteins. This can make it challenging to maintain the activity and integrity of certain proteins during the disruption process.
- Protein release influenced by various factors: The release of proteins during cell disruption using these techniques is influenced by multiple factors, including temperature, the location of enzymes inside the cell, the number of passages or cycles, and the operating pressure. Optimizing these parameters becomes crucial to ensure efficient protein release and minimize the loss of desired components.
- Biomass concentration: The concentration of biomass or cells in the sample can impact the efficiency of the disruption process. High biomass concentrations can lead to increased viscosity and resistance to flow, making it more difficult to achieve effective disruption and complete the desired number of passages.
- Scale limitations: The French press is typically considered a small-scale process, while high-pressure homogenizers can be used for both small-scale and large-scale production. However, scaling up the process using high-pressure homogenizers can present challenges, as the design and capabilities of homogenizers can vary significantly. Additionally, large quantities of solids, up to 50 percent, in certain food samples may pose challenges in achieving efficient disruption and maintaining homogeneity.
- Heat generation: Both the French press and high-pressure homogenizer techniques can generate significant heat during operation. The heat generation is proportional to the applied pressure, and it can be relatively high, with an increase in temperature of about 1.5°C per 1000 psi of pressure. Excessive heat can lead to thermal denaturation or degradation of sensitive biomolecules, impacting the overall efficiency and quality of the disruption process.
Uses of French press and high pressure homogeniser
The French press and high-pressure homogenizer techniques find diverse applications in various fields. Here are some common uses of these methods:
- Cell disruption: The French press and high-pressure homogenizer techniques are widely employed for cell disruption. They can effectively break down the cell walls or membranes of various microorganisms, making them particularly useful for disrupting yeast cells. The disruption of cells allows for the release of intracellular components, such as proteins, enzymes, and nucleic acids, which can be further utilized for downstream applications in research, biotechnology, and industrial processes.
- Protein extraction and purification: These techniques are utilized in protein extraction and purification processes. By applying high pressure, the French press and high-pressure homogenizers enable efficient disruption of cells, facilitating the extraction of desired proteins from cellular material. This is crucial in biotechnology and pharmaceutical industries, where the production of recombinant proteins or isolation of specific proteins is essential for research, therapeutics, or industrial applications.
- Homogenization of dairy products: High-pressure homogenizers play a significant role in the dairy production industry, particularly in the homogenization of milk. Milk naturally separates into cream and liquid layers due to differences in fat content. Homogenization helps create a uniform distribution of fat globules throughout the milk, resulting in a stable and consistent product. High-pressure homogenization disrupts and reduces the size of fat globules, preventing creaming and enhancing the sensory properties, texture, and shelf life of dairy products such as milk, cream, and yogurt.
- Food processing and formulation: French press and high-pressure homogenizer techniques are utilized in food processing and formulation to achieve desirable textures, emulsions, and product consistency. By subjecting food ingredients to high pressure, these techniques can effectively blend and mix ingredients, create stable emulsions, improve product stability, and enhance texture and mouthfeel. They are employed in the production of sauces, dressings, spreads, beverages, and other food products where a uniform and smooth consistency is desired.
- Nanoparticle synthesis: High-pressure homogenization is also employed in nanoparticle synthesis. The high shear forces generated during the process can effectively break down materials into smaller particle sizes, leading to the formation of nanoparticles with controlled properties. This has applications in fields such as nanotechnology, materials science, and drug delivery systems.
In summary, the French press and high-pressure homogenizer techniques are versatile tools with a wide range of applications. They are commonly used for cell disruption, protein extraction, homogenization of dairy products, food processing, and nanoparticle synthesis. These techniques play crucial roles in various industries, including biotechnology, pharmaceuticals, food, and nanotechnology, facilitating processes that require disruption, homogenization, and the extraction of valuable components from cellular material.
4. Mortar and pestle
The mortar and pestle is a widely used tool for grinding and breaking up plant cells manually. It is a traditional method employed to disrupt cell walls and extract desired components from plant tissues. Here’s some information about the mortar and pestle technique:
- Hand grinding: Grinding by hand using a mortar and pestle is a popular method for breaking up plant cells. The plant tissue is typically first frozen and stored in liquid nitrogen to preserve its integrity. This freezing step helps to maintain the structure of the cells and prevents enzymatic degradation of the cellular components. Once the tissue is sufficiently frozen, it is placed in a mortar.
- Crushing with a pestle: The mortar and pestle consist of two components—a bowl-shaped mortar and a rod-shaped pestle. The frozen plant tissue is crushed using the pestle, which is rotated and pressed against the tissue in a grinding motion. The repeated grinding action applied by the pestle breaks down the cell walls and disrupts the plant cells, allowing for the release of intracellular components.
- Cell wall composition: The effectiveness of the mortar and pestle technique in breaking up plant cells is attributed to the tensile force of cellulose and other polysaccharides that form the cell wall. The cell wall provides structural support to plant cells and is composed of various complex carbohydrates. By grinding and crushing the tissue using the mortar and pestle, the cell walls are ruptured, facilitating the extraction of cellular components.
The mortar and pestle technique is simple, cost-effective, and suitable for small-scale applications. It is commonly used in research laboratories and in fields such as plant biology, pharmacology, and natural product extraction. However, it is important to note that this method may not be suitable for large-scale processing due to limitations in scalability and consistency. In such cases, alternative methods like high-pressure homogenization or bead mills are often employed to achieve efficient and standardized cell disruption.
Overall, the mortar and pestle technique offers a manual approach for breaking up plant cells and extracting intracellular components. It relies on the mechanical force applied by grinding and crushing to disrupt the cell walls, allowing for the subsequent isolation and analysis of plant cell contents.
Advanatges of Mortar and pestle Method
The mortar and pestle method offers several advantages for certain applications, especially when working with difficult tissues such as plants. Here are some advantages of the mortar and pestle method:
- Effectiveness with difficult tissues: The mortar and pestle method is particularly effective for grinding and disrupting plant tissues. Plant cells often have rigid cell walls composed of cellulose and other complex polysaccharides, making them more challenging to break down. The manual grinding action provided by the mortar and pestle allows for the application of controlled force, enabling the effective disruption of these tough tissues and facilitating the extraction of intracellular components.
- Versatility: The mortar and pestle method offers versatility in terms of sample preparation and experimentation. It allows for the reconstitution of buffers directly in the mortar, providing flexibility in choosing the appropriate buffer for specific applications. This can be advantageous when working with sensitive samples or when specific buffer conditions are required for downstream analyses or experiments.
- Accessibility and affordability: Mortar and pestle sets are widely available and relatively affordable compared to more advanced cell disruption techniques. This makes them accessible to a wide range of researchers and laboratories, especially those with limited resources or smaller-scale projects. The simplicity of the method also makes it easy to learn and perform, requiring minimal specialized equipment.
- Preservation of sample integrity: The mortar and pestle method, particularly when combined with freezing the tissue in liquid nitrogen, helps preserve the integrity of the sample. Freezing the tissue prior to grinding helps maintain the structural integrity of the cells and minimizes enzymatic degradation, ensuring the retention of important cellular components.
- Limited contamination risk: Compared to some other cell disruption methods that involve mechanical or chemical processes, the mortar and pestle method presents a lower risk of sample contamination. The simplicity of the method and the absence of additional reagents or equipment minimize the potential for introducing foreign substances or contaminants into the sample during the disruption process.
While the mortar and pestle method has its advantages, it is important to note that it may not be suitable for all types of samples or for large-scale applications. For more efficient and standardized cell disruption on a larger scale, alternative methods such as high-pressure homogenization, bead mills, or ultrasonication may be preferred. However, for specific applications where plant tissues or difficult samples need to be disrupted manually, the mortar and pestle method remains a valuable and effective option.
Limitation of Mortar and pestle Method
The mortar and pestle method, while offering advantages in certain contexts, also has limitations that should be considered. Here are some limitations of the mortar and pestle method:
- Time-consuming: The mortar and pestle method can be a time-consuming process, especially when working with larger sample volumes or tougher tissues. The manual grinding and crushing require repetitive motions and can take a significant amount of time and effort to achieve the desired level of cell disruption. This can be a drawback when processing a large number of samples or when time is a limiting factor in the experimental workflow.
- Variability in reproducibility: The reproducibility of the results obtained using the mortar and pestle method may vary between different users or experimental runs. Manual grinding relies on the skill and technique of the operator, which can introduce variability in the level of cell disruption achieved. Factors such as the force applied, grinding time, and consistency of grinding can influence the reproducibility of the method. This can be a concern when consistency and standardization of results are crucial, especially in research or industrial settings.
- Limited scalability: The mortar and pestle method is more suitable for small-scale applications. Scaling up the process to handle larger sample volumes can be challenging and time-consuming. The manual nature of the method makes it impractical for processing large quantities of samples consistently. Alternative methods such as high-pressure homogenization or bead mills are often preferred for large-scale cell disruption due to their ability to provide more efficient and controlled disruption on a larger scale.
- Sample heating and degradation: The grinding action in the mortar and pestle method can generate heat due to friction, potentially leading to sample heating. Heat-sensitive components within the sample, such as enzymes or proteins, may be susceptible to denaturation or degradation during the grinding process. This can affect the quality and integrity of the extracted intracellular components, impacting downstream analyses or applications.
- Limited control over particle size: Achieving consistent and precise control over particle size is challenging with the mortar and pestle method. The manual grinding process may result in a range of particle sizes, leading to sample heterogeneity. Obtaining a uniform particle size distribution may require additional steps or modifications to the technique, which can further increase the complexity and time required for the method.
It’s important to consider these limitations when choosing the appropriate cell disruption method. While the mortar and pestle method may be suitable for certain applications, alternative techniques should be explored for large-scale processing, achieving greater reproducibility, and controlling particle size more precisely.
Use of Mortar and pestle Method
The use of the mortar and pestle method is considered highly efficient and rapid for accessing DNA and plant proteins. Here’s some information about the use of this method:
- DNA extraction: The mortar and pestle method is widely employed for DNA extraction from plant tissues. The process involves grinding the plant material in a mortar using the pestle, which helps disrupt the cell walls and release the cellular contents, including DNA. By grinding the tissue, the DNA becomes accessible for subsequent extraction and purification steps. The simplicity and effectiveness of the mortar and pestle method make it a popular choice for DNA extraction, especially in small-scale laboratory settings.
- Protein extraction: The mortar and pestle method is also commonly utilized for accessing plant proteins. By grinding the plant tissue, the cell walls are disrupted, allowing for the release of proteins into the extraction buffer. The grinding action of the mortar and pestle helps break down the cellular structures and facilitate the extraction of soluble proteins. This method can be particularly effective for small-scale protein extractions when immediate access to plant proteins is required.
- Efficiency and speed: The mortar and pestle method is known for its efficiency and speed in gaining access to DNA and plant proteins. The manual grinding process allows for rapid disruption of cell walls, enabling quick extraction of intracellular components. Compared to other cell disruption methods, such as high-pressure homogenization or bead milling, the mortar and pestle method can offer a more straightforward and time-efficient approach, especially for smaller-scale experiments or when immediate access to DNA and proteins is needed.
- Cost-effectiveness: The use of a mortar and pestle is a cost-effective option for gaining access to DNA and plant proteins. Mortar and pestle sets are readily available, affordable, and do not require additional specialized equipment or reagents. This makes the method accessible to researchers and laboratories with limited resources or those conducting smaller-scale studies. The simplicity of the technique also minimizes the cost associated with sample processing.
5. Microfluidizer
The microfluidizer principle is based on applying pressure to induce the production of small particles. This method is commonly utilized in laboratory-scale applications. The combination of shear force and pressure in the microfluidizer can lead to cell damage, making it useful for cell disruption.
Advantages:
- Efficient particle size reduction: The microfluidizer method is highly effective at reducing particle sizes. By subjecting the sample to intense shear forces and high-pressure conditions, it can achieve significant particle size reduction. This makes it valuable in various applications where precise control over particle size is crucial.
- Versatile: Microfluidizers offer versatility in terms of sample types. They can handle a wide range of materials, including cells, tissues, liposomes, emulsions, and suspensions. This versatility makes microfluidizers suitable for a broad spectrum of research and industrial applications.
- Enhanced homogenization: The microfluidizer principle facilitates efficient homogenization of samples. It can effectively disrupt cells and release intracellular components, including proteins, enzymes, and DNA. The homogenization capability of microfluidizers makes them valuable in applications where the extraction of cellular contents is required.
Limitations:
- Cost-intensive method: The microfluidizer technique can be relatively expensive due to the specialized equipment required. Microfluidizers are complex machines that involve high-pressure pumps and intricate fluidic systems. The initial investment and maintenance costs associated with microfluidizers can be a limitation, particularly for laboratories with budget constraints.
- Not suitable for large-scale production: Microfluidizers are generally more suitable for laboratory-scale or small-scale production. While they provide efficient particle size reduction and homogenization, scaling up the process to larger volumes can be challenging. The complexity and cost of large-scale microfluidizers, along with the associated challenges in maintaining consistent pressure and shear forces, make them less practical for large-scale production.
Uses:
- Cell disruption and homogenization: Microfluidizers are commonly used for disrupting cells and achieving efficient homogenization of samples. They can break down cell walls and release intracellular components for further analysis or extraction, making them valuable in biotechnology, pharmaceutical, and biochemical research.
- Particle size reduction: The microfluidizer principle is often employed to reduce particle sizes in various applications. It is utilized in industries such as food processing, cosmetics, and nanotechnology, where achieving a specific particle size distribution is crucial for product quality and performance.
- Emulsion and suspension processing: Microfluidizers are also employed in emulsion and suspension processing. They can effectively mix and disperse immiscible liquids or suspend solid particles in a liquid medium. This makes them valuable in the production of emulsions, suspensions, and encapsulated products.
In summary, the microfluidizer principle offers advantages such as efficient particle size reduction, versatility, and enhanced homogenization. However, it is a cost-intensive method and not well-suited for large-scale production. Microfluidizers find applications in cell disruption, particle size reduction, and emulsion/suspension processing in various industries and research fields.
6. Liquid homogenization
Liquid homogenization is a widely used method for cell disruption, particularly for small volumes of cultured cell lines. It involves pushing a suspension of tissue or cells through a narrow space, which leads to the disruption of cell membranes. There are three commonly used types of homogenizers in this method: Dounce homogenizer, Potter-Elvehjem homogenizer, and French press.
Principle:
- Dounce homogenizer: It consists of a round glass pestle that is manually inserted into a glass tube. By repeatedly grinding and shearing the sample against the tube walls, cell disruption is achieved. The efficiency of disruption in the Dounce homogenizer depends on the number of strokes and the speed at which the strokes are performed.
- Potter-Elvehjem homogenizer: This homogenizer features a mechanically or manually driven pestle made of PTFE (polytetrafluoroethylene), which fits into a round or conical vessel. Similar to the Dounce homogenizer, the sample is disrupted by the grinding and shearing action of the pestle against the vessel walls. The efficiency of disruption in the Potter-Elvehjem method is influenced by the number and speed of strokes.
- French press: This homogenizer consists of a piston that applies high pressure to the sample, typically ranging from 40 to 250 mL, and forces it through a small opening. The high pressures generated in this process allow for efficient cell lysis. Although French press equipment can be costly, it is considered a highly effective method for mechanical disruption of bacteria.
Advantages:
- Ease of use: Liquid homogenization methods, including Dounce and Potter-Elvehjem homogenizers, are relatively simple to use, even with smaller sample volumes. They do not require complex equipment or specialized training, making them accessible to a wide range of users.
Limitations:
- Low throughput: Liquid homogenization methods typically have a low throughput, making them less suitable for processing large sample volumes. These methods are better suited for smaller-scale applications where higher throughput is not a primary concern.
- Reproducibility variation: In the case of the Dounce homogenizer, reproducibility can vary between experiments and users. Achieving consistent results with the Dounce homogenizer may require careful attention to technique and practice.
Uses:
Liquid homogenization methods, such as Dounce and Potter-Elvehjem homogenizers, are commonly employed for cell disruption in liquid-based homogenization protocols. These methods are particularly useful for small-scale volumes of cultured cell lines. They are often utilized in various research applications, such as protein extraction, enzyme assays, and preparation of cell lysates for downstream analysis.
In summary, liquid homogenization methods offer advantages in their ease of use, especially for smaller quantities. However, they have limitations in terms of low throughput and potential reproducibility variations. Liquid homogenization is frequently utilized in research settings for processing small volumes of cultured cell lines to achieve cell disruption and extract cellular components for further analysis.
7. Sonication
Sonication is a cell disruption method that utilizes pulsed high-frequency sound waves to stimulate and lyse cells, bacteria, spores, and finely chopped tissues. The principle of sonication involves transmitting sound waves through an apparatus with vibrating probes that are immersed in a liquid cell suspension. The mechanical energy from the vibrating probes leads to the formation of small vapor bubbles that subsequently implode, generating shock waves that disrupt the cells.
Advantages:
- Independent of cell type: Sonication is effective for various types of cells and can be applied to a wide range of biological samples, regardless of their specific characteristics.
- High lysing effectiveness: Sonication is known for its efficient disruption capabilities, resulting in a high degree of cell lysis and release of cellular components.
- Shears chromosomes and eliminates the necessity for nuclease treatment: Sonication can effectively shear chromosomes, eliminating the need for additional nuclease treatment in certain applications.
Limitations:
- Produces heat: Sonication generates heat during the process, which needs to be carefully controlled to prevent damage to proteins that are sensitive to high temperatures. Cooling the sample in an ice bath during sonication helps mitigate excessive heating.
- Produces cellular debris: The disruptive forces of sonication can lead to the generation of cellular debris, which may require additional steps to remove unwanted particles from the lysate.
- Loud and requires sound reduction: Sonication is a noisy process, and precautions should be taken to reduce sound levels to protect the operator’s hearing and create a more comfortable working environment.
Uses:
Sonication is commonly employed to break open cells and extract cellular components for various applications. It is particularly useful for processes such as protein extraction, DNA or RNA isolation, enzyme activity assays, and preparation of cell lysates for downstream analysis. Sonication is widely utilized in research laboratories, biotechnology, and pharmaceutical industries for its efficient cell disruption capabilities and versatility in working with different sample types.
In summary, sonication is a widely used cell disruption method that utilizes high-frequency sound waves to disrupt cells and extract their contents. It offers advantages such as effectiveness across various cell types, high lysing efficiency, and the ability to shear chromosomes. However, it has limitations related to heat generation, production of cellular debris, and noise. Sonication finds extensive use in cell biology, molecular biology, and biotechnology for applications requiring cell disruption and extraction of cellular components.
B. Non-mechanical physical methods
It is divided into three categories such as;
- Physical Method
- Chemical Method
- Biological Method
1. Physical Method
A. Thermolysis/Microwave
Thermolysis, also known as microwave treatment, is a cell disruption method that utilizes heat generated by microwave radiation to lyse cells and release their intracellular components. The principle of thermolysis involves subjecting the cells to high temperatures for a specific duration to induce protein release and cell lysis.
Advantages:
- Suitable for large-scale production: Thermolysis has been demonstrated to be effective for large-scale production, making it a favorable choice for industrial applications.
- Rapid protein release: Thermolysis can achieve efficient protein release from bacterial cells. For example, periplasmic proteins from Gram-negative bacteria can be released by heating cells to 50°C, while cytoplasmic proteins from E.coli can be released in just 10 minutes at 90°C.
- Synergistic effects: Combining the freezing and thawing process with grinding of cells can enhance the efficiency of thermolysis, leading to impressive results in terms of cell disruption and extraction of cellular components.
Limitations:
- Expensive: Thermolysis can be a costly method due to the requirement for specialized equipment such as microwave ovens or reactors capable of generating controlled heat.
- Limited to small-scale labs: Despite its potential for large-scale production, the use of thermolysis is often limited to small-scale laboratories due to the associated costs and specific infrastructure requirements.
- Inconsistent enzyme activity: Some reports have indicated a lack of enzyme activity following thermolysis treatment, suggesting that the heat may denature or impair the functionality of certain enzymes.
Uses:
Thermolysis is commonly employed in research laboratories and industrial settings for the extraction of intracellular components such as proteins and enzymes. It can be applied to various cell types, including bacteria, to release their cellular contents. Thermolysis is particularly useful when large-scale production is required, as it offers a rapid and efficient method for cell disruption.
In summary, thermolysis or microwave treatment is a cell disruption method that utilizes heat generated by microwave radiation to lyse cells and release their intracellular components. It has advantages such as suitability for large-scale production, rapid protein release, and the potential for enhanced efficiency when combined with freezing/thawing and grinding. However, thermolysis can be expensive, limited to small-scale labs, and may exhibit inconsistent enzyme activity. It finds applications in research and industrial settings for the extraction of cellular components.
B. Decompression
Decompression, specifically explosive decompression, is a cell disruption method that involves subjecting a cell suspension to subcritical pressurized gas for a specific duration. The gas enters the cells and upon release, expands rapidly, leading to cell rupture or explosion.
Advantages:
- Gentle on cells: Decompression is a relatively gentle method compared to some other cell disruption techniques. It minimizes the potential for excessive damage or denaturation of intracellular components.
- Promising results: Decompression has shown promise in small-scale laboratory applications, particularly for the destruction of E. coli and the disruption of yeasts. It has the potential to effectively disrupt cells and release their intracellular contents.
- Removal of off-flavors: The use of supercritical CO2 in decompression methods can help remove off-flavors caused by lipids, improving the quality of the resulting products.
- Easy removal of debris: The explosive nature of decompression results in the generation of large amounts of debris, which can be easily separated and removed. This facilitates the extraction and purification of the desired cellular products.
Limitations:
- Low efficiency: Decompression may have relatively lower efficiency compared to other cell disruption methods. The effectiveness of the technique depends on factors such as the release of pressure and the duration of contact between the cell suspension and the gas.
- Dependency on pressure release and contact time: Achieving optimal disruption using decompression relies on carefully controlling the release of pressure and ensuring sufficient contact time between the cell suspension and the gas. Inadequate pressure release or insufficient contact time may lead to suboptimal results.

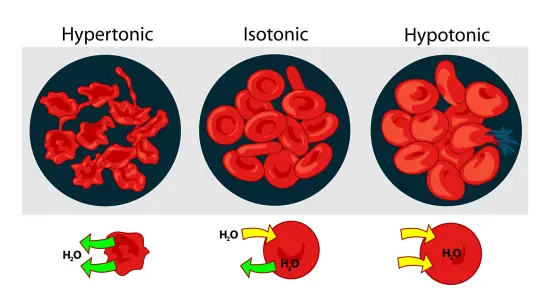
C. Osmotic shock
Osmotic shock is a cell disruption technique that involves subjecting cells to alternating salt concentrations, which leads to cell destruction. The principle behind osmotic shock is the rapid movement of water from low salt concentration environments to high salt concentration environments, causing changes in cell volume and pressure and ultimately resulting in cell rupture.
Advantages:
- Biotechnological applications: Osmotic shock can be utilized in various biotechnological applications to trigger cell destruction. It provides a means to disrupt cells and release their intracellular contents for further processing.
- Simple procedure: Osmotic shock is a relatively simple and straightforward technique that does not require complex equipment or specialized reagents. It can be performed using readily available salt solutions.
Limitations:
- Inefficiency: Osmotic shock is not widely employed as a method of cell disruption due to its relatively low efficiency. The effectiveness of disruption may vary depending on the cell type and its resistance to osmotic changes.
- Enzymatic pre-treatment: In some cases, enzymatic pre-treatment may be required to reduce the cell’s resistance and enhance the effectiveness of osmotic shock. This additional step can increase the complexity and time required for the overall process.
- High salt and water usage: Osmotic shock typically involves the addition of large amounts of salts to create the desired salt concentration gradients. This can lead to high salt usage and significant water consumption, which may not be environmentally or economically favorable.
- Dilution of the product: The osmotic shock process can result in the dilution of the desired product due to the influx or efflux of water from the cells. This can increase downstream processing costs to concentrate and purify the desired components.
In summary, osmotic shock is a cell disruption technique that utilizes alternating salt concentrations to induce cell destruction. It offers simplicity in procedure and finds applications in biotechnological processes. However, its limitations include inefficiency, the potential need for enzymatic pre-treatment, high salt and water usage, and the dilution of the product. These factors may affect its widespread use and efficiency in large-scale cell disruption processes.
D. Freeze-thaw lysis
The freeze-thaw lysis technique is a commonly used method to lyse bacterial and mammalian cells. It involves subjecting a cell suspension to cycles of freezing and thawing, which leads to cell rupture and release of cellular contents. The principle behind freeze-thaw lysis is the formation of ice crystals during freezing and the expansion of cells as the crystals grow, followed by cell disruption during thawing.
Advantages:
- Cost-effective: The freeze-thaw lysis technique is relatively inexpensive compared to other cell lysis methods. It does not require expensive equipment or reagents, making it accessible for researchers with limited resources.
Limitations:
- Sensitivity to temperature: Freeze-thaw lysis may not be suitable for the extraction of cellular components that are sensitive to temperature changes. Some proteins or biomolecules may become denatured or degraded during the freezing and thawing process, leading to loss of activity or functionality.
- Time-consuming: The freeze-thaw lysis method typically requires multiple cycles of freezing and thawing to achieve effective cell lysis. Each cycle takes time, and the overall process can be time-consuming, especially when dealing with large volumes or high cell densities.
Despite its limitations, the freeze-thaw lysis technique has been proven effective in releasing recombinant proteins from bacterial cytoplasm and has been suggested for lysing certain mammalian cells in specific protocols. It remains a valuable method in laboratories where cost-effectiveness and simplicity are prioritized. However, researchers need to consider the temperature sensitivity of their target cellular components and the time required for the lysis process when choosing this method.
E. Dessication
The principle of desiccation involves drying a suspension of cells either through vacuum drying or air drying. When cells are subjected to desiccation, the removal of water causes them to shrink. If the dried cells are subsequently rehydrated or exposed to a high moisture environment, the influx of water can lead to cell rupture and explosion. This cycle of drying and rehydration can be repeated multiple times to achieve the desired level of cell disruption.
Advantages:
- Slower process: Desiccation is a relatively slow process compared to other cell disruption methods. It may require multiple repetitions of drying and rehydration to achieve efficient cell lysis. This slow and controlled process allows for better regulation and adjustment of the disruption level.
- Complementary to other processes: Desiccation is often used in conjunction with other cell disruption methods. By combining desiccation with techniques such as grinding, sonication, or enzymatic treatment, the overall disruption efficiency can be enhanced. Desiccation can act as a preparatory step to further facilitate cell lysis in subsequent processes.
Limitations:
- Limited to certain cell types: Desiccation may not be suitable for all types of cells. Some cells may have greater resistance to desiccation-induced stress and may not undergo effective lysis through this method. The efficiency of desiccation can vary depending on the cell’s structural characteristics and composition.
- Time-consuming: Achieving complete cell disruption through desiccation can be a time-consuming process. The repetitive cycles of drying and rehydration, as well as the coordination with other disruption methods, may increase the overall time required for the cell lysis process.
While desiccation alone may not be the primary method for efficient cell disruption, it serves as a valuable technique in combination with other processes. The slow and controlled nature of desiccation allows for customized disruption conditions and can be tailored to specific cell types and experimental requirements. By integrating desiccation into a comprehensive cell lysis strategy, researchers can enhance the overall efficiency of cell disruption and achieve successful extraction of cellular components.
F. Electrolysis (Electroporation)
Electrolysis, also known as electroporation, is a technique that involves subjecting a cell suspension to an electric field with an intensity exceeding a certain threshold. This electric field leads to the formation of nanoscale pores on the surface of the cells. The development of these pores can be reversible or irreversible, depending on the applied field strength and its direction. Through these pores, intracellular substances can be released, allowing for further analysis or extraction.
Advantages:
- Highly efficient: Electrolysis is a highly efficient method of cell disruption. The formation of nanoscale pores on the cell membrane allows for the release of intracellular components, such as proteins, nucleic acids, and enzymes. This enables researchers to access and analyze the cellular content without complete cell lysis.
- Selective disruption: Electroporation can selectively disrupt specific types of cells within a heterogeneous cell population. By adjusting the parameters of the electric field, such as field strength and duration, it is possible to target specific cells and achieve cell-specific disruption. This selective disruption is advantageous in applications where precise control over cell manipulation is required.
- Versatile: Electroporation can be applied to various types of cells, including bacteria, yeast, mammalian cells, and plant cells. It is a versatile technique that can be adapted to different cell types and experimental needs.
Limitations:
- Heat generation: One limitation of electroporation is the generation of heat during the process. The electric field can cause heating, which may affect the viability of cells or the stability of sensitive biomolecules. Careful monitoring and optimization of the electroporation parameters are necessary to minimize heat-related issues.
- Cost-intensive process: The equipment and instrumentation required for electroporation can be costly, especially for high-throughput applications or large-scale experiments. Additionally, the optimization of parameters and the proper handling of samples may require specialized training and expertise.
Electrolysis, or electroporation, is a powerful technique for cell disruption and the release of intracellular substances. It offers high efficiency and selectivity, allowing for precise manipulation of cells. However, considerations regarding heat generation and the investment required for equipment should be taken into account when employing this method. Overall, electrolysis has become an essential tool in various fields of research, including molecular biology, biotechnology, and genetic engineering.
2. Biological methods or enzymatic methods of cell disruption
Biological methods, also known as enzymatic methods, are used for cell disruption and lysis by employing specific enzymes to break down the cell walls or membranes. These methods offer advantages such as controlled operation, low energy requirements, and moderate operating conditions. They can provide biological specificity and avoid the physical stress caused by mechanical breakdown. However, the selection of the appropriate enzyme and optimization of reaction conditions are crucial for efficient lysis.
There are three different approaches to enzymatic cell disruption:
A. Autolysis: Autolysis is a process used to extract compounds from yeast. The mechanism behind autolysis is not fully understood, making it challenging to control. It involves triggering the production of lytic enzymes in yeast through mild thermal or chemical shocks, although the classification of this process is not always clear.
B. Lytic Enzymes: Various enzymes like cellulase, lysozyme, and proteases are utilized for cell lysis. These enzymes can selectively degrade the cell walls or membranes of target cells. Lytic enzyme-based lysis is commonly employed on a small scale due to factors such as enzyme availability and cost.
C. Phage Lysis: Bacteriophages, such as T4-phage, OX174, and ssRNA phage, are viruses that infect bacteria. Phage lysis involves using these bacteriophages to enter and multiply within the target bacteria, ultimately leading to cell lysis. This approach is specific to bacterial cells and requires the presence of suitable bacteriophages.
The choice of enzyme depends on the type of cell wall or membrane being targeted. For example, lysozyme is commonly used to digest the cell wall of gram-positive bacteria by hydrolyzing β-1-4-glucosidic bonds in the peptidoglycan. Yeast and fungi have distinct cell walls, and enzymes like Zymolyase, which contains β-1,3 glucanase and β-1,3-glucan laminaripentao-hydrolase activities, are used for their degradation. Additionally, cellulases, pectinases, xylanases, chitinases, beta(1-6) and beta(1-3) glycanases, proteases, and mannases are commonly employed to disrupt the cell walls of yeast, fungi, and other microbes.
While enzymatic methods offer advantages in terms of biological specificity and controlled conditions, they may be limited by enzyme availability and cost when applied on a larger scale. Nonetheless, enzymatic cell disruption remains a valuable approach in various fields, including biotechnology, microbiology, and biochemistry, where selective and gentle cell lysis is desired.
3. Chemical Methods
Chemical methods are commonly employed for microbial cell disruption and lysis, relying on specific agents to break down the cell’s structure. Several agents, such as pH extremes (particularly alkaline conditions), solvents, detergents, chaotropic substances, chelating agents, peroxide, hypochloride, and antibiotics, can be used for this purpose.
- a. Alkaline Cell Lysis: Alkaline conditions with a pH range of 10.5-12.5 for a duration of 30 seconds to 30 minutes have been studied for bacterial systems like Erwinia carotovora, E. coli, and C. necator. However, the requirement for materials stable at high pH and the need for subsequent neutralization can affect the practicality of this method.
- b. Solvents: Solvents are used to extract lipids from cell membranes, leading to the release of intracellular components. Solvents like ethanol, isopropanol, butanol, dimethyl sulfoxide, toluene, and methyl ethylketone have been employed across various microorganisms. However, caution must be exercised due to their potential ignitability and the possibility of protein denaturation. Higher temperatures between 25 and 45 degrees Celsius may enhance release but can also lead to elevated costs and stability concerns.
- c. Detergent Treatment: Detergents are widely used in laboratory-scale cell lysis to permeate or lyse cells by disrupting protein-lipid interactions. Anionic detergents like sodium dodecyl sulfate (SDS) cause cell membrane disorganization, while cationic detergents like cetyltrimethylammonium bromide (CTAB) affect the lipopolysaccharide part of the cell envelope. Nonionic detergents like Triton X-100 and Pluronic F-68 partially solubilize proteins within the inner structure of the membrane, leading to permeabilization. However, the use of detergents can result in protein denaturation and may require additional purification steps, limiting their application in large-scale processes.
- d. Chaotropic Agents: Chaotropic agents disrupt hydrogen bonding and alter hydrophobic interactions, reducing cross-linking within cell walls. Common agents include guanidine chloride and urea, which can be enhanced when used in combination with a chelator like EDTA. EDTA chelates divalent ions, destabilizes lipopolysaccharide layers, and dissociates membranes.
- e. Chelating Agent: EDTA is an example of a chelating agent that binds to bivalent cations (such as Mg2+ and Ca2+), rendering them unusable for cells and causing cell membrane rupture.
- f. Peroxide and Hypochloride: H2O2 and HClO oxidize cell structures, causing damage to lipid bilayers and inhibiting -SH proteins. The oxidation of cell membranes leads to the release of cell constituents.
- g. Antibiotics: Antibiotics like polymyxin, azoles, and nystatin can inhibit cell membrane function and cause cell membrane disruption.
These chemical methods offer diverse options for cell disruption and lysis, but they also have limitations. Factors such as high chemical costs, stability concerns under alkali conditions, denaturation of proteins, disruption of downstream processing, and the need for additional purification steps should be considered when selecting and applying these methods. Nonetheless, chemical methods play a significant role in laboratory research and specific applications where targeted cell lysis is required.
Significance of Cell Disruption
The significance of cell disruption lies in its crucial role in biotechnology and the downstream processes involved in the production of biological products. Cell disruption is necessary for the extraction and retrieval of desired products, as it greatly enhances the recovery of biological substances.
- Increased Product Yield: Cell disruption techniques allow for the efficient release and recovery of intracellular components, such as proteins, enzymes, nucleic acids, and metabolites. By breaking down the cellular barriers, valuable products can be accessed and isolated in higher quantities, leading to increased yield during the manufacturing process.
- Enhanced Product Purity: Cell disruption methods not only facilitate the release of desired products but also aid in the separation and purification of these substances. Once the cells are disrupted, the target products can be separated from cellular debris, unwanted contaminants, and other impurities, resulting in higher product purity.
- Access to Intracellular Components: Many biological products of interest are located within the intracellular environment of cells. Cell disruption techniques allow researchers and biotechnologists to access these components, which may have important applications in various fields, including medicine, biopharmaceuticals, and research.
- Extraction of Recombinant Proteins: In biotechnology, the production of recombinant proteins often involves expressing foreign genes in host organisms. Cell disruption is employed to release and recover these recombinant proteins from the host cells. Efficient cell disruption methods ensure optimal protein extraction, enabling downstream processing and purification.
- Process Optimization: Cell disruption plays a vital role in process optimization and scale-up of biotechnological processes. By selecting the appropriate cell disruption method, researchers can improve the efficiency and productivity of downstream processes, leading to cost-effective and scalable production of biological products.
- Biological Studies and Research: Cell disruption techniques are essential in various biological studies and research areas. By breaking down cells, researchers can study intracellular processes, investigate cellular functions, analyze biomolecules, and explore cellular pathways, providing valuable insights into fundamental biological mechanisms.
In summary, cell disruption is of significant importance in biotechnology and related fields. It enables the extraction, retrieval, and purification of desired biological products, enhances product yield and purity, facilitates the study of intracellular components, and contributes to process optimization and scale-up. By employing effective cell disruption methods, scientists and biotechnologists can unlock the potential of cells and harness their valuable contents for various applications in medicine, industry, and research.
FAQ
What is cell disruption?
Cell disruption refers to the process of breaking open cells to release their intracellular contents, such as proteins, nucleic acids, enzymes, and metabolites.
Why is cell disruption necessary?
Cell disruption is necessary to access and retrieve intracellular components for various applications, including extraction of valuable products, purification, research studies, and biotechnological processes.
What are the common methods used for cell disruption?
Common methods for cell disruption include mechanical methods (e.g., homogenization, high-pressure homogenization, bead milling), enzymatic methods, chemical methods, physical methods (e.g., sonication, freeze-thaw cycles), and microbial methods.
How do mechanical methods of cell disruption work?
Mechanical methods apply physical force to break cells, such as shearing, grinding, or agitation. They can involve high-pressure homogenizers, bead mills, or other mechanical devices to disrupt cells.
What are enzymatic methods of cell disruption?
Enzymatic methods utilize specific enzymes that degrade cell walls or membranes, leading to cell lysis. Enzymes like lysozyme, cellulase, or zymolyase are commonly used in enzymatic cell disruption.
What are the advantages of using enzymatic methods?
Enzymatic methods offer biological specificity, operate under moderate conditions, require low energy and capital investment, and can be tailored for specific cell types or organisms.
What are chemical methods of cell disruption?
Chemical methods involve the use of chemicals, such as alkali solutions, solvents, detergents, chaotropic agents, chelating agents, or oxidizing agents, to disrupt cells by affecting their structure or membrane integrity.
How does sonication work in cell disruption?
Sonication applies high-frequency sound waves to generate cavitation bubbles, which collapse and create intense shear forces, leading to cell disruption.
What factors should be considered in choosing a cell disruption method?
Factors to consider include the type of cells or organisms, the desired product, the scalability of the method, the cost and availability of equipment and reagents, and the impact on product quality.
Are there any limitations or challenges associated with cell disruption?
Yes, some limitations include potential damage to sensitive intracellular components, difficulty in achieving complete disruption, high energy requirements for certain methods, and the need for subsequent purification steps to remove debris or contaminants.
References
- Harrison, S.T.L. (2011). Comprehensive Biotechnology || Cell Disruption. , (), 619–640. doi:10.1016/B978-0-08-088504-9.00127-6
- https://info.gbiosciences.com/blog/cell-lysis-5-common-cell-disruption-methods-g-biosciences
- https://www.thermofisher.com/in/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/traditional-methods-cell-lysis.html
- https://www.microfluidics-mpt.com/blog/what-is-cell-disruption
- https://en.wikipedia.org/wiki/Cell_disruption
- https://analytik.co.uk/what-is-cell-disruption/
- https://www.slideshare.net/AishwaryaBabu2/cell-disruption-methods
- https://www.bio-rad.com/en-in/applications-technologies/cell-disruption?ID=LUSP2D1FX
