Table of Contents
What is Immunoglobulin E (IgE Antibody)?
- Immunoglobulin E (IgE) is a unique antibody that belongs to the family of immunoglobulins, which includes IgM, IgG, IgD, and IgA. It was the last immunoglobulin to be discovered, and its structure and functions have been extensively studied. IgE plays a crucial role in various physiological processes, including Type I hypersensitivity reactions, immune responses against parasitic infections, autoimmune processes, and venom protection.
- The structure of IgE consists of two heavy chains called epsilon (ε) chains and two light chains. The ε chain contains four Ig-like constant domains (Cε1–Cε4). This antibody is primarily produced by plasma cells in mammals. One of its key functions is to protect against certain parasitic worms, such as Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. Additionally, IgE is involved in immune defense against protozoan parasites like Plasmodium falciparum. It is believed that IgE has evolved as a defense mechanism against venoms as well.
- Type I hypersensitivity reactions, commonly known as allergies, rely on the role of IgE. Allergic diseases, including allergic asthma, sinusitis, rhinitis, food allergies, chronic urticaria, and atopic dermatitis, are associated with IgE-mediated immune responses. IgE is essential in the response to allergens, triggering anaphylactic reactions to drugs, bee stings, and antigens used in desensitization immunotherapy.
- Despite being the least abundant isotype in the bloodstream, with levels of IgE in non-atopic individuals accounting for only 0.05% of the total Ig concentration, IgE can induce severe and rapid immunological reactions, such as anaphylaxis. Its discovery in 1966 and 1967 by separate research groups led by Kimishige and Teruko Ishizaka in the United States and Gunnar Johansson and Hans Bennich in Sweden highlighted its significance. Their collaborative work was published in 1969.
- IgE is present at very low concentrations in the circulation, and its half-life is approximately 2 days in serum. It is primarily produced by IgE plasma cells located in mucosal areas, especially in the respiratory and gastrointestinal tracts. In the respiratory tract, secreted IgE mediates allergic reactions, while in the gastrointestinal tract, it plays a role in expelling parasitic worm infestations. While the exact physiological role of IgE is not yet fully understood, its association with allergic reactions and defense against parasites, particularly helminths, is well-established.
- In summary, Immunoglobulin E (IgE) is a distinctive antibody class that plays a significant role in allergic reactions, immune responses against parasites, and other physiological processes. Its unique structure and functions make it a subject of extensive research and a target for developing new therapeutic interventions.
Definition of Immunoglobulin E (IgE Antibody)
Immunoglobulin E (IgE) is a type of antibody that is involved in allergic reactions and defense against parasites. It is the least abundant but highly potent class of immunoglobulins found in mammals.
Structure of Immunoglobulin E (IgE Antibody)
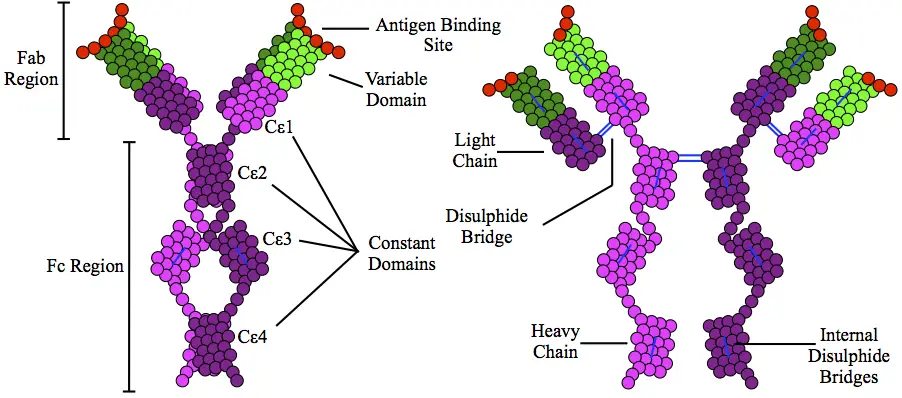
- The structure of Immunoglobulin E (IgE) antibody consists of two identical heavy chains of the epsilon (ε) class and two identical light chains. The heavy chains have a high carbohydrate content of about 12%. The antibody has two antigen-binding areas formed by the interaction of the light and heavy chains, giving it a valency of 2.
- Both the heavy and light chains have variable regions at their N terminal ends, which contribute to the diversity of antigen recognition. These chains are further divided into variable and constant regions. Disulfide bonds link the heavy and light chains together, and intrachain disulfide links divide each chain into domains.
- The light chains have two domains: one variable and one constant. The heavy chains, on the other hand, have five domains: one variable domain and four constant-region domains. IgE is unique in having an additional constant region called CH4. This CH4 region plays a crucial role in restricting IgE binding to high-affinity receptors (Fcε-RI) found on basophils and mast cells. These cells contain preformed granules of heparin and histamine.
- Unlike other antibody classes, IgE lacks a hinge region.
Molecular Level of Immunoglobulin E (IgE Antibody)
- At the molecular level, Immunoglobulin E (IgE) antibody shares a common structural framework with other immunoglobulin molecules. It consists of two identical small polypeptide chains called light chains and two identical large polypeptide chains called heavy chains. The Fc (crystallizable fragment) region, responsible for binding to cellular receptors, is composed solely of the two heavy chains. The Fab (antigen-binding fragment) region, where antigen recognition and binding occur, is made up of the continuous heavy chains with the Fc region and the light chains. Both the heavy and light chains have variable and constant regions.
- The antigen binding sites of IgE are located in the variable regions of the light and heavy chains. These regions have variable amino acid sequences that can be modified through affinity maturation for a specific antigen. The variable heavy and light chains are arranged in antiparallel B-pleated sheets, creating three-dimensional folds where antigens can bind. The remaining regions of the polypeptide chains are known as constant regions, which are held together by strong dipeptide bonds to provide stability to the two chains.
- Unlike some antibodies such as IgM, which can form pentamers capable of binding multiple antigens, IgE exists as a monomer. It is composed of two epsilon-heavy chains and two light chains, allowing it to bind a total of two antigens. The binding occurs through the unique antigen-specific binding sites created by the variable regions of the light and heavy chains. The C-terminal regions of the heavy chains consist of four C-epsilon dimers (C-epsilon 1-4). These dimers play a critical role in binding to specific cellular receptors of IgE, such as Fc-epsilon R1 and CD23.
- Unlike certain immunoglobulins that have a “hinge” region in the middle, IgE lacks this hinge region and instead has the C-epsilon2 domain. This absence of a hinge region gives IgE a more flexible conformation when interacting with its receptors. Additionally, IgE is heavily glycosylated compared to other antibodies, with each heavy chain containing seven N-linked glycosylations. These glycosylations are necessary for binding to the high-affinity cellular receptor Fc-epsilon-R1.
Clinical Significance of IgE
- Immunoglobulin E (IgE) has significant clinical significance in various disease processes. One important aspect to note is that not everyone exposed to environmental antigens will generate a strong immune reaction. Individuals with a genetic predisposition known as “atopics” are more prone to developing type I hypersensitivity responses to environmental antigens.
- The clinical manifestations of type I hypersensitivity reactions are primarily due to the actions of chemical mediators, including histamine, leukotrienes, and cytokines. These mediators cause increased vascular permeability, smooth muscle constriction, mucus secretion, and inflammation.
- In severe cases, they can lead to systemic effects such as hypotension, edema, anaphylactic shock, and even death if poorly managed.
- The manifestations of IgE-mediated hypersensitivity can occur in different areas of the body. In the upper airway, patients may experience mild seasonal allergies or allergic rhinitis, characterized by symptoms such as watery eyes, runny nose, cough, and others.
- In the lower airway, atopic asthma can manifest as airway constriction, increased mucus production, and inflammation, leading to obstructive lung disease.
- The skin is also commonly involved, and the release of histamine and dermal edema can result in urticaria or wheals. Atopic dermatitis or eczema, although a multifactorial disease, may also involve IgE-mediated hypersensitivity.
- Studies have shown a correlation between elevated IgE levels and atopic dermatitis, and some evidence suggests that IgE-mediated hypersensitivity may play a role in the disease. However, further research is needed to fully understand this relationship.
- IgE also plays a crucial role in the defense against parasitic organisms, particularly helminths. When the immune system encounters helminths, B-cells undergo a class switch to produce IgE antibodies. These IgE antibodies bind to the parasites, coating them and enabling recognition by effector cells such as eosinophils and mast cells. Through the Fc-epsilon RI receptor, these effector cells release cytokines, histamine, and other substances toxic to the helminths, leading to their destruction and clearance from the body.
- The clinical significance of IgE extends beyond understanding disease processes. It has led to the development of novel treatment modalities. For example, the humanized monoclonal antibody omalizumab has been engineered to target IgE, rendering it ineffective in producing associated symptoms. Omalizumab has shown benefits in patients with moderate to severe asthma and allergic rhinitis.
- While significant progress has been made in understanding the role of IgE in disease, there is still much to learn. Further research is necessary to unravel the precise functions of IgE in established IgE-related health disorders and to discover novel clinical relationships. By continuing to investigate IgE, we can deepen our understanding of these diseases and develop more effective treatments.
Functions of Immunoglobulin E (IgE Antibody)
IgE, or Immunoglobulin E, serves several important functions in the immune system:
- Allergic Reactions: The primary function of IgE is its involvement in allergic reactions. When a person is exposed to an allergen, such as pollen or certain foods, their immune system produces specific IgE antibodies against that allergen. Upon subsequent exposure, the allergen binds to the IgE antibodies, triggering the release of inflammatory mediators like histamine from mast cells and basophils. This immune response leads to the characteristic symptoms of allergies, such as itching, sneezing, and swelling.
- Immunity to Parasites: IgE plays a crucial role in defending the body against parasitic infections, particularly helminths (parasitic worms). When a person is infected with certain parasites like Schistosoma mansoni or Trichinella spiralis, their immune system produces IgE antibodies specific to those parasites. These IgE antibodies facilitate the immune response against the parasites, promoting their elimination.
- Asthma and Respiratory Diseases: IgE is closely associated with asthma and other respiratory diseases. In individuals with asthma, exposure to certain triggers, such as dust mites or pet dander, leads to the production of allergen-specific IgE antibodies. These IgE antibodies bind to mast cells in the airways, causing the release of inflammatory substances that result in bronchoconstriction and other asthma symptoms.
- Allergic Skin Conditions: IgE is involved in the development of certain allergic skin conditions, such as atopic dermatitis (eczema) and chronic urticaria (hives). In these conditions, IgE-mediated immune responses contribute to skin inflammation, itching, and the formation of rashes or welts.
- Immunotherapy: IgE plays a role in allergen immunotherapy, a treatment approach aimed at desensitizing individuals with allergies. During immunotherapy, small amounts of allergens are administered to the patient to gradually reduce their sensitivity. This process involves the modulation of IgE levels and the induction of IgG antibodies, which can compete with IgE for binding to allergens and attenuate the allergic response.
By understanding the various functions of IgE, researchers and healthcare professionals can develop strategies to manage allergies, enhance parasite immunity, and improve the treatment of related conditions.
FAQ
What is Immunoglobulin E (IgE)?
Immunoglobulin E (IgE) is a type of antibody, or immunoglobulin, produced by the immune system in response to allergens or parasitic infections.
What are the functions of IgE?
IgE has several functions, including triggering allergic reactions, providing immunity against parasites, playing a role in type I hypersensitivity, and participating in responses to allergens.
How does IgE contribute to allergic reactions?
IgE is involved in allergic reactions by binding to mast cells and basophils through the Fc epsilon RI receptor. Upon exposure to allergens, IgE antibodies attached to these cells trigger the release of inflammatory mediators, leading to allergic symptoms.
What is the role of IgE in parasite immunity?
IgE plays a crucial role in defending the body against parasitic infections, particularly helminths. It helps to eliminate parasites by initiating an immune response specific to the invading parasites.
What is the structure of IgE antibody?
IgE is composed of two identical heavy chains of the epsilon (ε) class and two identical light chains. It has variable and constant regions, with antigen-binding sites located in the Fab region and the Fc region responsible for binding to cellular receptors.
Which allergic diseases are associated with IgE?
IgE is associated with various allergic diseases, including allergic asthma, allergic rhinitis (hay fever), food allergies, sinusitis, chronic urticaria (hives), and atopic dermatitis (eczema).
Can IgE be involved in non-allergic conditions?
While IgE is primarily associated with allergies, it may also play a role in non-allergic conditions such as autoimmune processes and venom protection. However, more research is needed to fully understand these associations.
How are IgE levels measured?
IgE levels can be measured through blood tests, specifically the total IgE level or specific IgE testing for particular allergens. These tests help diagnose allergies and assess the immune response.
What is the significance of high IgE levels?
High IgE levels can indicate an overactive immune response to allergens, leading to allergic reactions. They may also suggest the presence of a parasitic infection or certain immune system conditions that require further evaluation.
Can IgE levels be modified or treated?
Various treatments aim to manage IgE-related conditions. Allergy medications, immunotherapy (such as allergy shots or sublingual tablets), and targeted therapies that inhibit IgE activity are available options to control allergic reactions mediated by IgE.
References
- Owen, J. A., Punt, J., & Stranford, S. A. (2013). Kuby Immunology (7 ed.). New York: W.H. Freeman and Company.
- Amarasekera, M. (2011). Immunoglobulin E in health and disease. Asia Pacific Allergy, 1(1), 12–15. http://doi.org/10.5415/apallergy.2011.1.1.12
- Lydyard, P.M., Whelan,A.,& Fanger,M.W. (2005).Immunology (2 ed.).London: BIOS Scientific Publishers.
- https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8159
- https://www.sciencedirect.com/science/article/pii/B9780323069472100094


