Table of Contents
What is Liposome?
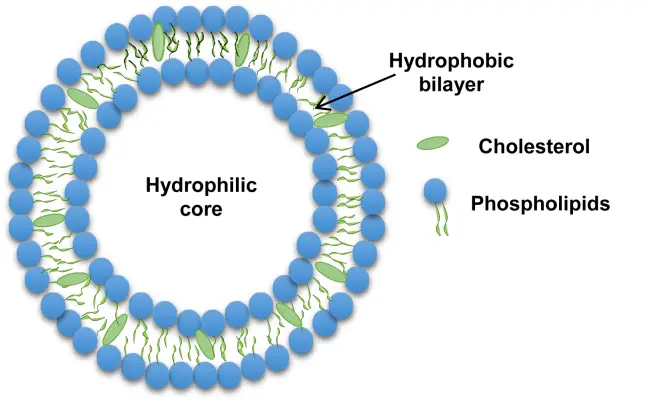
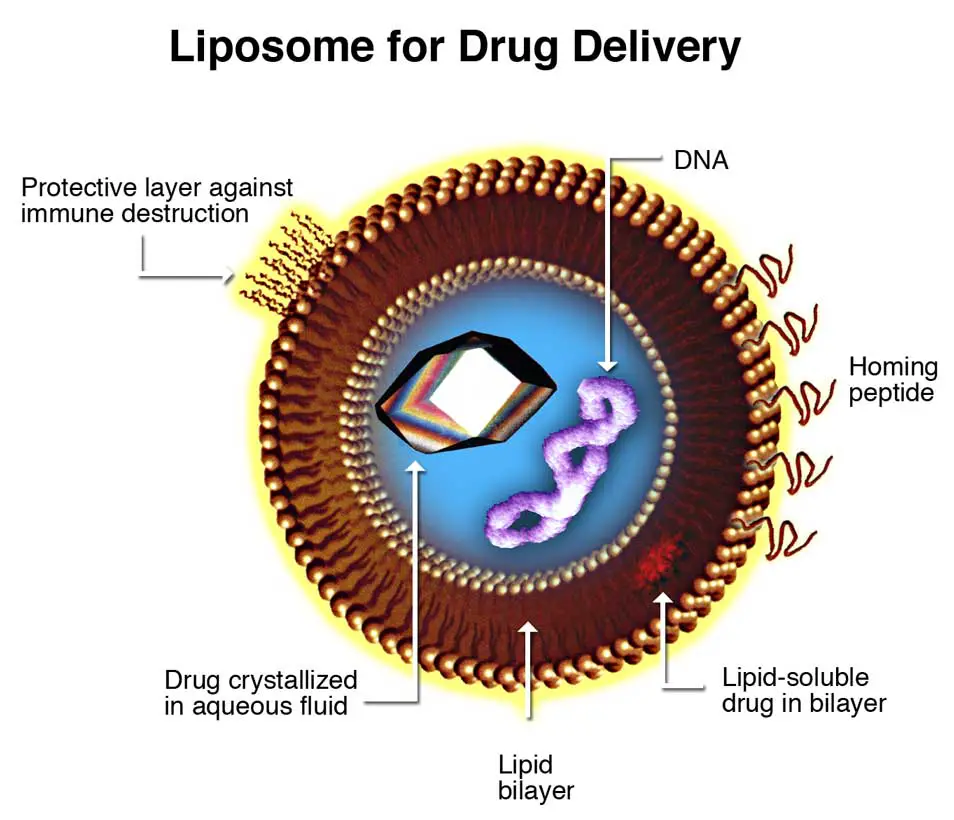
Liposomes, derived from the Greek words “lipo” (fat) and “soma” (body), are spherical vesicles characterized by at least one lipid bilayer. These structures are primarily composed of phospholipids, notably phosphatidylcholine, and often incorporate cholesterol. Other lipids, such as those present in eggs and phosphatidylethanolamine, can also be integrated, provided they are congruent with the bilayer structure. The inclusion of surface ligands in liposome design can further enable their attachment to specific tissues.
The genesis of liposomes can be traced back to 1961 when British haematologist Alec Douglas Bangham, along with colleagues, observed these structures while experimenting with dry phospholipids under an electron microscope. This discovery provided the first tangible evidence supporting the bilayer lipid structure of the cell membrane. Bangham, in collaboration with Standish and Weissmann, further elucidated the closed bilayer nature of liposomes and their ability to release contents upon exposure to detergents. The term “liposome” was coined during a discussion between Weissmann and Bangham, drawing inspiration from the lysosome, a cellular organelle.
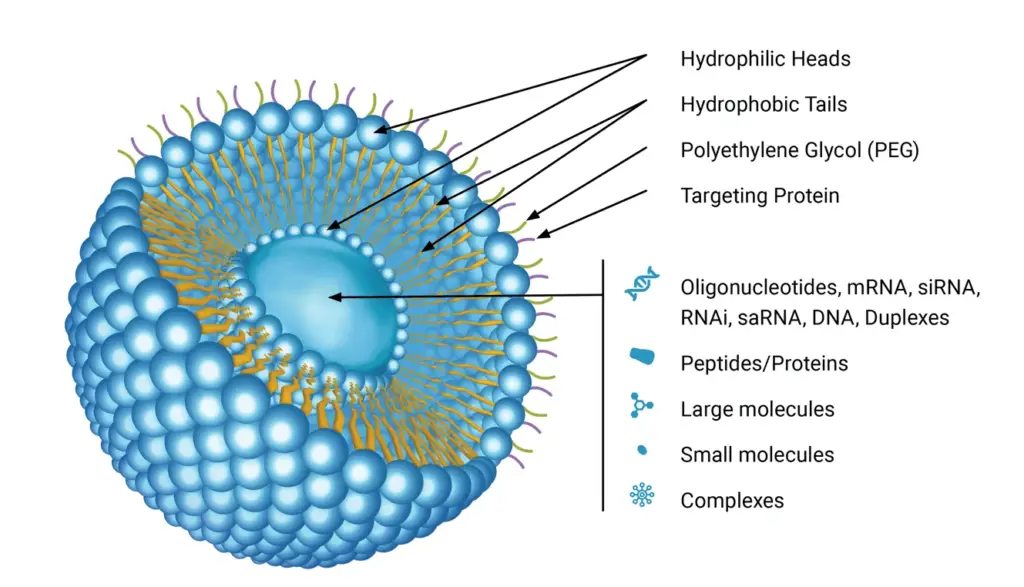
Liposomes exhibit a unique architecture, with their lipid bilayers forming a closed spherical structure that encloses an aqueous cavity. This configuration arises from the amphipathic nature of phospholipids, which possess both hydrophilic (water-attracting) heads and hydrophobic (water-repelling) tails. When these molecules align, the hydrophobic tails converge, while the hydrophilic heads interact with the surrounding aqueous environment, resulting in a bilayer that segregates the internal solution from the external milieu.
Liposomes are distinguishable from micelles, which are also phospholipid spheres but consist of a singular layer and lack an aqueous core. Additionally, liposomes should not be mistaken for lysosomes, cellular organelles equipped with specialized proteins that facilitate their intracellular functions.
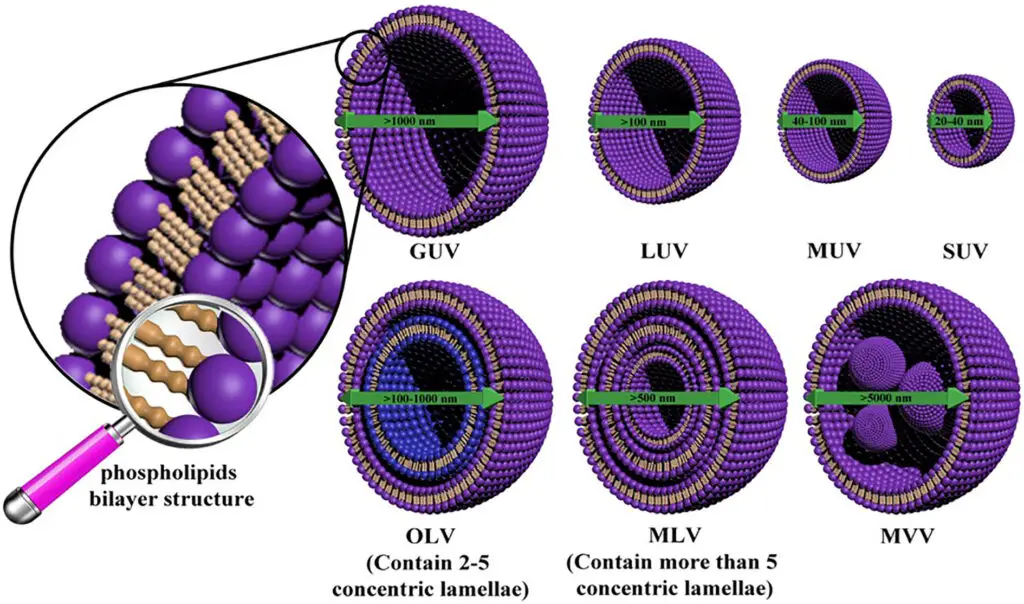
Based on their structural intricacies, liposomes can be categorized into several types:
- Small Unilamellar Vesicles (SUV): Single lipid bilayer structures.
- Large Unilamellar Vesicles (LUV): Single bilayer entities with a larger diameter.
- Multilamellar Vesicles (MLV): Multiple concentric lipid bilayers resembling an onion’s layers.
- Multivesicular Vesicles (MVV): Comprising numerous unilamellar vesicles nested within larger liposomes.
The encapsulation efficiency of liposomes is influenced by their size and the number of bilayers. Typically, for drug delivery applications, the preferred vesicle size ranges between 50 nm to 150 nm. The interaction of liposomes with cell membranes can be governed by various mechanisms, including endocytosis, phagocytosis, and membrane fusion. Factors such as liposome composition, size, surface charge, and the presence of targeting ligands can significantly impact these interactions.
In conclusion, liposomes, with their versatile structure and biocompatibility, have emerged as promising vehicles for the delivery of pharmaceutical agents, nutrients, and genetic material. Their ability to encapsulate and transport aqueous solutions offers immense potential in the realm of therapeutic applications.
Definition of Liposome
A liposome is a spherical vesicle composed of at least one lipid bilayer, primarily used as a vehicle for the delivery of pharmaceutical drugs and nutrients due to its ability to encapsulate and transport aqueous solutions.
Liposomes compositions
Liposomes, by definition, are vesicular structures formed by the self-assembly of diacyl-chain phospholipids in aqueous solutions, resulting in a lipid bilayer. This bilayer is characterized by a hydrophilic head and a hydrophobic tail, giving rise to an amphiphilic nature. The choice of lipids and phospholipids, whether natural or synthetic, plays a pivotal role in determining the properties of the liposome, such as size, stability, fluidity, and electrical charge.
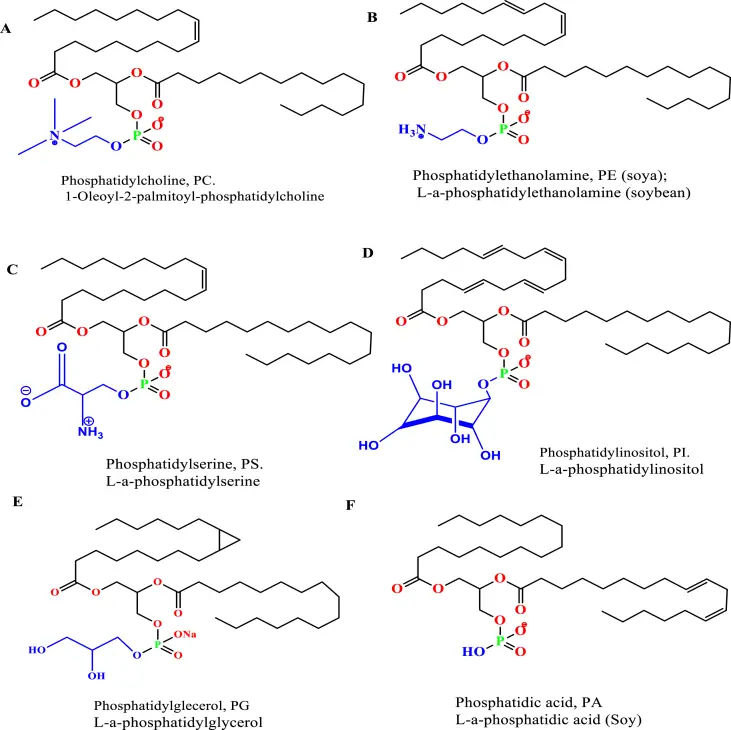
- Natural Lipids: The primary constituents of the membrane bilayer in cells are glycerophospholipids. These phospholipids comprise a glycerol unit linked to a phosphate group and two fatty acid molecules. Depending on the polar head groups, phospholipids can be categorized into phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylglycerol (PG), and phosphatidic acid (PA). Natural sources like soybean and egg yolk are rich in these phospholipids. However, natural phospholipids tend to be less stable than their synthetic counterparts due to the unsaturated nature of their hydrocarbon chain.
- Synthetic Lipids: Synthetic phospholipids are crafted through specific chemical alterations to the polar and non-polar regions of natural phospholipids. This modification allows for a vast array of well-defined phospholipids. Typically, these synthetic lipids are based on stearic or palmitic fatty acids. Furthermore, synthetic phospholipids can be designed with mixed or unsaturated fatty acids.
- Steroids: Steroids, characterized by a four-ring structure, differ based on the functional groups attached to these rings. Cholesterol is a predominant steroid incorporated into liposomes to enhance their rigidity and stability. Its inclusion in the lipid bilayer of liposomes aids in their structural integrity.
- Surfactants: Surfactants are introduced into liposome formulations to modify encapsulation and release properties by reducing surface tension between immiscible phases. These single acyl-chain amphiphiles can destabilize the lipid bilayer, enhancing the deformability of the liposomal nanoparticles. Common surfactants in liposome formulations include sodium cholate, Span 60, and Tween 80. Surfactant-based liposomes, known as transfersomes, have shown promise in transdermal drug delivery due to their increased deformability.
In conclusion, the composition of liposomes, whether derived from natural sources or synthesized, significantly influences their characteristics and potential applications. The integration of various lipids, steroids, and surfactants allows for the tailoring of liposomes to meet specific therapeutic needs.
Liposomes types
Liposomes, as vesicular structures, have garnered significant attention in the realm of drug delivery and therapeutics. Their classification is based on their composition, application, and size. Herein, we delve into the various types of liposomes and their distinctive characteristics.
A. Classification Based on Composition:
- Conventional Liposomes: These are the pioneering generation of liposomes, synthesized from natural or synthetic phospholipids, with or without cholesterol. Cholesterol’s inclusion aims to modulate the bilayer’s fluidity, thereby influencing liposome stability. However, their rapid clearance from the bloodstream and susceptibility to the mononuclear phagocyte system (MPS) limit their efficacy.
- pH-sensitive Liposomes: Comprising pH-responsive lipid components, these liposomes release their contents upon encountering an acidic environment. Their specificity to tissues with a lower pH makes them particularly suitable for targeted cancer treatments.
- Cationic Liposomes: Characterized by their positive charge, these liposomes can associate with negatively charged nucleic acids, making them ideal for gene delivery applications.
- Immuno Liposomes: These liposomes are adorned with antibodies or antibody fragments, enabling them to target specific tissues or tumor cell-specific receptors, enhancing their specificity in drug delivery.
- Long Circulating Liposomes (LCLs): Modified with components like polyethylene glycol (PEG) to evade the reticuloendothelial system (RES), LCLs have an extended circulation time, making them apt for tumor imaging and therapy.
B. Classification Based on Size:
- Multilamellar Vesicles: Resembling an “onion-like” structure, these vesicles consist of multiple phospholipid bilayers and are typically larger than 500 nm. Their high encapsulation capacity for lipophilic drugs and stability make them suitable for targeting the RES.
- Giant Unilamellar Liposomes: Ranging between 0.1-1 µm in size, these liposomes consist of a singular phospholipid bilayer. Their design allows for the encapsulation of hydrophilic materials within their aqueous core.
- Small Unilamellar Liposomes: Often referred to as SUVs, these vesicles are less than 100 nm in diameter. Their thermodynamic instability makes them prone to fusion, and they generally encapsulate smaller molecules.
In conclusion, liposomes, with their diverse types and classifications, offer a versatile platform for drug delivery and therapeutic applications. Their design and composition can be tailored to meet specific therapeutic needs, making them a cornerstone in modern pharmaceutical research.
Methods of liposome preparation
Liposomes, vesicular structures composed of lipid bilayers, have emerged as pivotal entities in drug delivery systems. The methodology employed in their preparation significantly influences their final characteristics, including size, lamellarity, and encapsulation efficiency. This article elucidates various techniques employed in liposome fabrication.
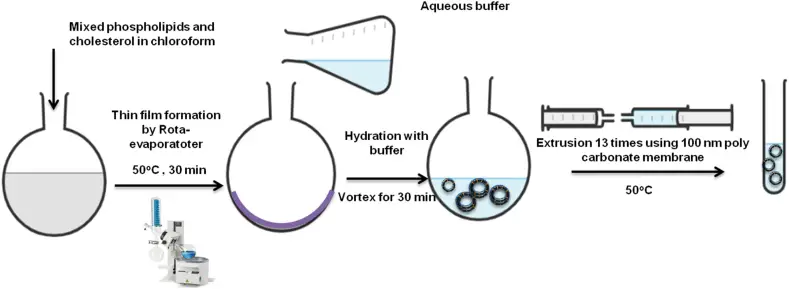
- Thin Film Hydration Method (Bangham Method): This method involves dissolving lipids and hydrophobic drugs in an organic solvent, which is subsequently evaporated under reduced pressure to form a thin lipid film. This film is hydrated using an aqueous buffer, potentially containing a hydrophilic drug. The hydration rate is pivotal, with slower rates leading to higher encapsulation efficiencies. The resultant liposomes can be resized and their lamellarity controlled through extrusion or sonication.
- Reverse-Phase Evaporation Method: Here, lipids are dissolved in an organic solvent, which is then combined with an aqueous buffer containing the hydrophilic drug to form a water-in-oil emulsion. Post solvent evaporation, lipid vesicles are dispersed in the aqueous solution. The vesicle size and distribution can be further refined through extrusion.
- Solvent Injection Methods: In this technique, an organic solvent containing dissolved lipids and hydrophobic agents is rapidly injected into an aqueous phase. Depending on the solvent used, different procedures are employed to evaporate it, leading to the formation of liposomes.
- Detergent Removal Method: Lipids and a high CMC surfactant are dissolved in an organic solvent to form a thin film. Upon hydration, mixed micelles are formed. The surfactant is subsequently removed through various techniques, resulting in the formation of Large Unilamellar Vesicles (LUVs).
- Dehydration-Rehydration Method: This solvent-free method involves dispersing lipids in an aqueous solution containing drug molecules, followed by sonication. A dehydration step is then employed, followed by rehydration to form large vesicles encapsulating the drug molecules.
- Heating Method: Another solvent-free technique, this method involves direct lipid hydration with an aqueous solution, followed by heating. The presence of hydrating agents ensures stability and prevents nanoparticle coagulation.
- pH Jumping Method: In this solvent-free approach, an aqueous solution of phosphatidic acid and phosphatidylcholine undergoes a rapid pH increase, leading to the breakdown of MLVs into SUVs.
- Microfluidic Channel Method: A contemporary method, it involves the dissolution of lipids in ethanol or isopropanol, which is then injected into micro-channels containing an aqueous medium. The continuous mixing of the two phases within the channels results in liposome formation.
- Supercritical Fluidic Method: This innovative method employs supercritical carbon dioxide (CO2) as a solvent for lipids. The dissolved phospholipids undergo a phase transition upon introduction to an aqueous phase, leading to liposome formation upon a rapid pressure decrease.
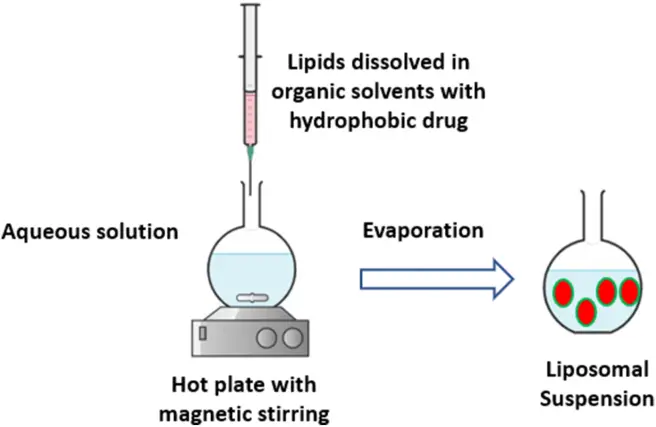
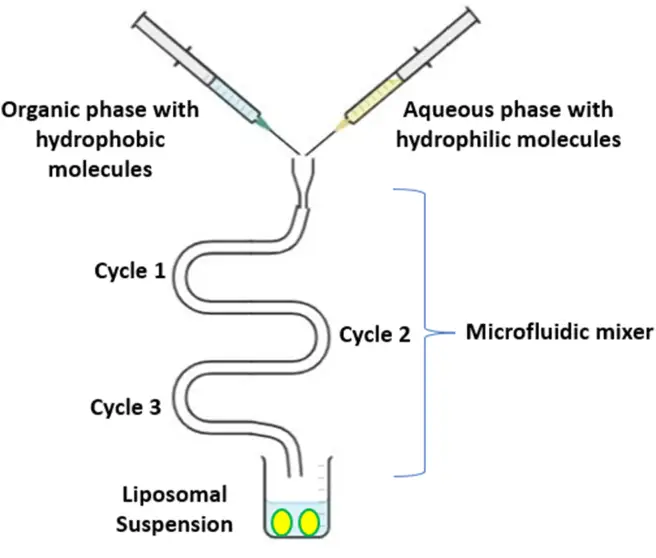
In summation, the choice of liposome preparation method is contingent upon the desired characteristics of the resultant liposomes and the specific application in mind. Each method offers unique advantages and potential limitations, underscoring the importance of method selection in liposome-based drug delivery research.
Post preparation handlings
Once liposomes are prepared, certain post-preparation procedures are often employed to optimize their characteristics and enhance their stability. These treatments can significantly influence the encapsulation efficiency, lamellarity, and shelf life of the liposomal products. Here, we delve into two primary post-preparation handlings:
- Freeze-Thaw Cycles: This method is integral to refining the properties of liposomes post-preparation. By subjecting the liposomes to cycles of freezing and thawing, one can achieve enhanced encapsulation efficiency and improved lamellarity. The procedure typically involves alternating between temperatures as low as -196 °C, using liquid nitrogen, and temperatures slightly below the transition temperature of the incorporated phospholipids. The freeze-thaw cycles serve to reorient the lipid molecules, thereby optimizing the structure and function of the liposomes.
- Freeze-Drying (Lyophilization): To ensure the long-term stability and preservation of liposomal products, freeze-drying is often employed. This process involves the deep freezing of the liposomal suspension, usually after the addition of a cryoprotective agent such as 5–10% sucrose or trehalose. Following this, the frozen samples undergo sublimation under specific conditions: extremely low temperatures and reduced vacuum. This results in the transformation of the liquid liposomal suspension into a dry, powdery form. Notably, lyophilization is particularly crucial for liposomes that encapsulate thermo-sensitive biomolecules, as it ensures their stability and prevents degradation over time.
In conclusion, post-preparation handlings play a pivotal role in ensuring the optimal performance and longevity of liposomal products. By employing techniques like freeze-thaw cycles and lyophilization, researchers can enhance the efficiency and stability of liposomes, making them more suitable for various therapeutic and diagnostic applications.
Liposomes characterization
Liposomes, as vesicular structures composed of lipid bilayers, have been extensively utilized in drug delivery due to their ability to encapsulate both hydrophilic and hydrophobic agents. Their physicochemical properties play a pivotal role in determining their efficacy, stability, and interaction with biological systems. Herein, we delve into the key characteristics of liposomes and the methodologies employed to assess them:
- Average Particle Size:
- Importance: The size of liposomes can influence their biodistribution, cellular uptake, and pharmacokinetics.
- Measurement Techniques: The predominant method for determining the size of liposomes is Dynamic Light Scattering (DLS). Additionally, microscopic techniques, including Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Cryogenic-TEM (Cryo-TEM), and Atomic Force Microscopy (AFM), provide visual insights into the size and structure of these vesicles.
- Zeta Potential/Surface Charge:
- Importance: The surface charge can affect liposome stability, interaction with cells, and overall biodistribution.
- Measurement Techniques: The zeta potential, indicative of the surface charge, is typically measured using electrophoretic mobility and DLS.
- Particle Shape/Morphology:
- Importance: The morphology of liposomes can influence their stability and interaction with biological membranes.
- Measurement Techniques: Advanced microscopic techniques, such as TEM, Cryo-TEM, and AFM, are employed to visualize and determine the shape and structural morphology of liposomes.
- Lamellarity:
- Importance: The number of lipid bilayers (lamellarity) in liposomes can affect their encapsulation efficiency and release profile.
- Measurement Techniques: Cryo-TEM provides visual insights into the lamellarity of liposomes. Additionally, 31P-NMR can be used to determine the arrangement of phospholipids within the vesicles.
- Phase Behavior:
- Importance: The phase behavior of liposomes can influence their stability, encapsulation efficiency, and drug release kinetics.
- Measurement Techniques: Techniques such as X-ray Diffraction (XRD), Differential Scanning Calorimetry (DSC), and Thermogravimetric Analysis (TGA) are employed to study the phase transitions and behavior of liposomal formulations.
- Encapsulation Efficiency/Drug Release:
- Importance: The efficiency with which drugs are encapsulated and subsequently released from liposomes determines their therapeutic efficacy.
- Measurement Techniques: Centrifugation and dialysis are commonly used to separate free drugs from liposome-encapsulated drugs. The drug content
Liposomes drug loading
Liposomal drug loading is a pivotal step in the formulation process, ensuring the effective encapsulation of therapeutic agents within these vesicular systems. The loading techniques can be broadly categorized into passive and active methods, each with its distinct advantages and challenges.
1. Passive Loading: Passive loading is a straightforward approach wherein drugs are encapsulated during the formation of the liposomal bilayer.
- Hydrophilic Drugs: These are entrapped within the aqueous core of the liposomes as the lipid bilayer forms [103].
- Hydrophobic Drugs: These tend to localize within the hydrophobic domain of the lipid bilayer, given their affinity for non-polar environments [187, 188, 189]. However, passive loading can present challenges, such as bilayer destabilization, a high drug-to-lipid ratio, and uncontrolled drug release [101]. To address the issue of hydrophobic drug solubility, cyclodextrin host-guest complexation has been employed. This approach enhances the aqueous solubility of hydrophobic drugs, allowing them to be loaded within the liposome’s aqueous core, leading to the formation of a drug-in-cyclodextrins-in-liposomes system [190].
2. Active (Remote) Loading: Active loading, also known as remote loading, is a more sophisticated approach developed to achieve higher encapsulation efficiencies, especially for valuable chemotherapeutic agents [191].
- Mechanism: This method capitalizes on pH gradients or potential ionic differences across the liposomal bilayer membranes to facilitate drug loading post-liposome formation [101, 187, 192].
- Success Factors: The efficiency of remote loading is contingent upon two primary factors: the drug’s aqueous solubility and the presence of an ionizable functional group within the drug molecule [192, 193, 194].
- Hydrophobic Drug Loading: Active loading techniques have been devised to encapsulate hydrophobic drugs within the liposome’s core, independent of the liposome preparation conditions [101, 194, 195]. This is particularly advantageous as it decouples the drug loading process from the liposome formulation parameters.
- Weak Base Drugs: Many potential therapeutic agents are weak bases, often possessing primary, secondary, or tertiary amine functional groups. These can be actively loaded in response to pH gradients [196]. For drugs that are not weak bases or lack an ionizable functional group, strategies such as conversion to weak base prodrugs or encapsulation with amino-modified carriers like cyclodextrins have been employed. These modifications facilitate drug encapsulation and retention within the liposome [193, 197, 198].
In conclusion, the choice between passive and active drug loading hinges on the physicochemical properties of the drug and the desired therapeutic outcomes. Both methods offer unique advantages, and their judicious application can optimize the therapeutic potential of liposomal drug delivery systems.
Advantages of liposome
- Enhanced Efficacy and Therapeutic Index: Liposomes can enhance the efficacy of certain drugs, such as actinomycin-D, leading to a better therapeutic index.
- Increased Stability: Drugs that are inherently unstable can benefit from encapsulation within liposomes, which can offer a protective environment and enhance their stability.
- Biocompatibility and Safety: Liposomes are recognized for their biocompatibility. They are non-toxic, flexible, and completely biodegradable. Moreover, they are non-immunogenic, making them suitable for both systemic and non-systemic administrations.
- Reduced Drug Toxicity: By encapsulating toxic agents like amphotericin B and Taxol within liposomes, their systemic toxicity can be significantly reduced, leading to safer therapeutic profiles.
- Site Avoidance Effect: Liposomes can help in reducing the exposure of sensitive tissues to toxic drugs, ensuring that the drug is delivered primarily to the target site.
- Active Targeting Potential: The flexibility of liposomes allows them to be coupled with site-specific ligands, facilitating active targeting to specific tissues or cells.
Disadvantages of liposome
- Low Solubility: Some drugs may have solubility issues when encapsulated within liposomes.
- Short Half-life: Despite their protective nature, liposomes might have a short half-life in the bloodstream, necessitating frequent dosing.
- Phospholipid Degradation: The phospholipids constituting the liposomes can sometimes undergo degradation reactions, such as oxidation and hydrolysis, compromising the stability of the formulation.
- Leakage and Fusion Issues: There’s a potential for the encapsulated drug or molecules to leak out of the liposomes or for the liposomes to fuse with other structures, leading to reduced drug delivery efficiency.
- High Production Cost: The sophisticated techniques required for liposome production can lead to higher costs, making the final product expensive.
- Stability Concerns: Some liposomal formulations might be less stable, posing challenges in storage and transportation.
Applications of Liposomes
Liposomes, due to their unique structural and functional attributes, have found a wide range of applications in various fields of science and medicine. Here are some of the prominent applications of liposomes:
- Drug Delivery:
- Targeted Delivery: Liposomes can be engineered to target specific cells or tissues, improving the therapeutic index of drugs and reducing side effects.
- Co-delivery: Liposomes can encapsulate both hydrophilic and hydrophobic agents, allowing for the simultaneous delivery of multiple drugs.
- Controlled Release: The release of drugs from liposomes can be controlled, leading to sustained drug release and prolonged therapeutic effects.
- Gene Therapy:
- Liposomes can encapsulate nucleic acids, such as DNA, RNA, and siRNA, facilitating their delivery into cells for therapeutic purposes.
- Vaccine Delivery:
- Liposomes can act as adjuvants, enhancing the immune response to antigens. They can encapsulate and protect antigens, ensuring their effective delivery to immune cells.
- Diagnostics:
- Liposomes can be loaded with contrast agents and used in imaging techniques like MRI (Magnetic Resonance Imaging) and ultrasound.
- Topical Applications:
- In dermatology and cosmetics, liposomes are used to enhance the penetration of active ingredients into the skin, ensuring deeper and more effective delivery.
- Liposomal Antioxidants:
- Liposomes can encapsulate antioxidants, protecting them from degradation and ensuring their effective delivery to target sites.
- Enzyme Replacement Therapy:
- Liposomes can deliver enzymes to specific organs or tissues, especially useful in treating certain genetic disorders where a particular enzyme is deficient or absent.
- Agriculture:
- In agriculture, liposomes are used to deliver nutrients, herbicides, and pesticides. They ensure the controlled release of these agents, reducing the amount required and minimizing environmental impact.
- Research Tool:
- In cell biology and biochemistry, liposomes are used as model membranes to study membrane dynamics, protein-lipid interactions, and other membrane-associated phenomena.
- Cosmetics and Personal Care:
- Liposomes are used in cosmetic formulations to encapsulate and deliver active ingredients, ensuring deeper skin penetration and prolonged release.
- Nutraceutical Delivery:
- Liposomes can enhance the bioavailability of certain nutrients, ensuring their effective absorption in the gastrointestinal tract.
- Antimicrobial Agents:
- Liposomes can be used to deliver antimicrobial agents, ensuring targeted and effective killing of pathogens.
In conclusion, the versatility of liposomes, combined with their biocompatibility and ability to encapsulate a wide range of molecules, has made them a valuable tool in various scientific and medical fields. As research progresses, it’s likely that new and innovative applications for liposomes will continue to emerge.
Quiz
What are liposomes primarily composed of?
a) Carbohydrates
b) Proteins
c) Phospholipids
d) Nucleic acids
Which of the following is NOT a primary application of liposomes?
a) Drug delivery
b) Gene therapy
c) Enzyme catalysis
d) Vaccine delivery
Which method involves the formation of a thin lipid film for liposome preparation?
a) Reverse-phase evaporation method
b) Solvent injection method
c) Thin film hydration method
d) Detergent removal method
Which of the following techniques is used to determine the average particle size of liposomes?
a) Dynamic light scattering (DLS)
b) X-ray diffraction (XRD)
c) Thermogravimetric analysis (TGA)
d) Electrophoretic mobility
Which property of liposomes allows them to encapsulate both water-soluble and fat-soluble drugs?
a) Biodegradability
b) Amphiphilic nature
c) Positive charge
d) Lamellarity
Which of the following is a disadvantage of liposomes?
a) High solubility
b) Biocompatibility
c) Potential for phospholipid oxidation
d) Increased drug efficacy
Liposomes that are designed for prolonged circulation in the bloodstream are often modified with:
a) Cholesterol
b) Polyethylene glycol (PEG)
c) Oleic acid
d) Cyclodextrin
Which of the following liposomes is characterized by multiple lipid bilayers?
a) Small unilamellar vesicles
b) Giant unilamellar vesicles
c) Multilamellar vesicles
d) Micellar vesicles
Which method for liposome preparation is solvent-free and involves a change in pH to break down MLVs into SUVs?
a) pH jumping method
b) Heating method
c) Dehydration-rehydration method
d) Microfluidic channel method
In which application are liposomes used as model membranes to study membrane dynamics?
a) Drug delivery
b) Vaccine delivery
c) Research tool in cell biology
d) Nutraceutical delivery
FAQ
What are liposomes?
Liposomes are tiny spherical vesicles primarily made up of phospholipids. They have an aqueous core surrounded by one or more lipid bilayers, making them suitable for encapsulating both hydrophilic and hydrophobic substances.
How are liposomes formed?
Liposomes can be formed through various methods, including the thin-film hydration method, reverse-phase evaporation, solvent injection, and detergent removal, among others.
Why are liposomes used in drug delivery?
Liposomes can enhance the therapeutic index of drugs by improving their solubility, stability, and bioavailability. They can also reduce the toxicity of certain drugs and provide targeted delivery to specific cells or tissues.
What is the difference between unilamellar and multilamellar liposomes?
Unilamellar liposomes have a single lipid bilayer surrounding the aqueous core, while multilamellar liposomes consist of multiple concentric lipid bilayers with aqueous spaces in between.
How are liposomes characterized?
Liposomes can be characterized based on their size, surface charge, shape, lamellarity, phase behavior, and encapsulation efficiency using techniques like dynamic light scattering, electron microscopy, and X-ray diffraction.
Are liposomes safe for human use?
Liposomes are generally considered biocompatible, biodegradable, and non-immunogenic, making them suitable for various medical applications. However, the safety profile may vary depending on their composition and the encapsulated substance.
How do liposomes enhance drug stability?
By encapsulating drugs within their bilayers or aqueous core, liposomes can protect them from degradation, thereby increasing their stability.
Can liposomes be used for gene therapy?
Yes, liposomes can encapsulate and deliver nucleic acids to cells, making them potential vectors for gene therapy applications.
What factors affect the drug-loading efficiency in liposomes?
Factors such as the method of preparation, lipid composition, drug-to-lipid ratio, and the physicochemical properties of the drug can influence the encapsulation efficiency in liposomes.
Do liposomes have any limitations?
While liposomes offer several advantages, they also have limitations like potential phospholipid oxidation, drug leakage, high production cost, and stability concerns in some cases.
References
- Rooijen, N. va. (1998). Liposomes. Encyclopedia of Immunology, 1588–1592. doi:10.1006/rwei.1999.0407
- Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022 May 13;8(5):e09394. doi: 10.1016/j.heliyon.2022.e09394. PMID: 35600452; PMCID: PMC9118483.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013 Feb 22;8(1):102. doi: 10.1186/1556-276X-8-102. PMID: 23432972; PMCID: PMC3599573.
