Table of Contents
What is Keto Enol Tautomerization?
- Keto enol tautomerization is a specific type of tautomerization that involves the interconversion between a ketone and an enol molecule. Tautomerization refers to the dynamic equilibrium process in which two molecular structures undergo interconversion by the movement of a chemical group between different sites on the molecule.
- To better understand tautomerization, let’s consider an example involving a molecule called Molecule A with a functional group X attached to site A. However, there is another suitable location on the molecule, called site B, where group X can also attach. Tautomerization occurs when group X moves between site A and site B, resulting in the conversion of Molecule A to its tautomeric form, Molecule B. The movement of group X between the two sites leads to the alteration of the molecular structure.
- In a system where multiple molecules of Molecule A are present, such as a mole dissolved in a solvent, tautomerization takes place continuously, with Molecule A converting to Molecule B and vice versa. However, due to the opposing rates of conversion, the relative proportions of Molecule A and Molecule B remain constant over time. This balanced state is referred to as dynamic equilibrium.
- In tautomer pairs, it is often observed that one form of the molecule is more stable than the other. This stability difference results in an unequal distribution of the tautomeric forms at equilibrium, with the more stable form predominating. The factors determining the stability and the distribution of the tautomers can vary depending on the specific molecules involved.
- In the case of keto enol tautomerization, the interconversion occurs between a ketone and an enol. Ketones are carbonyl compounds with a carbon double-bonded to an oxygen atom (C=O), while enols are compounds that possess both an alkene (-C=C-) and an alcohol (-OH) group on adjacent carbon atoms. The keto form represents the more stable tautomer, where the carbonyl group is intact, while the enol form is the less stable tautomer, characterized by the presence of a double bond between adjacent carbon atoms and an alcohol group.
- The conversion between the keto and enol forms in keto enol tautomerization is driven by various structural factors, such as the nature of the substituents attached to the carbon atoms involved in the tautomeric interconversion and the presence of suitable catalytic or solvent conditions. These factors influence the relative stability of the tautomers and the rates of interconversion, ultimately determining which form predominates at equilibrium.
- In summary, keto enol tautomerization is a dynamic equilibrium process in which ketones and enols interconvert through the movement of a chemical group. The stability of the tautomers and the specific structural factors involved play crucial roles in determining the distribution of the tautomeric forms at equilibrium. Understanding this tautomerization process is important in various areas of chemistry, including organic synthesis, biochemistry, and pharmaceutical research.
Keto Enol Tautomer Structure
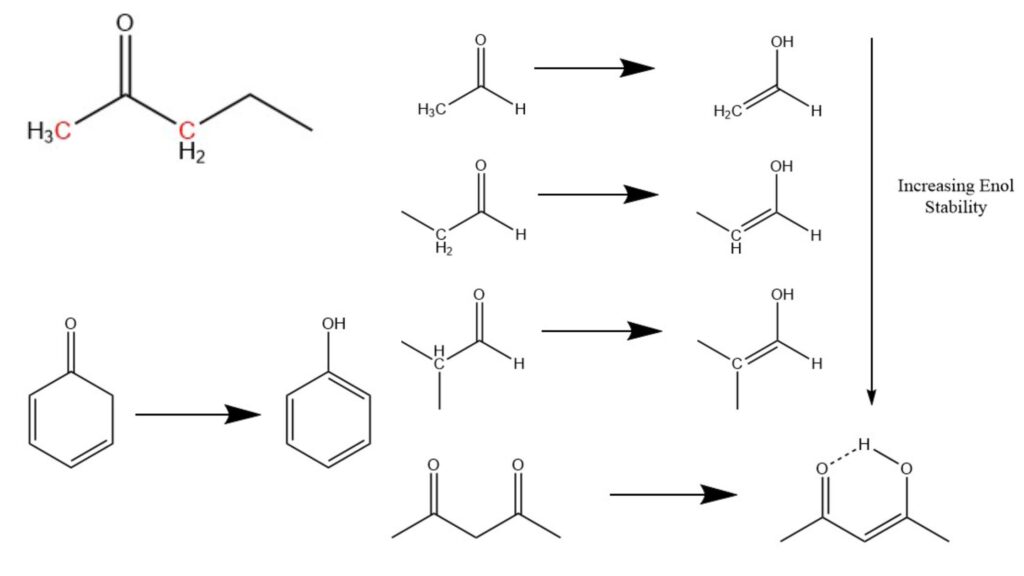
The structure of a molecule plays a significant role in determining the favorability and stability of keto and enol forms during keto enol tautomerization. In general, ketones are more favored over enols in many common molecular structures. However, the presence and nature of the α-carbon (the carbon atom adjacent to the carbonyl carbon) can influence the stability and preference for the enol form.
A structural trend suggests that α-carbons with more non-hydrogen substituents tend to exhibit greater stability as enols. For example, an α-carbon with a methyl group, having three hydrogen atoms, is less favorable for enol formation compared to an α-carbon with a methylene group, which has two hydrogen atoms. Additionally, enols formed by methylene α-carbons are less stabilized compared to those formed by methine α-carbons, which have one hydrogen and two hydrocarbon attachments.
While ketones are generally more stable, there are certain molecules where the enol form predominates. One such example is 1,3-dicarbonyl molecules, commonly formed through aldol condensations. In these molecules, an enol can be generated by transferring a hydrogen to the oxide ion, leading to the stabilization of the resulting hydroxide structure through a hydrogen bond from the adjacent carbonyl group. This unique arrangement forms a self-stabilizing structure resembling a six-membered ring.
Aromaticity also plays a significant role in the stability of enols. Aromatic enols tend to be more stable than their corresponding ketones because aromaticity provides substantial stability that outweighs the stability of the ketone form. Specifically, enols are favored when the resulting alkene completes an aromatic electron cycle. This phenomenon explains why phenols, which exhibit aromaticity, tend to form stable structures, while 2,4-cyclodienones, which lack complete aromatic electron cycles, are less commonly observed.
In summary, the structure of a molecule, particularly the nature of the α-carbon and the presence of aromaticity, influences the stability and preference for the keto or enol form during keto enol tautomerization. Understanding these structural factors is crucial in predicting and explaining the behavior of tautomeric transformations and their implications in various areas of chemistry.
Keto Enol Tautomerization Mechanism
The mechanism of keto enol tautomerization can vary depending on the conditions, specifically whether the reaction occurs under acidic or basic conditions. Let’s explore the mechanisms for both scenarios:
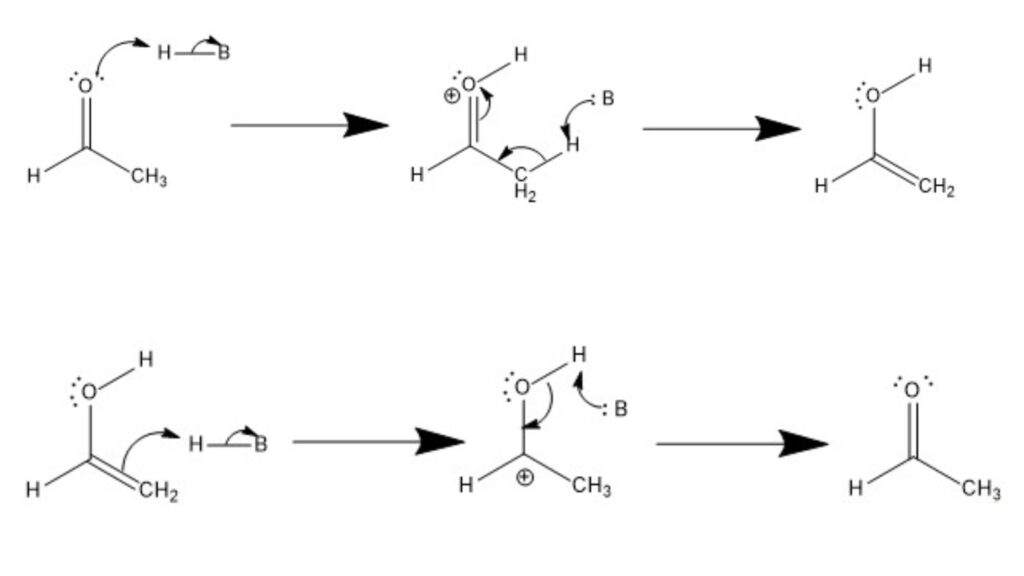
- Tautomerization under Acidic Conditions: In the acidic conditions, protonation drives the conversion between ketones and enols.
- Formation of Enol: The process starts with an acid protonating a lone electron pair on the carbonyl oxygen, generating a positively charged oxygen atom. Subsequently, the conjugate base of the acid deprotonates the α-carbon adjacent to the carbonyl carbon. This deprotonation leads to the formation of a carbanion intermediate. Finally, the electrons from the π bond of the carbonyl group undergo a rearrangement to form a new π bond with the deprotonated α-carbon, resulting in the formation of an enol.
- Formation of Ketone: To convert the enol back to a ketone, the acid protonates the alkene moiety of the enol, creating a C-H bond between the α-carbon and the proton. The conjugate base of the acid then deprotonates the hydroxyl group, generating an oxide anion. The lone pair of electrons on the oxide anion forms a π bond with the carbocation, resulting in the restoration of the carbonyl group and the formation of a ketone.
- Tautomerization under Basic Conditions: In the basic conditions, deprotonation is the driving force behind the conversion between ketones and enols.
- Formation of Enol: In this case, a generic Brønsted-Lowry base deprotonates the α-carbon adjacent to the carbonyl carbon, resulting in the generation of a carbanion. The π electrons of the carbonyl group then shift to form an alkene π bond with the deprotonated carbon, leading to the formation of an enol. Finally, the conjugate acid of the base protonates the oxide anion, resulting in the stabilization of the enol form.
- Formation of Ketone: To revert from the enol to the ketone form, the base deprotonates the hydroxyl group, liberating a lone pair of electrons. These electrons subsequently form a carbonyl group, pushing a lone pair of electrons onto the α-carbon, generating a carbanion intermediate. The conjugate acid of the base then protonates the α-carbon, regenerating the carbonyl group and forming a ketone.
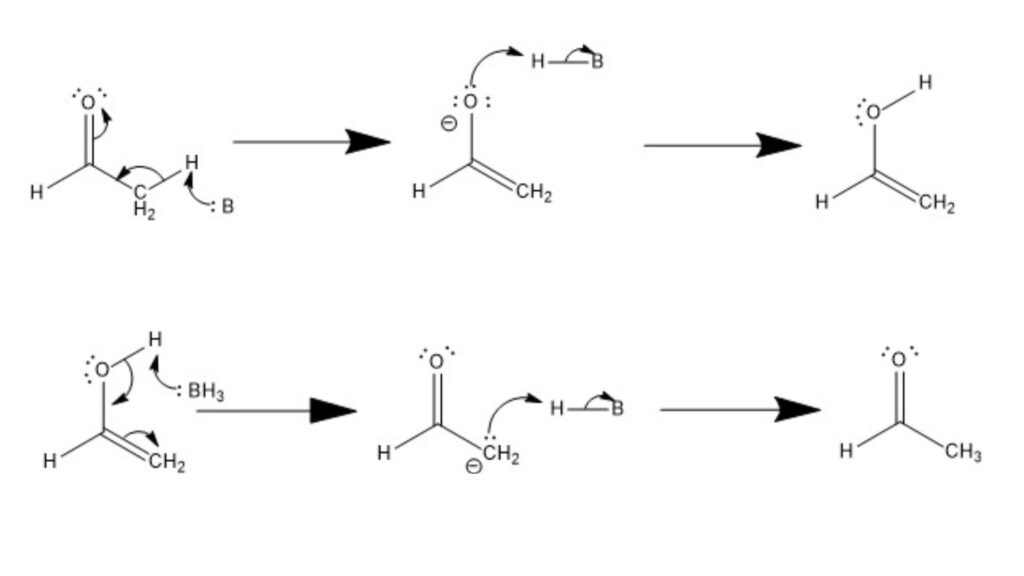
In both acidic and basic conditions, the tautomeric interconversion occurs through a series of protonations and deprotonations, with the involvement of conjugate acids and bases. The specific mechanism depends on the nature of the conditions and the specific molecules involved in the tautomerization process.
Overall, understanding the keto enol tautomerization mechanism provides insights into the dynamic equilibrium between ketone and enol forms, which is relevant in various areas of chemistry, including organic synthesis, biochemistry, and pharmaceutical research.
Factors That Affect Keto-Enol Equilibria
The equilibrium between the keto and enol forms in aldehydes and ketones is typically strongly biased towards the keto form due to the greater stability of the C-O pi bond compared to the C-C pi bond. This preference for the keto form is even more pronounced in carboxylic acids and esters.
However, there are several factors that can significantly influence the keto-enol ratio:
- Substitution: Enols are a type of alkene, and substituted alkenes tend to be more stable. Therefore, the presence of substituents on the alpha-carbon adjacent to the carbonyl group can increase the stability of the enol form, thereby shifting the equilibrium towards the enol.
- Conjugation: When the pi bond of the enol is conjugated with a neighboring pi system, such as in a conjugated carbonyl compound or a molecule with an extended conjugated system, the enol form becomes more stable. The resonance effects associated with conjugation help delocalize the electrons, leading to increased stability of the enol and a higher proportion of the enol form in the equilibrium mixture.
- Hydrogen bonding: Intramolecular hydrogen bonding between the hydroxyl group of the enol and a suitable hydrogen bond acceptor in the molecule can stabilize the enol form. This interaction enhances the stability of the enol and can significantly influence the keto-enol equilibrium, favoring the enol form.
- Aromaticity: If the enol form is part of an aromatic ring, it can exhibit significant stability due to aromaticity. Aromatic enols, such as those found in phenols or enols participating in aromatic electron cycles, are particularly favored over the keto form. Aromaticity provides a strong stabilization effect, resulting in a higher proportion of the enol form in the equilibrium mixture.
It is important to note that these factors influence the keto-enol equilibrium to varying extents. Substitution and conjugation have relatively weaker influences on the equilibrium, while hydrogen bonding and aromaticity exert stronger effects. Understanding these factors is crucial for predicting and explaining the relative abundance of the keto and enol forms in different molecular systems.
In summary, while the keto form is generally favored over the enol form in aldehydes, ketones, carboxylic acids, and esters, several factors can significantly affect the keto-enol equilibrium. Substitution, conjugation, hydrogen bonding, and aromaticity all play important roles in determining the relative proportions of the two forms. The interplay of these factors contributes to the dynamic nature of keto enol tautomerization and its impact on chemical reactivity and properties.
Factor #1: Substitution
Substitution, or the presence of substituents on the alpha-carbon adjacent to the carbonyl group, plays a significant role in determining the stability and preference of enols. Similar to Zaitsev’s rule in elimination reactions, where the more substituted alkene is favored due to its greater thermodynamic stability, the same trend applies to enols.
In the case of enols, the more substituted the enol is, the more thermodynamically stable it tends to be. Although the difference in stability between different enols may not be substantial (typically around 1-2 kcal/mol), even a small difference of 1 kcal/mol can result in a significant change in the equilibrium ratio, approximately 80:20 in favor of the more substituted enol. This stability trend is essential to consider, particularly when contrasting kinetic and thermodynamic enolates.
To illustrate the influence of substitution on enol stability, let’s compare the stability of aldehyde enols:
Aldehyde Enol 1: H | R1-C=C-OH
Aldehyde Enol 2: R2 | R1-C=C-OH
In this case, Aldehyde Enol 2, which has a substituent R2 on the alpha-carbon, is more substituted compared to Aldehyde Enol 1, which has only a hydrogen atom. As a result, Aldehyde Enol 2 is expected to be more thermodynamically stable than Aldehyde Enol 1.
This trend of increased stability with increased substitution in enols is important to consider in various reactions and processes where enol formation or interconversion is involved. Understanding the influence of substitution on enol stability provides insights into the relative abundance of different enol forms and their impact on the overall equilibrium in keto enol tautomerization reactions.
Note: The specific stability trend of enols with respect to substitution can vary depending on the specific molecules and substituents involved. The magnitude of the stability difference may also depend on the nature of the substituents and the specific molecular context.
Factor #2: Conjugation / Resonance
Conjugation, or resonance, is another important factor that influences the stability and preference of enols. In a similar way to how π bonds in molecules tend to connect and participate in conjugated systems for increased stability, enols also benefit from conjugation with neighboring pi systems.
When considering the preference for the enol form, it is crucial to determine which ketone allows for conjugation with a neighboring pi system. The presence of conjugation enhances the stability of the enol form and can significantly influence the equilibrium between the keto and enol forms.
To illustrate the impact of conjugation on enol stability, let’s compare two ketones:
Ketone 1: R1-C(=O)-R2
Ketone 2 (with a neighboring pi system): R1-C(=O)-C=C-R3
In this case, Ketone 2 has a neighboring pi system represented by the C=C bond connected to the carbonyl group. This allows for conjugation between the pi electrons of the enol and the pi system of the neighboring double bond. As a result, Ketone 2 will have a greater preference for the enol form compared to Ketone 1, which lacks a neighboring pi system for conjugation.
Conjugation provides additional stability to the enol form by delocalizing electrons and distributing the charge more evenly throughout the molecule. This stabilization effect contributes to a higher proportion of the enol form in the equilibrium mixture.
It is important to note that the presence of conjugation or the availability of a neighboring pi system can vary depending on the specific molecular structure and substituents involved. The extent and nature of conjugation can significantly influence the stability and preference of enols in keto enol tautomerization reactions.
Understanding the role of conjugation in enol stability helps explain the relative abundance of different enol forms and their impact on the overall equilibrium. The interplay between conjugation, substitution, and other factors provides a comprehensive understanding of the factors that affect keto-enol equilibria.
Factor #3: Hydrogen Bonding
Hydrogen bonding is an intriguing factor that significantly influences the stability and preference of the enol form in keto-enol tautomerization. Enols possess an O-H bond, which exhibits high polarization, with the hydrogen atom carrying a partial positive charge. This polarity allows for hydrogen bonding interactions to occur when a suitable Lewis base, such as the oxygen of a carbonyl group, is present in close proximity to the enol.
The formation of intramolecular hydrogen bonds between the O-H group of the enol and the nearby Lewis base leads to the stabilization of the enol form. This stabilization arises from the attractive electrostatic interactions between the partially positive hydrogen and the lone pair of electrons on the Lewis base. The presence of hydrogen bonding in the enol form enhances its stability relative to the keto form.
In some cases, the proportion of the enol form can even be equal to or greater than the amount of the keto tautomer in solution. For instance, the NMR spectrum of 2,4-pentanedione demonstrates approximately a 1:1 mixture of the keto and enol tautomers. This suggests that hydrogen bonding interactions in these systems play a significant role in favoring the enol form.
The presence of intramolecular hydrogen bonding can lead to unique properties and reactivity in molecules undergoing keto-enol tautomerization. It not only stabilizes the enol tautomer but also affects the overall equilibrium between the keto and enol forms. Understanding the impact of hydrogen bonding on the stability and prevalence of enols provides insights into the behavior and properties of compounds undergoing such tautomerization reactions.
It is worth noting that the extent and strength of hydrogen bonding can vary depending on the specific molecular structure and the nature of the participating groups. Different functional groups and substituents can influence the availability and strength of hydrogen bonding interactions, ultimately affecting the equilibrium between keto and enol forms.
Factor #4 : Aromaticity
Aromaticity is a highly influential factor that strongly favors the enol tautomer in keto-enol tautomerization. The presence of aromaticity can significantly shift the equilibrium towards the enol form, making it the dominant tautomer.
Let’s compare two ketones to understand the impact of aromaticity on the preference for the enol tautomer:
Ketone 1: R1-C(=O)-R2
Ketone 2 (containing an aromatic ring): R1-C(=O)-C=C-C(=O)-R3
In this case, Ketone 2 possesses an aromatic ring, represented by the C=C-C(=O)- moiety. The presence of this aromatic system imparts considerable stability to the enol form, favoring its prevalence over the keto form. The resonance energy associated with aromaticity, which can exceed 20 kcal/mol in the case of phenol, contributes to the enhanced stability of the enol tautomer.
Due to the overwhelming influence of aromaticity, the keto tautomer may not even be detectable in solution. This emphasizes the significant preference for the enol tautomer in systems where aromaticity is present.
Aromaticity offers remarkable stability to molecules by delocalizing electrons over the conjugated pi system, resulting in lowered energy and enhanced thermodynamic favorability. The delocalization of electrons in the enol form, when coupled with an aromatic system, leads to a more stable structure compared to the corresponding keto tautomer.
Understanding the impact of aromaticity on keto-enol equilibria provides insights into the behavior of compounds with aromatic systems and their propensity for enolization. Aromaticity represents a powerful driving force that promotes the enol tautomer and can significantly influence the reactivity and properties of molecules undergoing keto-enol tautomerization.
Examples of Keto-Enol Tautomerism
Keto-enol tautomerism is a common phenomenon observed in various organic compounds. Here are a few examples of keto-enol tautomerism:
- Acetone (Ketone) and Enol Form: The compound acetone, also known as propanone, can undergo keto-enol tautomerism. In its keto form, acetone exists as a stable ketone with a carbonyl group. However, under specific conditions, such as in the presence of an acid or base, acetone can tautomerize to its enol form. The enol form of acetone involves the migration of a hydrogen atom from the α-carbon to the oxygen atom, resulting in the formation of an alkene-like structure.
- Pyruvate (Ketone) and Enol Form: Pyruvate, an important intermediate in metabolism, exhibits keto-enol tautomerism. In its keto form, pyruvate contains a carbonyl group. However, under certain conditions, such as in the presence of an enzyme called pyruvate tautomerase, pyruvate can convert to its enol form. The enol form of pyruvate is stabilized by intramolecular hydrogen bonding between the hydroxyl group and the carbonyl oxygen.
- 2,4-Pentanedione (Diketone) and Enol Form: 2,4-Pentanedione, also known as acetylacetone, is a diketone that exhibits keto-enol tautomerism. In its keto form, 2,4-pentanedione consists of two carbonyl groups. However, the enol form can be favored in certain solvents or under acidic conditions. The enol form of 2,4-pentanedione involves the migration of a hydrogen atom to the oxygen atom, resulting in the formation of an enolic double bond.
- Phenol (Phenolic Compound) and Enol Form: Phenol, a common aromatic compound, can undergo keto-enol tautomerism. In its keto form, phenol exists as a stable compound with a hydroxyl group attached to an aromatic ring. However, under certain conditions, such as in the presence of strong acid or base, phenol can tautomerize to its enol form. The enol form of phenol involves the migration of a hydrogen atom to the oxygen atom, resulting in the formation of an enolic double bond.
These are just a few examples of compounds that exhibit keto-enol tautomerism. Many other ketones, aldehydes, and compounds containing α-hydrogens can also undergo this type of tautomerization, leading to the interchange between keto and enol forms. The relative stability and proportion of the keto and enol forms depend on various factors, including substitution, conjugation, hydrogen bonding, and aromaticity.
FAQ
What is keto-enol tautomerism?
Keto-enol tautomerism is a reversible chemical phenomenon in which a molecule undergoes conversion between the keto form (containing a carbonyl group) and the enol form (containing an alkene-like structure with a hydroxyl group).
What drives the keto-enol tautomerization?
The conversion between the keto and enol forms is driven by the distribution of electron density and the stability of the resulting structures. Factors such as substitution, conjugation, hydrogen bonding, and aromaticity can influence the equilibrium between the two forms.
What are the key factors that affect the keto-enol equilibrium?
The key factors influencing the keto-enol equilibrium include substitution (more substituted alkenes are more stable), conjugation (conjugation with neighboring pi systems stabilizes the enol form), hydrogen bonding (intramolecular hydrogen bonding stabilizes the enol form), and aromaticity (aromatic systems strongly favor the enol form).
How is keto-enol tautomerism influenced by pH?
The pH of the solution can affect the keto-enol tautomerism. Under acidic conditions, protonation can drive the conversion to the enol form, while under basic conditions, deprotonation can favor the keto form. The pH-dependent equilibrium can shift the relative proportions of the keto and enol forms.
Are both keto and enol forms stable?
The stability of the keto and enol forms can vary depending on the specific compound and its structural factors. In general, the keto form is more stable due to the strength of the C=O bond. However, under certain conditions, such as conjugation or hydrogen bonding, the enol form can be stabilized and exist in significant proportions.
How can the keto-enol equilibrium be experimentally determined?
Various experimental techniques can be employed to determine the keto-enol equilibrium. These include spectroscopic methods such as nuclear magnetic resonance (NMR), infrared (IR) spectroscopy, and mass spectrometry. The relative intensities of specific peaks or signals can provide insights into the equilibrium between the two tautomeric forms.
Can keto-enol tautomerism affect the chemical reactivity of a compound?
Yes, keto-enol tautomerism can significantly influence the chemical reactivity of a compound. The different tautomeric forms can exhibit distinct reactivity towards various reactions, such as nucleophilic additions, electrophilic substitutions, and enzyme-catalyzed transformations. The choice of tautomer can impact the reaction pathway and product formation.
Are there any biological implications of keto-enol tautomerism?
Keto-enol tautomerism can have biological implications as it can affect the structure and function of biomolecules. For example, tautomeric forms of nucleic acid bases can impact DNA replication and mutation processes. Understanding the tautomeric equilibria is essential for studying biological systems and designing drugs targeting specific tautomeric forms.
Can keto-enol tautomerism be influenced by external factors?
Yes, external factors such as temperature, solvent polarity, and the presence of catalysts or additives can influence the keto-enol equilibrium. Changes in these factors can alter the stability and relative proportions of the keto and enol forms.
Is keto-enol tautomerism reversible?
Yes, keto-enol tautomerism is a reversible process. The conversion between the keto and enol forms can occur in both directions, depending on the conditions and equilibrium constants. The tautomeric interconversion continues until an equilibrium is reached between the two forms.


