Table of Contents
What is Protein?
- Gluconeogenesis is a pivotal metabolic pathway that facilitates the synthesis of glucose from non-carbohydrate precursors, primarily amino acids. Therefore, understanding the role of amino acids in this process is essential for a comprehensive grasp of cellular metabolism.
- Proteins, composed of amino acid residues, are fundamental biomolecules that execute a myriad of functions within organisms. These functions range from catalyzing metabolic reactions to providing structural support to cells. Besides, proteins are responsible for DNA replication, responding to stimuli, and transporting molecules. Then, it’s crucial to note that the sequence of amino acids in a protein is determined by the gene’s nucleotide sequence, leading to a specific 3D structure essential for its activity.
- Amino acids, the building blocks of proteins, are linked together by peptide bonds, forming a structure known as a polypeptide. A protein may contain one or more of these polypeptides. When these chains are shorter, containing less than 20–30 residues, they are typically referred to as peptides rather than proteins. The genetic code generally specifies these 20 standard amino acids. However, in specific organisms, variations like selenocysteine and pyrrolysine can be found. Besides, post-translational modification can chemically modify the residues in a protein shortly after or during its synthesis. This modification can influence various attributes of the protein, including its function, stability, and activity.
- Furthermore, some proteins possess non-peptide groups, known as prosthetic groups or cofactors, attached to them. These proteins can collaborate to perform a specific function, often forming stable protein complexes. However, proteins do not last indefinitely. They undergo degradation and recycling through a process termed protein turnover. The lifespan of a protein can vary significantly, with an average duration of 1–2 days in mammalian cells. Misfolded or abnormal proteins are typically degraded at a faster rate.
- In the context of metabolism, many proteins serve as enzymes that catalyze biochemical reactions. These enzymes are indispensable for metabolic processes, including gluconeogenesis. Additionally, proteins play structural roles, such as actin and myosin in muscles, and form the cytoskeleton, ensuring the cell maintains its shape. They are also involved in cell signaling, immune responses, cell adhesion, and the cell cycle.
- For animals, dietary proteins are crucial as they provide essential amino acids that the body cannot synthesize. Upon digestion, these proteins are broken down, making amino acids available for metabolic use, including gluconeogenesis.
- In conclusion, amino acids, derived from proteins, play a pivotal role in gluconeogenesis. Their intricate structure, determined by the genetic code, and their diverse functions, from enzymatic to structural, underscore their significance in cellular metabolism. As research advances, techniques such as X-ray crystallography and mass spectrometry continue to provide deeper insights into protein structure and function, further emphasizing the importance of amino acids in gluconeogenesis and other metabolic pathways.
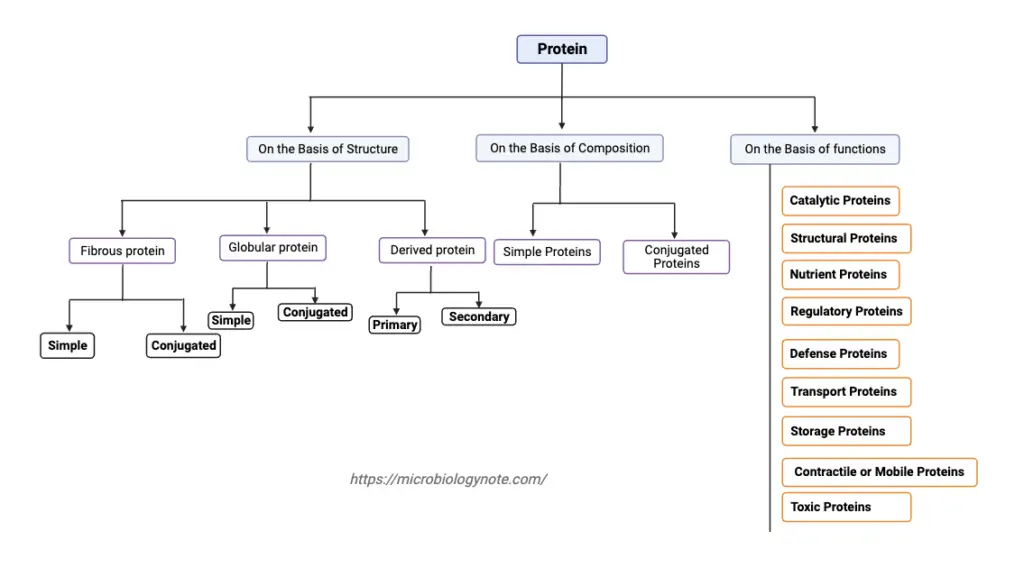
Classification of protein On the Basis of Structure
1. Fibrous protein
Fibrous proteins are a distinct category of proteins characterized by their elongated or fiber-like structure. These proteins are primarily found in animals and play specific roles in structural support. Therefore, understanding their nature, structure, and function is essential for a comprehensive grasp of protein biology.
- Physical Characteristics
- Elongated Structure: As the name suggests, fibrous proteins have an elongated or fiber-like appearance.
- Axial Ratio: One of the defining characteristics of fibrous proteins is their axial ratio, which refers to the length-to-breadth ratio. For these proteins, this ratio is typically greater than 10.
- Static Nature: Fibrous proteins are static in nature, implying that they do not undergo significant conformational changes.
- Simplicity in Structure: These proteins possess a relatively simple structure compared to their globular counterparts.
- Biological Functions Fibrous proteins have limited biological functions. Their primary role is structural, providing support and rigidity to various tissues in animals. Therefore, while they might not be involved in diverse enzymatic or regulatory activities, their contribution to maintaining the integrity of tissues is paramount.
- Presence in Organisms These proteins are predominantly found in animals. Their presence is crucial for various structural components, from hair and nails to connective tissues.
- Classification of Fibrous Proteins Fibrous proteins can be broadly categorized into two types:
i. Simple Fibrous Proteins:- Examples: This category includes scleroproteins such as keratin, elastin, collagen, and fibroin. Scleroproteins or Albuminoids: These proteins form the skeletal framework of animals. One of their defining characteristics is their water-insolubility, making them ideal for providing structural support.
- Examples: An example of conjugated fibrous proteins is the pigments found in chicken feathers. These proteins are complexed with other non-protein entities, enhancing their functionality.
2. Globular protein
Globular proteins are a specific type of proteins characterized by their spherical or globular shape. These proteins stand in contrast to fibrous proteins, which are elongated in structure.
- Physical Characteristics
- Shape and Axial Ratio: Globular proteins have a shape that is spherical, and their axial ratio is consistently less than 10.
- Nature and Structure: These proteins are dynamic in nature, meaning they can flow or move. Their structure exhibits a higher degree of complexity compared to fibrous proteins.
- Biological Functions Globular proteins have a wide variety of biological functions. Examples of such proteins include enzymes and hormones, which play critical roles in various metabolic processes and signaling pathways.
- Classification of Globular Proteins Globular proteins can be classified based on their composition or solubility into:
i. Simple or Homoglobular Proteins- Composed solely of amino acids.Protamine:
- Positively charged proteins found mainly in animals and fishes, especially in sperm.Binds with DNA during the embryonic stage and is later replaced by histone.Exhibits specific solubility and coagulation properties.Rich in arginine and lysine but lacks sulfur-containing and aromatic amino acids.
- A basic protein, albeit weaker in its basic nature compared to protamine.Found in nucleic acids as nucleohistone binding with DNA.
- Abundantly found in nature, present in seeds of plants and in the blood and muscles of animals.Exhibits specific molecular weight, solubility, and coagulation properties.
- Classified into Pseudoglobulin and Euglobulin based on solubility.
- Water-insoluble proteins with examples like Glutenin and Oryzenin.
- Storage proteins found in seeds with specific solubility and coagulation properties.
- Composed of both protein and non-protein components, with the non-protein component termed the prosthetic group.
- Metalloprotein:
- Contains a metal as the prosthetic group.
- Examples include Ceruloplasmin and Calsequestrin.
- Chromoprotein:
- Contains a colored prosthetic group.
- Examples include Haemoprotein and Flavoprotein.
- Glycoprotein/Mucoprotein:
- Contains carbohydrate as the prosthetic group.
- Examples include antibodies and heparin.
- Phosphoprotein:
- Contains a phosphate group as the prosthetic group.
- Examples include Caesein and Ovovitellin.
- Lipoprotein:
- Contains lipid as the prosthetic group.
- Examples include Lipovitelline and chylomicrons.
- Composed solely of amino acids.Protamine:
3. Derived protein
Derived proteins are a unique category of proteins that result from the modification of either simple or complex proteins. These modifications can arise due to various factors, including heat, enzymes, and chemicals. Some proteins in this category are also artificially produced.
- Characteristics of Derived Proteins Derived proteins differ from their original forms in terms of molecular arrangement or size. The changes in these proteins can be attributed to external factors that cause them to undergo structural alterations.
- Classification of Derived Proteins Derived proteins can be broadly categorized into two main types:
i. Primary Derived Proteins- These proteins undergo changes in their molecular arrangement without significant alterations in their size.Proteans:
- These are the initial products formed after proteins are subjected to the action of acids, enzymes, or water.They are water-insoluble.Examples include Edestan and Myosin.
- Produced by further treating proteins with acids or alkalis at temperatures ranging from 30-60°C.While they are insoluble in water, they can dissolve in dilute acids or alkalis.They are also referred to as Infraproteins.An example is Curd.
- These proteins form due to the action of heat or alcohol on the original protein.They are characterized by their water-insolubility.An example is coagulated egg.
- In these proteins, the size of the original protein molecules is altered, typically becoming smaller.
- This change in size is a result of hydrolysis.
- Proteoses:
- Formed when proteins undergo hydrolysis beyond the metaprotein level, typically due to the action of dilute acids or digestive enzymes.
- They are water-soluble and do not coagulate when exposed to heat.
- Examples include Albumose and Globulose.
- These proteins undergo changes in their molecular arrangement without significant alterations in their size.Proteans:
Classification of Proteins based on Composition
- Simple Proteins
- Description: These proteins are solely composed of amino acid residues without any additional non-protein components.
- Nature: They can manifest as either fibrous or globular structures, depending on their specific roles and organization.
- Function: Simple proteins primarily exhibit structural roles in organisms.
- Examples: Collagen (found in connective tissues), Myosin (a muscle protein), Insulin (a hormone regulating glucose), and Keratin (present in hair and nails).
- Conjugated Proteins
- Description: These are complex proteins that consist of amino acid chains combined with non-amino acid units, termed as prosthetic groups.
- Nature: The protein component can be either tightly or loosely associated with the non-protein parts, determining their specific functions.
- Function: The prosthetic group is essential for the biological activity of these proteins. Many enzymes fall under this category, with the majority being globular and water-soluble.
- Sub-classification:
- Phosphoproteins: Characterized by the presence of phosphoric acid as a prosthetic group.
- Examples: Casein in milk and Vitellin in egg yolk.
- Glycoproteins: These proteins contain carbohydrate chains.
- Examples: Many membrane proteins and Mucin, which is a component of saliva.
- Nucleoproteins: These are associated with nucleic acids.
- Examples: Proteins found in chromosomes and structural components of ribosomes.
- Chromoproteins: Characterized by the presence of pigments or chromes.
- Examples: Haemoglobin (oxygen transport), Phytochrome (plant pigment), and Cytochrome (involved in electron transport).
- Lipoproteins: These proteins have lipids as their prosthetic group.
- Example: Certain proteins in cell membranes.
- Flavoproteins: Characterized by the presence of FAD (Flavin Adenine Dinucleotide).
- Function: They play a role in the Electron Transport System (ETS).
- Phosphoproteins: Characterized by the presence of phosphoric acid as a prosthetic group.
Classification of protein on the basis of biological functions
- Catalytic Proteins
- Function: These proteins play a crucial role in catalyzing biochemical reactions within cells, ensuring that metabolic processes occur efficiently and timely.
- Examples: Enzymes and co-enzymes are prime examples of catalytic proteins that facilitate various chemical reactions.
- Structural Proteins
- Function: Structural proteins are responsible for forming the various structural components of living organisms. They provide rigidity, support, and shape to cells and tissues.
- Examples: Collagen, which forms bones; Elastin, which constitutes ligaments; and Keratin, which is the primary component of hair and nails.
- Nutrient Proteins
- Function: These proteins have significant nutritional value. When consumed, they provide essential nutrients to the body.
- Example: Casein, a protein found in milk, is a primary source of nutrition for many mammals.
- Regulatory Proteins
- Function: Regulatory proteins oversee and control various metabolic and cellular activities within cells and tissues. They ensure that biological processes are well-coordinated.
- Example: Hormones are regulatory proteins that modulate various physiological processes.
- Defense Proteins
- Function: Defense proteins are integral to the body’s immune response. They offer protection against pathogens and foreign invaders.
- Examples: Antibodies and complement proteins play pivotal roles in neutralizing and eliminating pathogens.
- Transport Proteins
- Function: These proteins facilitate the movement of nutrients and other molecules from one organ or tissue to another, ensuring that cells receive the necessary compounds for their functions.
- Example: Haemoglobin is a transport protein that carries oxygen from the lungs to the rest of the body.
- Storage Proteins
- Function: Storage proteins hold onto various molecules and ions within cells, ensuring that they are available when needed.
- Example: Ferritin is a storage protein that retains iron, making it available for processes like hemoglobin synthesis.
- Contractile or Mobile Proteins
- Function: These proteins are involved in movement and locomotion of various body parts. They enable muscles to contract and relax, facilitating movement.
- Examples: Actin, Myosin, and Tubulin are proteins that play roles in muscle contraction and cellular movements.
- Toxic Proteins
- Function: Toxic proteins can cause harm and damage to tissues. They are often used as defense mechanisms by certain organisms.
- Examples: Snake venom and bacterial exotoxins are toxic proteins that can cause significant harm upon exposure.
Functions of Protein
- Enzymatic Activity
- Catalysis: Proteins, specifically enzymes, accelerate biochemical reactions, ensuring that metabolic processes occur efficiently. For instance, digestive enzymes like amylase and lipase help break down food components.
- Structural Support
- Framework: Proteins provide structural support to cells and tissues. For example, collagen and elastin are essential for the integrity of connective tissues, while keratin is a primary component of hair, nails, and the outer layer of skin.
- Transport
- Molecule Carriers: Some proteins transport vital molecules around the body. Hemoglobin, for instance, carries oxygen from the lungs to tissues, while lipoproteins transport lipids in the bloodstream.
- Storage
- Reservoirs: Certain proteins store essential molecules. Ferritin, for example, stores iron in the body, while ovalbumin in egg whites stores amino acids for developing embryos.
- Signaling
- Communication: Proteins play a role in transmitting signals within cells and between cells. Hormones like insulin regulate glucose levels in the blood, while receptor proteins on cell surfaces receive signals from other parts of the body.
- Regulatory Functions
- Control: Some proteins regulate the activity of other molecules. For instance, transcription factors control gene expression, determining which genes are turned on or off in a cell.
- Movement
- Locomotion and Contraction: Proteins like actin and myosin are responsible for muscle contraction, allowing for movement. Additionally, proteins in cilia and flagella enable cell movement.
- Defense
- Protection: Proteins play a crucial role in the immune response. Antibodies, for instance, bind to foreign invaders like bacteria and viruses, marking them for destruction. Complement proteins assist in eliminating pathogens.
- Growth and Maintenance
- Repair and Development: Proteins are essential for tissue growth, repair, and maintenance. They ensure that damaged tissues are repaired and that growth processes in developing organisms proceed correctly.
- Regulation of pH and Fluid Balance
- Homeostasis: Proteins like albumin maintain the osmotic balance between blood and interstitial fluid, ensuring fluid balance. Additionally, certain proteins act as buffers, helping to maintain the body’s pH within a narrow range.
- Energy Source
- Fuel: While not a primary energy source, in certain situations, proteins can be broken down to provide energy for the body.
Applications of Protein
- Medical Applications
- Therapeutic Proteins: Many proteins, such as insulin, growth hormones, and monoclonal antibodies, are used as therapeutic agents to treat diseases like diabetes, growth disorders, and various cancers.
- Vaccines: Some vaccines are based on protein subunits of pathogens, which stimulate the immune system without causing the disease.
- Diagnostics: Proteins like antibodies are used in diagnostic tests, including ELISA and rapid antigen tests.
- Enzymatic Applications
- Industrial Enzymes: Proteins, especially enzymes, are used in various industries. For instance, proteases are used in detergents, and amylases are used in brewing.
- Biocatalysis: Enzymes are employed as catalysts in the production of biofuels, pharmaceuticals, and other chemicals.
- Food Industry
- Nutritional Supplements: Proteins, especially from whey and soy, are used as dietary supplements to enhance muscle growth and recovery.
- Food Processing: Proteins like rennet are used in cheese-making, and other enzymes are used in bread-making and meat tenderization.
- Research and Biotechnology
- Protein Engineering: Scientists modify proteins to create versions with desired properties, leading to better drugs, enzymes, and other products.
- Proteomics: This is the large-scale study of proteins, especially their functions and structures. It’s crucial for understanding diseases and developing new therapies.
- Cosmetics and Personal Care
- Hair and Skin Care: Keratin-based products are used for hair treatments, and collagen is used in skin creams for its anti-aging properties.
- Enzymatic Exfoliants: Proteases are used in skincare products to help exfoliate and renew the skin.
- Textile and Leather Industry
- Processing: Enzymes like proteases are used in the leather industry for dehairing hides and in the textile industry for removing impurities from raw materials.
- Environmental Applications
- Bioremediation: Certain proteins can bind or break down environmental pollutants, aiding in cleaning up contaminated sites.
- Biosensors: Proteins can be used in sensors to detect pollutants or toxins in the environment.
- Agriculture
- Crop Protection: Certain proteins are used in biopesticides to protect crops from pests without the harmful effects of chemical pesticides.
- Animal Feed: Protein supplements are added to animal feed to enhance growth and health.
