Table of Contents
Lassa virus is an RNA virus that is spread to humans through contact with the urine or feces of an infected Mastomys rat. It is a type of virus known as an arenavirus, and it is endemic in West Africa. The virus is named after the town of Lassa, in Borno State, Nigeria, where it was first identified in 1969.
The tiny (80–200 nm) enclosed virion of the LASV looks to be packed with sand grains. The function, if any, of the host ribosomes is unknown. Their existence resulted in the name Arenaviridae, derived from the Latin word for sand, arenos, for the viral family that contains LASV and other significant human infections, such as lymphocytic choriomeningitis virus.
Symptoms of Lassa fever include fever, weakness, headache, muscle aches, sore throat, and chest and abdominal pain. In severe cases, the virus can cause multi-organ failure and bleeding, leading to death. The virus can also cause deafness in some cases.
There is no specific treatment for Lassa fever, and supportive care is typically the mainstay of treatment. This can include measures such as fluid and electrolyte management, oxygen therapy, and blood transfusions as needed. There are also antiviral medications that can be used to treat the virus, but they are not always effective.
The best way to prevent Lassa fever is to avoid contact with Mastomys rats and to practice good hygiene, such as washing hands frequently and properly disposing of garbage to reduce the risk of exposure to the virus.
The vector of Lassa Virus
- The virus’ natural hosts are multimammate rats (Mastomys natalensis), which reproduce often and are extensively spread in west, central, and eastern Africa.
- Probably the most abundant rodent in tropical Africa, they are primarily found in rural regions, and in homes more frequently than in the surrounding countryside.
- The members of this genus are continuously infected and excrete the virus. Humans get infected by contact with the rats or by eating them (in some regions, up to 90 percent of the population consumes rats as a delicacy).
- Rats in the homes of sick individuals are 10 times more likely to be seropositive for the virus than rats in control homes.
- Those who consume rats are twice as likely to have virus antibodies during a febrile illness as those who do not, and Lassa fever-related deafness occurs four times more frequently.
Structure of Lassa Virus
- Lassa virus is an RNA virus that belongs to the family Arenaviridae. It is an enveloped virus, meaning it has a protective outer layer called the envelope.
- The envelope surrounds the virus’s nucleocapsid, which is a structure that contains the virus’s genetic material.
- The nucleocapsid is made up of the virus’s RNA and proteins called nucleoproteins.
- The envelope of Lassa virus is derived from the host cell’s membrane and contains proteins called envelope glycoproteins. These proteins play a role in the virus’s ability to enter host cells and replicate.
- Lassa virus particles are typically spherical in shape and range in size from 80 to 100 nanometers in diameter. They are generally smaller than other enveloped viruses, such as influenza virus or HIV.
- With the exception of the unexplained presence of ribosomes, LASV appears to be modest. Each virion is composed of two segments of single-stranded RNA, and each segment encodes two proteins.
- The four LASV-encoded proteins are multifunctional. The nucleoprotein (NP) envelopes viral genome segments and is required for transcription of viral mRNAs and replication of genome segments for inclusion into offspring viruses.
- The glycoprotein complex (GPC) mediates the attachment and entrance of viruses into cells. Large (L) is an RNA polymerase that plays a role in transcription and replication.
- Cap-snatching is one of the extra functions of L protein.
- The Zinc-binding (Z) protein functions as the matrix protein and is necessary for viral assembly and budding.
- Z protein negatively regulates viral replication and transcription and is essential for both viral and host cell translation suppression.
Genome of Lassa Virus
- Lassa virus is an RNA virus, which means that its genome is made up of ribonucleic acid (RNA). The RNA of Lassa virus is a single-stranded, negative-sense molecule, which means that it is complementary to the virus’s mRNA (messenger RNA). The Lassa virus genome is approximately 19,000 nucleotides long and is organized into three major segments.
- The first segment, called the small (S) segment, codes for the nucleoproteins and the viral polymerase, which is an enzyme that helps the virus replicate its RNA.
- The second segment, called the medium (M) segment, codes for the viral envelope glycoproteins.
- The third segment, called the large (L) segment, codes for the matrix and membrane glycoproteins and the viral RNA-dependent RNA polymerase.
- The Lassa virus genome is highly variable, with many different strains of the virus existing in nature. This genetic variability is thought to be due in part to the high error rate of the viral polymerase, which can lead to the accumulation of mutations in the viral genome.
- The gene encoding the nucleoprotein is composed of 1,710 nucleotides, and the protein has 569 amino acids.
- The glycoprotein-encoding gene consists of 1,473 nucleotides.
Replications and Cell Entry of Lassa Virus
The Lassa virus (LASV) virion contains host ribosomes and two segments of single-stranded RNA that encode four proteins.
- Steps in LASV replication include: LASV enters cells via receptor-mediated endocytosis (step 1);
- LASV binds α-dystroglycan (α-DG) at the cell surface — as the endosomal pH drops, the LASV glycoprotein complex (GPC) undergoes a conformational shift that enables binding to lysosomal-associated membrane protein 1 (LAMP1) (step 2).
- LASV undergoes pH-dependent membrane fusion releasing the viral genome segments into the cytoplasm (step 3).
- Both segments use an ambisense strategy — early transcription results in the synthesis of the Large (L) protein, an RNA-dependent RNA polymerase and the nucleoprotein (NP), and late transcription additionally involves synthesis of the GPC and the Zinc-binding (Z) protein (step 4).
- GPC translation and post-translational processing occur in the Golgi apparatus and result in association of GPC with the plasma membrane (step 5).
- LASV acquires its membrane by budding at the cell surface (step 6). ER, endoplasmic reticulum; GP1, glycoprotein 1; IGR, inter-gene region; L RNA, large RNA; S RNA, small RNA.
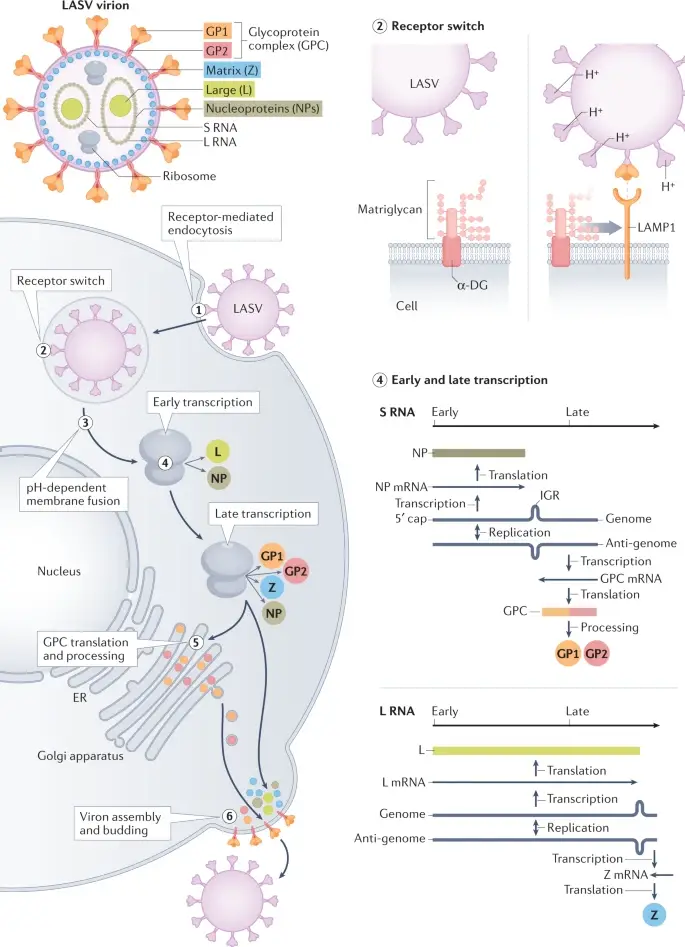
Matriglycan, an extended oligosaccharide situated on the widely distributed peripheral membrane protein α-dystroglycan (α-DG), is the major LASV entrance receptor. Following attachment, LASV virions are absorbed through endocytosis. GPC responds to the acidic endosomal environment by experiencing a conformational change known as “GPC priming,” which results in its separation from α-DG. The activated GPC interacts to the endosomal receptor lysosomal-associated membrane protein 1 (LAMP1). Following LAMP1 binding, GPC undergoes further conformational changes that promote virus–endosomal membrane fusion and allow LASV genome fragments to be released into the cytosol40.
Both parts of the LASV genome are ambisense, meaning that one portion is positive sense, the same sense as mRNA, while the other is negative sense, the complement of mRNA41. The bidirectional transcription is terminated by a stem–loop structure between positive and negative sense genes. The ambisense method helps to regulate the expression of LASV genes and genome replication. L protein’s endonuclease activity cleaves the 5′ ends of cellular mRNAs, which then stimulate the synthesis of mRNAs transcribed utilising the antisense regions of the small and large genome segments as templates. Thus, NP and L proteins are the first viral proteins produced. Through RNA-dependent RNA polymerization, freshly generated NP and L proteins enable the synthesis of complementary strands of the genome segments known as antigenomes. The antigenomes are subsequently used as templates for the mRNAs that code for the GPC precursor and Z protein. Additionally, the antigenomes act as templates for the synthesis of additional genome segments that are integrated into progeny virions.
More than thirty glycans are introduced to GPC trimer of LASV. GPC is disassembled by the host cell subtilase SKI-1/S1P into receptor-binding glycoprotein 1 (GP1), GP2, which is a class I membrane fusion protein, and a myristoylated stable signal peptide (SSP). The viral genome is encased by the amino-terminal domain of NP. Incorporating cleaved glycoproteins into the virion envelope. Z protein regulates the packing and bud formation of infectious particles. The genomic RNA, NP, Z protein, and L protein are assembled and released from the cell membrane.
Epidemiology of Lassa Virus
- Lassa virus is an RNA virus that is endemic in West Africa, where it is the cause of a disease called Lassa fever.
- The virus is transmitted to humans through contact with the urine or feces of infected Mastomys rats, which are common in the region.
- The virus can also be transmitted from person to person through contact with the blood, secretions, or bodily fluids of an infected individual.
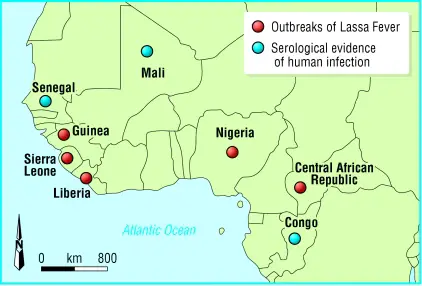
- Lassa fever is most common in Nigeria, where it is estimated to affect between 100,000 and 300,000 people each year. The virus is also found in other West African countries, including Sierra Leone, Liberia, and Guinea.
- Lassa fever is generally more common in rural areas, where people are more likely to come into contact with Mastomys rats. The virus is most active during the dry season, when food is scarce and rats are more likely to venture into homes in search of food.
- Lassa fever is generally a mild to moderate illness, but in severe cases, it can be life-threatening.
- The case-fatality rate for Lassa fever is estimated to be between 1% and 15%, with higher rates in individuals who are pregnant or have underlying health conditions.
Transmission of Lassa Virus
- Human transmission of Lassa virus typically happens through ingestion or inhalation. The virus is transmitted by Mastomys rodents in their urine and droppings, and direct contact with these materials can result in infection through handling filthy things, consuming infected food, or exposure to open wounds.
- Because Mastomys rodents frequently inhabit residential areas and feed on human food scraps or improperly stored food, direct contact transmission is widespread. Mastomys rodents are occasionally used as food, and infection can arise during capture and preparation. A person may also come into contact with the virus by inhaling airborne particles contaminated with rat faeces. This aerosol or airborne transfer may occur during sweeping or other cleaning tasks.
- Person-to-person transmission of Lassa virus is possible after exposure to virus in the blood, tissue, secretions, or excretions of a Lassa virus-infected individual. Lassa virus is not transmitted through casual touch (including skin-to-skin contact without exchange of body fluids). Nosocomial transmission is prevalent in health care settings where personal protective equipment (PPE) is unavailable or not used. The Lassa virus may be transmitted by infected medical supplies, such as overused needles.
Pathogenesis of Lassa Virus
- When commencing an infection, the Lassa virus links the glycoprotein GP-1 to a cell surface receptor.
- It replicates initially in dendritic cells and macrophage-monocyte cells before being distributed throughout the entire body.
- Infected DC are incapable of secreting proinflammatory cytokines, do not upregulate costimulatory molecules such as CD40, CD80, and CD86, and promote T cell proliferation weakly.
- The Lassa virus inhibits the innate immune system of its host via NP activity.
- LASV-infected patients produce the antibody isotypes IgM and IgG.
- Typically, neutralising antibodies arise months after an acute infection has cleared up, and their titers are modest.
- Even several months after convalescence has been established, neutralising antibody titers continue to rise, which may imply ongoing activation of B cells due to low levels of virus persistence.
- Antibodies in seroconverted people are specific to GPC, NP, and maybe Z protein.
Clinical manifestations of Lassa Virus
- Typically, the onset of Lassa fever symptoms occurs between one and three weeks after exposure to the virus.
- Approximately 80% of Lassa fever virus infections are characterised by mild, unexplained symptoms. Among the mild symptoms are a little fever, general malaise and lethargy, and a headache.
- In 20% of infected persons, the condition may proceed to more severe symptoms, including as bleeding (in the gums, eyes, or nose), respiratory trouble, frequent vomiting, face swelling, chest, back, and abdominal discomfort, and shock.
- Neurological issues, such as hearing loss, tremors, and encephalitis, have also been recorded. Multi-organ failure can result in death within two weeks of symptom onset.
- Deafness is the most prevalent consequence of Lassa fever. Approximately one-third of infections result in varying degrees of deafness, and in many cases, hearing loss is permanent. As far as is known, the disease’s severity has no effect on this complication: deafness can develop in both moderate and severe cases.
- 15 to 20 percent of hospitalised individuals with Lassa fever die from the disease. However, just one percent of Lassa virus infections are fatal.
- The death rates for pregnant women in their third trimester are very high. There is an estimated 95% mortality rate among foetuses of infected expectant moms whose pregnancies end in spontaneous abortion.
- Given that the symptoms of Lassa fever are so diverse and ambiguous, clinical diagnosis is frequently challenging. Occasionally, Lassa fever is connected with outbreaks in which the case-fatality rate among hospitalised patients might exceed 50 percent.
The most common symptoms of Lassa fever include:
- Fever
- Weakness
- Headache
- Muscle aches
- Sore throat
- Chest and abdominal pain
In severe cases, the virus can cause multi-organ failure and bleeding, leading to death. The virus can also cause deafness in some cases.
The symptoms of Lassa fever can be similar to those of other viral illnesses, such as influenza or dengue fever, so it is important to seek medical attention if you suspect you may have been exposed to the virus. A healthcare provider can order tests to confirm the diagnosis of Lassa fever.
Diagnosis of Lassa Virus
There are several methods that can be used to diagnose Lassa fever, including:
- Enzyme-linked immunosorbent assay (ELISA): This test detects antibodies to the Lassa virus in the blood. It is generally used to confirm the diagnosis of Lassa fever after the virus has been detected by other means.
- Polymerase chain reaction (PCR): This test detects the genetic material of the Lassa virus in samples of blood or other bodily fluids. PCR is highly sensitive and can detect the virus even in low concentrations.
- Virus isolation: This test involves attempting to grow the virus in cell culture. It is generally used to confirm the diagnosis of Lassa fever when other tests are negative or inconclusive.
- Serological tests: These tests detect antibodies to the Lassa virus in the blood. They can be used to confirm the diagnosis of Lassa fever or to determine whether an individual has been exposed to the virus in the past.
It is important to seek medical attention if you suspect you may have been exposed to the Lassa virus. A healthcare provider can order the appropriate tests to confirm the diagnosis and determine the appropriate course of treatment.
Treatment of Lassa Virus
- Ribavirin, an antiviral medication, has been successfully administered to individuals with Lassa fever. It has been demonstrated to be most effective when administered early in the disease’s progression.
- Patients should also get supportive care, which includes the maintenance of an appropriate fluid and electrolyte balance, oxygenation, and blood pressure, as well as the treatment of any other infections that may be aggravating.
Prevention and control of Lassa Virus
There are several measures that can be taken to prevent and control the spread of Lassa fever:
- Rat control: One of the most effective ways to prevent Lassa fever is to reduce the population of Mastomys rats, which are the main vector for the virus. This can be achieved through the use of rat traps and the proper disposal of garbage, which can attract rats.
- Personal protective measures: To reduce the risk of exposure to the virus, it is important to practice good hygiene, such as washing hands frequently and avoiding contact with the blood or bodily fluids of infected individuals. It is also important to avoid sharing needles or other medical equipment.
- Isolation and quarantine: To prevent the spread of Lassa fever, infected individuals should be isolated and their contacts should be quarantined to prevent further transmission of the virus.
- Supportive care: The mainstay of treatment for Lassa fever is supportive care, which can include measures such as fluid and electrolyte management, oxygen therapy, and blood transfusions as needed.
- Antiviral medications: There are antiviral medications that can be used to treat Lassa fever, but they are not always effective.
It is important to seek medical attention if you suspect you may have been exposed to the Lassa virus. A healthcare provider can provide guidance on the appropriate course of treatment and precautions to take to prevent further transmission of the virus.
I hope this information helps! Let me know if you have any other questions.
References
- Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003 Nov 29;327(7426):1271-5. doi: 10.1136/bmj.327.7426.1271. Erratum in: BMJ. 2004 Jan 10;328(7431):96. PMID: 14644972; PMCID: PMC286250.
- Garry, R.F. Lassa fever — the road ahead. Nat Rev Microbiol (2022). https://doi.org/10.1038/s41579-022-00789-8
- Grant, D. S., Khan, H., Schieffelin, J., & Bausch, D. G. (2014). Lassa Fever. Emerging Infectious Diseases, 37–59. doi:10.1016/b978-0-12-416975-3.00004-2
- Frame JD, Baldwin JM Jr, Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970 Jul;19(4):670-6. doi: 10.4269/ajtmh.1970.19.670. PMID: 4246571.
- https://www.medicalnewstoday.com/articles/306886
- https://openwho.org/courses/lassa-fever-introduction
- https://africacdc.org/disease/lassa-fever/
- https://www.who.int/news-room/fact-sheets/detail/lassa-fever
- https://www.sciencedirect.com/topics/immunology-and-microbiology/lassa-virus
- https://www.cdc.gov/vhf/lassa/index.html#:~:text=Lassa%20fever%20is%20an%20animal,vector%20lives%20throughout%20the%20region.
- https://www.who.int/health-topics/lassa-fever#tab=tab_1
