Table of Contents
What is Immunofluorescence Assay?
- Immunofluorescence, as the name suggests, is a fusion of immunology and fluorescence. Delving into its etymology, “immuno” pertains to immunity or the immune system, while “fluorescence” denotes molecules that emit visible or invisible radiation upon excitation. Therefore, immunofluorescence is a technique that harnesses the power of fluorescence to study immune responses, specifically the interaction between antibodies and antigens.
- In the realm of molecular biology and immunology, the immunofluorescence assay stands out as a pivotal tool. Its primary function is to detect and map the distribution of specific proteins or antigens within a sample. This is achieved by using antibodies that are tagged with fluorescent molecules. These labeled antibodies, upon introduction to a sample, bind specifically to their target protein or antigen. Once bound, the fluorescent tag can be visualized under a fluorescence microscope, revealing the location and distribution of the target molecule.
- The crux of this technique lies in the specificity of antibodies. Each antibody recognizes a unique region on an antigen, termed an epitope. It’s worth noting that multiple antibodies can bind to the same epitope, but with varying affinities. Therefore, epitope mapping has been a subject of extensive research to understand these binding dynamics. Besides, it’s crucial that the attached fluorophore does not hinder the antibody’s immunological specificity or its ability to bind to its antigen.
- Immunofluorescence is not just limited to detecting proteins. It can be employed on a range of samples, from tissue sections and cultured cell lines to individual cells. This versatility allows researchers to study the distribution of proteins, glycans, and even small biological and non-biological molecules. Furthermore, structures like intermediate-sized filaments can be visualized using this technique. In instances where the structure of a cell membrane is ambiguous, epitope insertion combined with immunofluorescence can shed light on the topology.
- One of the salient features of immunofluorescence is its adaptability. It can be paired with other fluorescent staining methods. For instance, DAPI, a fluorescent stain, can be used alongside to label DNA. In terms of microscopy, while the epifluorescence microscope is the most basic tool for analyzing immunofluorescence samples, the confocal microscope offers a more detailed view. For even higher resolution, various super-resolution microscope designs are available.
- Historically, the roots of immunofluorescence can be traced back to 1942, with significant refinements made by Coons in 1950. The technique leverages the property of certain dyes that absorb light at specific wavelengths and emit them at different ones, a phenomenon termed fluorescence. In the context of the immunofluorescence test, a dye like fluorescein isothiocyanate, which emits a yellow-green fluorescence, is commonly used. This dye illuminates under UV light, highlighting the specific interaction between an antigen and antibody. Owing to this, immunofluorescence tests are also referred to as fluorescent antibody tests (FAT).
- In conclusion, immunofluorescence is a robust and versatile technique that has revolutionized the way researchers visualize and understand cellular processes, structures, and interactions. Its ability to provide detailed, sequential, and objective insights into the intricate world of cells makes it an invaluable tool in the scientific community.
Definition of Immunofluorescence Assay
Immunofluorescence is a laboratory technique used to detect specific proteins or antigens in biological samples by using antibodies labeled with fluorescent molecules, allowing visualization under a fluorescence microscope.
Requirements of Immunofluorescence Assay
- Specific Antibodies: The cornerstone of immunofluorescence is the use of antibodies that can specifically recognize and bind to the antigen of interest, forming the antigen-antibody (Ag-Ab) complex. There are two main types of antibodies utilized:a. Primary Antibody: This is the initial antibody that has a direct affinity for the target antigen. It binds directly to the specific region or epitope of the antigen.b. Secondary Antibody: Acting subsequent to the primary antibody, the secondary antibody recognizes and binds to the Fc region of the primary antibody, which is already attached to the antigen. Its versatility allows it to be employed across various assays.
- Fluorochromes or Fluorophores: These are fluorescent dyes conjugated to antibodies, enabling visualization of the antigen-antibody interaction under specific light conditions. Some commonly used fluorochromes include:
- Fluorescein: A green-emitting dye.
- Rhodamine: Known for its red fluorescence.
- Phycoerythrin: A protein-based dye that emits yellowish-orange fluorescence.
- Immunofluorescence Microscope: A specialized microscope equipped to detect the emitted fluorescence from the fluorochromes. This microscope allows for the visualization of the specific locations where the antigen-antibody complexes are formed within the sample.
- Wash Buffers: Essential for the removal of unbound antibodies and other extraneous components, wash buffers play a pivotal role in the immunofluorescence process. One of the commonly used buffers is:
- Phosphate Buffered Saline (PBS): A balanced salt solution that aids in washing away any unattached antibodies, ensuring that only specific bindings are visualized.
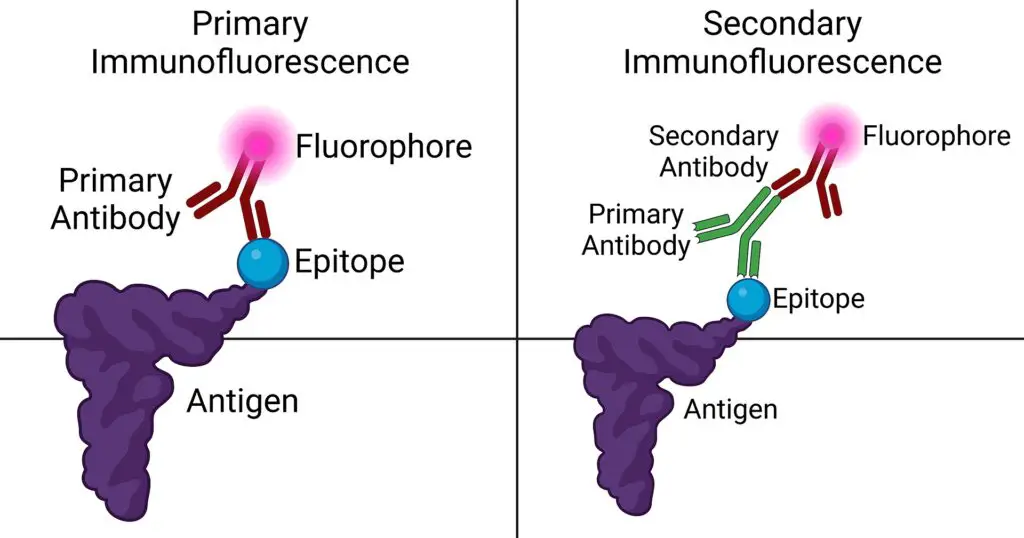
Objective of Immunofluorescence Assay
- Visualization of Specific Molecules: At the heart of immunofluorescence lies the principle of using fluorescent dyes. These dyes are specifically designed to bind to antibodies, which are proteins produced by the immune system in response to the presence of a particular antigen, a foreign invader in the body.
- Detection of Antigen-Antibody Interactions: Once an antigen enters the body, the immune system mounts a response by producing specific antibodies. These antibodies have a unique ability to recognize and bind to their corresponding antigen. Through immunofluorescence, this binding, whether direct or indirect, can be visualized. Therefore, it provides a means to observe the interaction between the foreign invader (antigen) and the body’s defense mechanism (antibody).
- Microscopic Analysis: After the antibody-antigen interaction has been labeled with the fluorescent dye, the next step involves visualization. Using specialized microscopes, such as confocal or fluorescence microscopes, researchers can study the now illuminated antibody-antigen pair in detail. This allows for a deeper understanding of the behavior of the foreign substance and the immune response it elicits.
- Quantification of Specimens: Besides mere visualization, immunofluorescence also offers the advantage of quantification. By employing tools like array scanners, flow cytometers, or automated imaging instruments, scientists can obtain a precise count of the specimens present in their sample.
Principle of Immunofluorescence
- Immunofluorescence is a sophisticated technique employed in biological research and clinical diagnostics to visualize specific proteins or antigens in biological samples. The principle behind this technique is both intricate and fascinating. This expository explanation will methodically elucidate the principle of immunofluorescence, emphasizing the functions of its core components.
- At the heart of immunofluorescence lies the principle of specificity. Specific antibodies, which are proteins produced by the immune system, have the unique ability to bind selectively to their corresponding antigens. Antigens, in this context, are typically proteins or other molecules that the immune system recognizes as foreign or non-self. Therefore, in the realm of immunofluorescence, these specific antibodies are employed to target and bind to the protein or antigen of interest within a given biological sample.
- Following the binding process, the next pivotal step involves labeling these antibodies. For this purpose, molecules known as fluorochromes or fluorophores are utilized. These molecules possess the remarkable property of fluorescence. When light of a specific wavelength illuminates a fluorochrome, it absorbs this light and, in response, emits light of a different wavelength. This emitted light, which is fluorescent in nature, can then be visualized using a fluorescence microscope.
- Besides the fundamental binding and labeling processes, it’s essential to understand the significance of the emitted light. Each fluorophore or fluorescent label emits light at a distinct wavelength. This characteristic is invaluable as it allows researchers and clinicians to identify and differentiate between various targets within the sample. For instance, different proteins or antigens can be labeled with different fluorophores, each emitting a unique color, enabling simultaneous visualization of multiple targets.
- Furthermore, the immunofluorescence assay is not just a tool for visual exploration. Its applications extend to clinical diagnostics, where it proves instrumental in detecting specific antigens or markers associated with various diseases or conditions. By visualizing these markers, clinicians can make informed decisions regarding diagnosis and treatment.
- In conclusion, the principle of immunofluorescence revolves around the specificity of antibodies and the fluorescence properties of fluorochromes. Through a detailed and sequential process, specific proteins or antigens within biological samples are targeted, labeled, and visualized. This technique, with its emphasis on the functions of antibodies and fluorescent labels, stands as a testament to the precision and sophistication of modern biological research and diagnostics.
General Steps of Immunofluorescence Assay
- Sample Preparation: The initial step involves preparing the biological sample. For cells, they are typically cultured on glass coverslips or chamber slides. In the case of tissues, they undergo a process of fixation, embedding, and sectioning to prepare thin tissue sections suitable for the assay.
- Fixation: To preserve the cellular and tissue structures and immobilize proteins, a chemical fixative, often formaldehyde, is employed. This ensures that the cellular components remain intact during the subsequent steps.
- Permeabilization: To allow antibodies to access intracellular targets, the cellular membranes are permeabilized using detergents or other agents. This step ensures that antibodies can penetrate the cells or tissues effectively.
- Blocking: To minimize nonspecific binding of antibodies to the sample, a blocking step is essential. The sample is incubated with a blocking solution, commonly bovine serum albumin or normal serum, which prevents unwanted interactions.
- Primary Antibody Incubation: The sample is then exposed to the primary antibody, which is specific to the target protein of interest. This antibody selectively binds to its target within the sample.
- Washing: To remove any unbound primary antibodies, the sample is washed with a buffer solution, ensuring that only the specifically bound antibodies remain.
- Secondary Antibody Incubation: A secondary antibody, conjugated to a fluorescent dye, is introduced. This antibody is designed to recognize and bind to the primary antibody, thereby attaching the fluorescent label to the target protein.
- Washing: Similar to the earlier washing step, the sample is again washed with a buffer solution to remove excess secondary antibodies, leaving behind only those bound to the primary antibodies.
- Mounting: To preserve the fluorescence signal and prepare the sample for microscopy, it is mounted using a medium that often contains anti-fading agents.
- Imaging: The final step involves examining the sample under a fluorescence microscope equipped with the appropriate filters. The fluorescent signal emitted by the bound secondary antibody illuminates the location of the target protein. The use of different fluorophores, each emitting a distinct color of light, allows for multicolor imaging, providing a comprehensive view of the sample.
Types of Immunofluorescence Assay
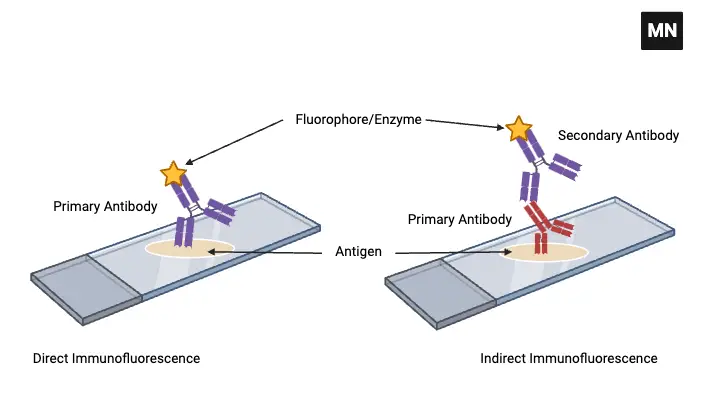
There are present two Types of Immunofluorescence
- Direct Immunofluorescence Test
- Indirect Immunofluorescence Test
1. Direct Immunofluorescence Test
- Direct Immunofluorescence Test, a pivotal technique in the realm of immunology and diagnostics, is designed to detect and visualize specific antigens in biological samples. This expository explanation will delve into the core principle and components of the Direct Immunofluorescence Test, emphasizing their functions and significance in the process.
- At the heart of the Direct Immunofluorescence Test is the use of a single antibody, known as the primary antibody. This antibody plays a crucial role in the detection process, and understanding its function is paramount.
- Primary Antibody: This is a specialized protein that has been meticulously designed to recognize and bind to a specific antigen. In the Direct Immunofluorescence Test, the primary antibody is chemically linked or conjugated to a fluorochrome. This conjugation is vital for the visualization process. The primary function of this antibody is to search for and bind directly to its target antigen if present within the biological sample.
- Following the binding process, the next step involves visualization.
- Fluorochrome Conjugation: The fluorochrome, to which the primary antibody is linked, is a molecule with the unique property of fluorescence. When exposed to light of a specific wavelength, the fluorochrome absorbs this light and subsequently emits light of another wavelength. This emitted light is what is observed under a fluorescence microscope. Therefore, if the antigen of interest is present in the sample, the primary antibody, now bound to the antigen and conjugated with the fluorochrome, will emit a fluorescent signal. This signal can then be visualized under the microscope, indicating the presence and location of the antigen.
- In essence, the Direct Immunofluorescence Test is a straightforward yet powerful technique. By using just one antibody that is directly conjugated to a fluorochrome, it eliminates the need for secondary antibodies, streamlining the process. The emphasis here is on the direct interaction between the primary antibody and its target antigen, leading to immediate visualization if the antigen is present.
- In conclusion, the Direct Immunofluorescence Test is rooted in the principle of direct detection using a fluorochrome-conjugated primary antibody. Through a detailed understanding of its components and their respective functions, one can appreciate the efficiency and precision of this diagnostic technique.
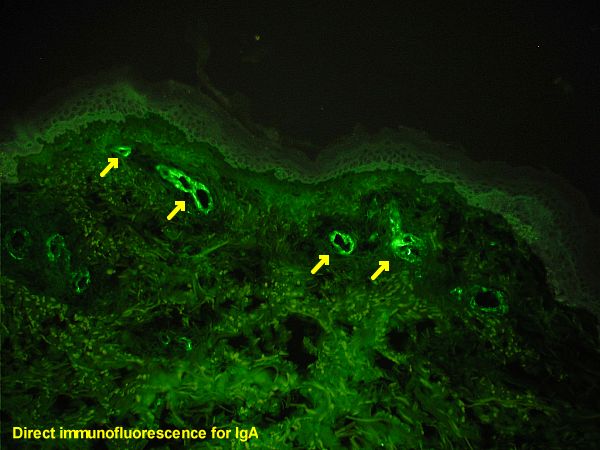
Procedure of Direct Immunofluorescence Test
- Fixing of Specimen (Antigen) onto the Slide: The initial step in the procedure involves the fixation of the specimen, which contains the antigen of interest, onto a slide. This fixation process is essential as it preserves the structural integrity and morphology of the specimen, ensuring that the antigen remains in its native state. Fixation also immobilizes the antigen, allowing for subsequent steps to be carried out more effectively.
- Application of Fluorochrome Labeled Antibodies: Following fixation, the next step involves the addition of fluorochrome-labeled antibodies to the slide. These antibodies, as previously mentioned, are primary antibodies that have been chemically conjugated to a fluorochrome. Their primary function is to recognize and bind specifically to the target antigen present in the fixed specimen.
- Incubation and Washing: After the application of the fluorochrome-labeled antibodies, the slide is incubated for a specific period. This incubation allows the antibodies ample time to bind to their respective antigens. Following incubation, the slide undergoes careful washing using wash buffers, such as PBS (Phosphate Buffered Saline). This washing step is of paramount importance as it removes any unbound antibodies and other extraneous components from the slide. Therefore, only the complex of the antigen and the fluorochrome-labeled antibody remains on the slide.
- Observation Under a Fluorescence Microscope: The final step in the procedure is the visualization of the slide under a fluorescence microscope. If the antigen of interest is present in the specimen, the fluorochrome-labeled antibody bound to it will emit fluorescence when exposed to specific wavelengths of light. This emitted light can be observed under the microscope, providing a clear and detailed view of the location and distribution of the antigen within the specimen.
Uses of Direct Immunofluorescence Test
- Detection of Rabies Virus Antigen: One of the pivotal uses of the Direct Immunofluorescence Test is in the detection of the rabies virus antigen. This application is particularly crucial in both human and veterinary medicine. In humans, skin smears collected from the nape of the neck serve as the specimen for this test. The presence of the rabies virus antigen in this specimen can indicate an active rabies infection, which requires immediate medical intervention. Besides its application in humans, this test is also employed to detect the rabies virus antigen in the saliva of dogs. Given the zoonotic nature of rabies, this test plays a vital role in controlling and preventing the spread of this deadly disease.
- Detection of Specific Pathogens in Clinical Specimens: Another significant use of the Direct Immunofluorescence Test is in the detection of specific pathogens directly in appropriate clinical specimens. Some of these pathogens include:
- N. gonorrhoeae: Neisseria gonorrhoeae is the causative agent of gonorrhea, a sexually transmitted infection. The Direct Immunofluorescence Test can swiftly detect this bacterium in clinical specimens, aiding in the timely diagnosis and treatment of the infection.
- C. diphtheriae: Corynebacterium diphtheriae is responsible for diphtheria, a severe respiratory disease. Early detection through the Direct Immunofluorescence Test can facilitate prompt medical intervention, reducing the risk of complications.
- T. pallidum: Treponema pallidum is the bacterium that causes syphilis. The Direct Immunofluorescence Test can be employed to detect this pathogen in clinical specimens, ensuring early diagnosis and treatment.
Advantages of Direct Immunofluorescence Test
- Shorter Protocols: One of the foremost advantages of the Direct Immunofluorescence Test is the brevity of its protocols. Since this method utilizes a primary antibody that is already conjugated to a fluorophore, it eliminates the need for additional labeling steps. Therefore, the entire procedure is streamlined, leading to quicker results. This shortened protocol not only saves time but also reduces the potential for errors that might arise during extended procedures.
- Minimized Species Cross-reactivity: Another significant advantage of the Direct Immunofluorescence Test is the reduction in species cross-reactivity. In many immunological tests, cross-reactivity can pose challenges, leading to false-positive or ambiguous results. However, in the Direct Immunofluorescence Test, the fluorophore is directly conjugated to the primary antibody. This direct conjugation minimizes the chances of the antibody reacting with non-target species, ensuring that the results are specific to the antigen of interest. Besides ensuring specificity, this also enhances the reliability and accuracy of the test results.
Disadvantages of Direct Immunofluorescence Test
- Preparation of Separately Labeled Antibodies: One of the primary challenges of the Direct Immunofluorescence Test is the necessity to prepare separately labeled antibodies for each pathogen under investigation. This requirement can be labor-intensive and time-consuming, especially when dealing with a wide array of pathogens. Besides the effort involved, this also means that researchers and diagnosticians need to maintain a comprehensive inventory of labeled antibodies, which can be logistically challenging.
- Higher Consumption of Primary Antibody: Another significant limitation is the requirement for a larger quantity of the primary antibody. The Direct Immunofluorescence Test relies on the direct binding of the fluorochrome-conjugated primary antibody to the target antigen. Therefore, a sufficient amount of this antibody is essential to ensure accurate results. Given that primary antibodies, especially those conjugated to fluorochromes, are often expensive, this can escalate the overall cost of the test, making it less economical in certain settings.
- Reduced Sensitivity: While the Direct Immunofluorescence Test is efficient in many respects, it is generally considered less sensitive than its counterpart, the Indirect Immunofluorescence Test. The direct method’s reduced sensitivity can sometimes lead to false-negative results, especially when the antigen concentration in the sample is low. This limitation can pose challenges in clinical settings where early detection of a pathogen is crucial for timely intervention and treatment.
2. Indirect Immunofluorescence Test
The Indirect Immunofluorescence Test is a sophisticated technique employed in the domain of immunology and diagnostics. This expository explanation will provide a detailed and sequential overview of the Indirect Immunofluorescence Test, emphasizing the roles and functions of its key components.
At the core of the Indirect Immunofluorescence Test is the use of two distinct antibodies: the primary and the secondary antibodies. Understanding the roles and interactions of these antibodies is pivotal to grasping the essence of this test.
- Primary Antibody: The primary antibody serves as the initial point of interaction with the antigen. This antibody is specifically designed to recognize and bind to a particular antigen of interest present in the sample. It’s worth noting that in the Indirect Immunofluorescence Test, the primary antibody is not labeled with a fluorochrome. Instead, its primary function is to seek out and bind to its target antigen, ensuring specificity in the detection process.
- Secondary Antibody: Following the binding of the primary antibody to its target antigen, the secondary antibody comes into play. This antibody is conjugated or chemically linked to a fluorochrome, enabling it to emit fluorescence when exposed to specific wavelengths of light. The primary role of the secondary antibody is to bind to the Fc region of the primary antibody, which is already bound to the antigen. This binding amplifies the fluorescent signal, enhancing the visualization of the antigen-antibody complex.
- Visualization: Once the secondary antibody, labeled with the fluorochrome, binds to the primary antibody-antigen complex, the sample is ready for visualization. Under a fluorescence microscope, the fluorochrome emits light at a specific wavelength, allowing for the clear and detailed observation of the target antigen’s location and distribution within the sample.
In conclusion, the Indirect Immunofluorescence Test offers a two-tiered approach to antigen detection, leveraging the specificity of the primary antibody and the amplification capabilities of the fluorochrome-labeled secondary antibody. By understanding the functions and interactions of these components, one can appreciate the precision and sensitivity of the Indirect Immunofluorescence Test in the realm of immunodiagnostics.
Procedure of Indirect Immunofluorescence Test
- Fixation of Known Antigen: The procedure commences with the fixation of a known antigen onto a slide. This step ensures that the antigen remains immobilized and retains its structural integrity throughout the subsequent steps. Fixation provides a stable platform for the antibodies to interact with the antigen.
- Application of the Test Specimen: Following fixation, the specimen to be tested, which contains the primary antibodies of interest, is applied to the slide. These antibodies, if present, are specific to the known antigen fixed on the slide and will bind to it during the incubation period.
- Incubation and Washing: After the application of the test specimen, the slide is incubated for a specific duration. This incubation period allows the primary antibodies in the specimen to bind to their respective antigens on the slide. Post incubation, the slide undergoes a careful washing process using PBS (Phosphate Buffered Saline). This washing step removes any unbound antibodies and extraneous components, ensuring that only the antigen-primary antibody complexes remain on the slide.
- Addition of Secondary Antibody: Subsequent to the washing process, a secondary antibody is introduced to the slide. This antibody, typically labeled with a fluorochrome (e.g., fluorescently labeled anti-IgG), is designed to bind to the Fc region of the primary antibody. The fluorochrome conjugation facilitates the visualization of the antigen-primary antibody complex.
- Further Incubation and Washing: The slide, now treated with the secondary antibody, is incubated once more. This allows the secondary antibody to bind effectively to the primary antibody-antigen complexes. Following this incubation, another careful washing step with PBS is carried out to remove any unbound secondary antibodies.
- Visualization Under a Fluorescence Microscope: The final step in the procedure involves observing the slide under a fluorescence microscope. If the primary antibodies specific to the known antigen were present in the test specimen, the slide will exhibit fluorescence due to the fluorochrome-labeled secondary antibodies. This fluorescence indicates the presence and distribution of the primary antibodies in the specimen.
Uses of Indirect Immunofluorescence Test
- Detection of Specific Antibodies for Disease Diagnosis: One of the paramount uses of the Indirect Immunofluorescence Test is in the diagnosis of a myriad of diseases by detecting the presence of specific antibodies in the patient’s specimen. Some of these diseases include:
- Syphilis: Caused by the bacterium Treponema pallidum, syphilis is a sexually transmitted infection. The Indirect Immunofluorescence Test can detect antibodies specific to this bacterium, aiding in its timely diagnosis.
- Amoebiasis: This parasitic infection, caused by the protozoan Entamoeba histolytica, affects the intestines. The test can identify antibodies produced in response to this parasite, facilitating its diagnosis.
- Leptospirosis: A bacterial disease caused by the Leptospira species, leptospirosis can be diagnosed by detecting specific antibodies using the Indirect Immunofluorescence Test.
- Toxoplasmosis: This disease, resulting from the Toxoplasma gondii parasite, can be identified by detecting antibodies that the immune system produces in response to the parasite.
- Detection of Autoantibodies in Autoimmune Disorders: Besides its role in infectious disease diagnosis, the Indirect Immunofluorescence Test also finds application in the realm of autoimmune disorders. In autoimmune conditions, the body’s immune system mistakenly produces antibodies, termed autoantibodies, against its own tissues and organs. These autoantibodies can cause damage and inflammation. The Indirect Immunofluorescence Test can detect the presence of these autoantibodies, aiding in the diagnosis of various autoimmune disorders.
Advantages of Indirect Immunofluorescence Test
- Use of a Single Fluorochrome-Labeled Secondary Antibody: A standout advantage of the Indirect Immunofluorescence Test is the ability to use a single fluorochrome-labeled secondary antibody for detecting multiple antigen-antibody (Ag-Ab) interactions. This means that, regardless of the primary antibody’s specificity, a single type of labeled secondary antibody can be employed for visualization. Therefore, this approach is both cost-effective and efficient, as it reduces the need for multiple labeled antibodies.
- Enhanced Sensitivity: When compared to the Direct Immunofluorescence Test, the Indirect method exhibits superior sensitivity. This heightened sensitivity ensures that even minute quantities of specific antibodies in a sample can be detected, making it particularly valuable in scenarios where early detection is crucial for timely intervention and treatment.
- Amplification of Fluorescence Signal: The design of the Indirect Immunofluorescence Test allows for multiple secondary antibodies to bind to the Fc region of a single primary antibody. This multiplicity in binding results in an amplification of the fluorescence signal. The amplified signal not only enhances the visibility of the antigen-antibody complex under a fluorescence microscope but also improves the test’s accuracy and reliability.
Disadvantages of Indirect Immunofluorescence Test
- Complexity and Time Consumption: One of the primary drawbacks of the Indirect Immunofluorescence Test is its inherent complexity. Unlike the Direct Immunofluorescence (IF) method, which involves a single step of antibody binding, the indirect method necessitates a two-tiered approach. This involves the binding of the primary antibody to the antigen, followed by the binding of the fluorochrome-labeled secondary antibody to the primary antibody. Therefore, this multi-step procedure can be more time-consuming, requiring meticulous attention to detail and precision at each stage to ensure accurate results.
- Cross-Reactivity Concerns: Another significant challenge associated with the Indirect Immunofluorescence Test is the potential for cross-reactivity of the secondary antibody. Since the secondary antibody is designed to bind to the Fc region of primary antibodies, there exists a possibility that it might also bind to other agents or proteins present in the sample. This cross-reactivity can lead to false-positive results, complicating the interpretation of the test outcomes. Besides, it underscores the importance of selecting secondary antibodies with high specificity to minimize such interactions.
Result interpretation of Immunofluorescence
- Presence of Specific Antigen or Antibody: The foundational principle of Immunofluorescence revolves around the formation of an antigen-antibody (Ag-Ab) complex. When a specific antigen or antibody of interest is present in the sample, it binds with its corresponding counterpart, leading to the formation of this complex. In the context of the test, if this complex is formed, the fluorochrome-conjugated antibody remains steadfastly bound to the preparation, even after rigorous washing. Therefore, upon visualization through a fluorescent microscope, one can observe a distinct fluorescence. The color of this fluorescence, be it yellow-green, green, or red, is contingent on the type of fluorochrome used. Observing this fluorescence is indicative of a positive test result, signifying the presence of the specific antigen or antibody in the sample.
- Absence of Specific Antigen or Antibody: Conversely, if the sample lacks the specific antigen or antibody of interest, the Ag-Ab complex will not form. This absence means that the fluorochrome-conjugated antibodies, having nothing to bind to, will be effectively washed away during the washing steps of the procedure. As a result, when the preparation is viewed under a fluorescent microscope, no fluorescence will be discernible. The absence of fluorescence denotes a negative test result, indicating that the specific antigen or antibody of interest is not present in the sample.
Applications of Immunofluorescence
- Detection of Biological Molecules in Tissues or Cell Sections: One of the foundational applications of Immunofluorescence is its ability to determine the presence of diverse biological molecules within tissues or cell sections. This includes not only proteins but also other vital biomolecules such as carbohydrates. Therefore, researchers and clinicians can utilize this technique to gain insights into the molecular composition of specific tissues or cells, aiding in both basic research and disease diagnosis.
- Visualization of Cytoskeletons: In the field of molecular biology, Immunofluorescence plays a pivotal role in visualizing intricate cellular structures, notably the cytoskeleton. The cytoskeleton, comprising structures like intermediate filaments, is essential for maintaining cellular shape and facilitating various cellular functions. Through Immunofluorescence, researchers can obtain detailed images of these structures, furthering our understanding of cellular morphology and function.
- Detection of Autoimmune Disorders: Immunofluorescence also finds significant application in the realm of clinical diagnostics, especially in the detection of autoimmune disorders. In these conditions, the body’s immune system produces antibodies against its own tissues. Immunofluorescence can detect these autoantibodies, providing a clear diagnosis and aiding in the management of such disorders.
- Combination with Non-Antibody Fluorescent Staining Methods: Besides its traditional applications, Immunofluorescence can also be combined with other non-antibody methods of fluorescent staining. A notable example is the use of DAPI (4′,6-diamidino-2-phenylindole), a fluorescent stain that specifically labels DNA. This combination allows for simultaneous visualization of specific proteins and DNA, offering a comprehensive view of cellular components.
Benefits of Immunofluorescence
- High Specificity: One of the primary benefits of immunofluorescence is its high specificity. The technique leverages antibodies that bind with precision to the target antigen, ensuring accurate detection of specific proteins.
- Sensitivity: The use of fluorescent dyes in immunofluorescence amplifies the detection signal. This heightened sensitivity facilitates the identification of proteins even in samples where their abundance is low.
- Visualization of Protein Distribution: Immunofluorescence provides a window into the cellular world, revealing the distribution and location of proteins within cells and tissues. This offers invaluable insights into the spatial organization of various cellular structures, including organelles, membranes, and subcellular compartments.
- Studying Protein Interactions: The technique is instrumental in exploring protein-protein interactions, allowing researchers to discern co-localization within the same cellular compartment.
- Multiplexing Capability: Certain immunofluorescence protocols enable the simultaneous detection of multiple proteins and antigens within a single sample, enhancing the depth of analysis.
- Versatility: Immunofluorescence is adaptable to a wide array of biological samples, spanning cells, tissues, and even whole organisms. Its applications are vast, encompassing fields such as cell biology, immunology, neuroscience, pathology, and diagnostics.
- Quantification: Beyond mere visualization, immunofluorescence can be harnessed for quantifying proteins or antigens, especially when paired with sophisticated imaging and analysis tools.
- Detailed Imaging: Coupled with fluorescence microscopy, the technique yields intricate images of cellular structures, offering a detailed view of proteins in their native environment.
- Compatibility: Immunofluorescence seamlessly integrates with other techniques, such as electron microscopy, facilitating the ultrastructural visualization of cells and tissues.
- Stable Signals: The fluorescence signals are inherently stable, ensuring that they can be preserved for extended periods. This stability allows for the reanalysis of samples, ensuring that no detail is overlooked.
Limitations of Immunofluorescence
- Photobleaching: A primary concern with Immunofluorescence is photobleaching, which refers to the degradation of fluorochromes upon exposure to light. This degradation can compromise the fluorescence signal. However, this issue can be mitigated by using higher concentrations of fluorochromes and reducing the exposure time to light.
- Extraneous Fluorescence: At times, unnecessary fluorescence can manifest due to the impurity of the targeted antigen. This extraneous fluorescence can interfere with the specific signal, leading to potential misinterpretations.
- Autofluorescence: Certain agents present in the specimen might inherently possess fluorescent properties, leading to autofluorescence. This can further complicate the interpretation of results.
- Application Limitations: Immunofluorescence is predominantly employed for fixed or dead cells, limiting its utility in studying live cell dynamics.
- Cost and Expertise: The technique can be expensive and demands a high level of expertise for accurate execution and interpretation.
- False Results: Non-specific binding of antibodies to unrelated molecules can yield false positive results. Conversely, low antigen expression or epitope masking might result in false negatives.
- Cross-Reactions: Antibodies might cross-react with antigens that are closely related. Therefore, validating the specificity of antibodies and conducting specificity tests are crucial.
- Antibody Selection: Choosing appropriate and high-quality antibodies is both challenging and essential for the success of the immunofluorescence assay.
- Background Signal Interference: The inherent background signal from the sample can overshadow the specific signal. Implementing proper controls and background subtraction methods is vital to address this challenge.
- Tissue-Based Challenges: When working with tissues, the methods of fixation and permeabilization can influence results. Fixation can alter protein structures, potentially affecting antibody binding.
- Fluorescent Dye Sensitivity: Fluorescent dyes are susceptible to photobleaching, which can diminish the fluorescence signal over time. Minimizing exposure to intense light is essential to preserve signal integrity.
- Quantification Challenges: Accurately quantifying immunofluorescence signals can be a daunting task due to variations in fluorescence intensity, background noise, and detection range.
Quiz
FAQ
What is immunofluorescence?
Immunofluorescence is a technique used to visualize and detect specific antigens or antibodies within cells, tissues, or organisms by utilizing fluorescently labeled antibodies.
How does immunofluorescence work?
Immunofluorescence involves the binding of fluorescently labeled antibodies to specific antigens or antibodies of interest, allowing for their visualization under a fluorescence microscope.
What are the different types of immunofluorescence?
There are two main types of immunofluorescence: direct immunofluorescence (DIF), where the primary antibody is directly labeled with a fluorochrome, and indirect immunofluorescence (IIF), which uses a secondary antibody labeled with a fluorochrome.
What are the applications of immunofluorescence?
Immunofluorescence has a wide range of applications, including protein localization, detection of specific antibodies in diseases, cytoskeleton visualization, and identification of cellular structures and organelles.
What are the advantages of immunofluorescence over other techniques?
Immunofluorescence offers high sensitivity, specificity, and the ability to visualize and localize antigens or antibodies within the cellular context, making it a valuable tool in research and diagnostics.
What are the limitations of immunofluorescence?
Some limitations of immunofluorescence include photobleaching of fluorochromes, extraneous fluorescence, autofluorescence, its suitability for fixed or dead cells, and the need for expertise and specialized equipment.
How can photobleaching in immunofluorescence be minimized?
Photobleaching can be reduced by using higher concentrations of fluorochromes, decreasing exposure time to light, using photostable fluorochromes, and employing antifade mounting media.
How can background fluorescence be minimized in immunofluorescence?
Background fluorescence can be minimized by optimizing the immunofluorescence protocol, including careful selection of antibodies, appropriate washing steps, and the use of negative controls.
Can immunofluorescence be used for live-cell imaging?
Immunofluorescence is primarily used for fixed cells, but it can be combined with live-cell imaging techniques such as fluorescent protein tagging or antibody uptake assays for dynamic studies.
What are the key considerations when performing immunofluorescence experiments?
Important considerations include choosing appropriate antibodies, optimizing staining conditions, selecting suitable fluorochromes, using appropriate controls, and careful image acquisition and analysis.

🙂 Excellent Article, Excellent Blog , Excellent Site ✅✅✅
This is a really nice page. It’s very informative and well organized. I’ll come back from time to time for more posts like this one.