Table of Contents
Nagler Reaction or Lecithinase Test
Nagler’s Reaction or Lecithinase test is a test in biochemistry used to detect organisms that produce the phospholipases (lecithinases) e.g. Clostridium perfringens. Its alpha (a) toxin from C. perfringens exhibits the phospholipase enzyme activity, which aids in the distinction between C. perfringens and the other Clostridium species that generate the enzyme lecithinase (C.baratti, C.absonum, C.bifermantans, C.sordelli, and C.novyi) through neutralization of lecithin C activity with an antitoxin.
Bacillus cereus is also characterized by significant lecithinase activity, however it can be distinguished by its strong hemolytic properties on sheep blood agar as well as mobility. In the Bacillus varieties, B. thuringiensis and B.anthracis are either lecithinase positive, or moderately positive. B. anthracis however is a nonmotile bacterium and is able to produce nonhemolytic colonies.
Objective of Nagler Reaction or Lecithinase Test
- To test the capability of microorganisms to make the enzyme lecithinase.
- To determine which bacteria can produce lecithinase enzyme.
Principle of Nagler Reaction or Lecithinase Test
Bacterial lecithinases are of particular significance due to the possibility of a involvement that these enzymes play in the pathogenicity. Lecithinases and phospholipases are the enzymes produced by bacteria that are capable of eliminate animal tissues. Phospholipids are typically emulsifying agents found in serum, tissues, and egg yolks. Lecithin is an essential ingredient in the yolk of eggs. Bacterial lecithinases breakdown the lecithin into insoluble diglycerides, resulting with an opaque and halo around the colony, when it is grown on egg yolk medium.
The egg’s yolk is agar it is the component of lipoprotein. Lecithovitellin is also split by lecithinase to form phosphorylcholine as well as diglyceride that is insoluble, which leads to an appearance of a precipitate within the medium. This precipitate appears as an opaque white halo surrounding the colony that is producing lecithinase enzyme. The opalescence is caused because of the release fats free. Lecithinase activity is utilized to identify Gram positive and negative bacteria.
Egg Yolk Agar Modified is a differentiated and enriched media used in the separation and presumptive differentiation of various species according to their lecithinase or lipase activity and production. This egg-yolk suspension contained in the medium can be used to determine the presence of the activity of lecithinase as well as lipase in the microorganism. Lecithin’s degradation inside the egg’s yolk leads to the formation of an opaque precipitate that surrounds the colonies. It is the Lipase enzyme hydrolyzes fats contained in the egg yolk that result in an iridescent sheen that appears on the surface of the colony.
Another frequent reaction is the proteolysis process of the egg yolk. This is evident by a clear media around the colonies. Enzymatic digests of casesin and soybean meal provide amino acids, as well as other nitrogenous compounds. It is the main ingredient in the Yeast extract that provides B-complex vitamins. Hemin enhances the growth of microorganisms that live in anaerobic environments. L-cystine is a degrading agent as well as an amino acid.
Lecithinase Producing Organism
Bacillus cereus, a pathogen that is often present in poultry and meat products. It is a very positive and powerful producer of the lecithinase enzyme. It is hemolytic on agar for sheep blood, and has a cellular activity. Other lecithinase lecithinase positive strains could be B. Thuringiensis or B.anthracis. B.anthracis however is a non-motile organism that produces colony that is not hemolytic. Bacillus Sphaericus is a negative maker of Lecithinase.
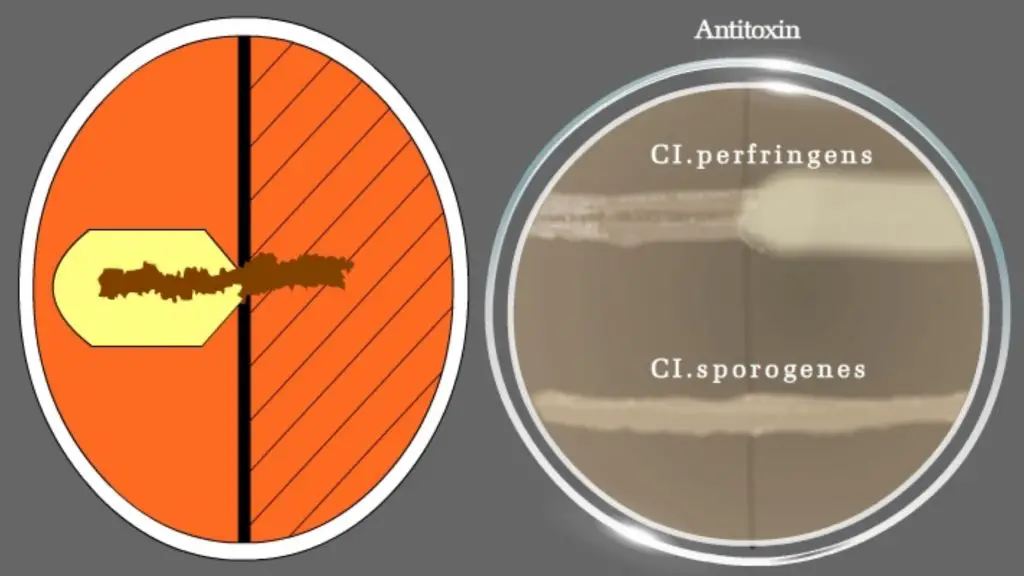
Clostridium perfringens has lecitinase positivity unlike the majority of varieties of Clostridium are lecithinase-negative (Clostridium difficult, Cl.sporogenes). Nagler’s reaction can be used to distinguish the perfringens group from other Clostridia that have lecithinase positivity (eg: Clostridium baratii, Clostridium absonum, Clostridium bifermentans, Clostridium Novyi) by adding an antitoxin specific to neutralizing lecithinase C.
Lecithinase can also distinguish Pseudomonas putida (negative) from Pseudomonas fluorescens (positive). This test is used as a replacement for gelatin hydrolysis.
Identification of Bacillus species using Mannitol Egg Yolk Polymixin agar.
Bacillus cereus is a powerful lecithinase-positive organism is easily identified by Mannitol egg yolk-polymyxin (MYP) Agar plates. The organism is an aerobic spore-producing organism that is often found in soil, as well as in many raw and processed foods, and on vegetables, and is often an agent responsible for food poisoning. MYP Agar contains beef extract and peptone, which are sources of carbon nitrogen, vitamins, and minerals. D-Mannitol provides the source of carbohydrate. The color red of Phenol is used as a indicator of pH. Agar is the agent used to solidify. Egg Yolk Enrichment 50 percent provides lecithin. The antimicrobial ingredient is Polymyxin B which inhibits the growth of many other bacteria. Bacteria that ferment mannitol create acid products and create colonies which are yellow. Lecithinase-producing bacteria hydrolyze the lecithin, and a layer of white precipitate is formed surrounding the colonies. B. cereus, which is generally mannitol-negative and produces colonies of pink-red in MYP Agar, with a layer of precipitate that surrounds the colonies, which indicates lecithinase positive activity.
Bacillus subtilis, also an aerobic spore-producing bacteria, which is not pathogenic to humans. The bacterium ferments mannitol which alters the color the medium to yellow, however it is not equipped with the ability to create the enzyme Lecithinase. Instead, Bacillus subtilis is believed to produce the proteolytic enzyme Subtilisin which can be used in a variety of ways in the leather industry.

Differentiating Clostridium perfringens from other Clostridium species: Naglers reaction
When Clostridia species is detected in a clinical specimen, the egg-yolk agar plates can be used to test for the production of Lecithinase as well as Lipase activity in the suspect sample. AnaeroGRO(tm) Egg Yolk Agar Modified is an enriched non-selective, non-selective and differentiating medium. It can also be employed for the Nagler Test for the presumptive confirmation for Clostridium perfringens. The agar is packaged in an an oxygen reduced, oxygen-free state to stop the development of toxic oxidized byproducts that could harm the anaerobes that are obligate and hinder the growth of the more aggressive species.
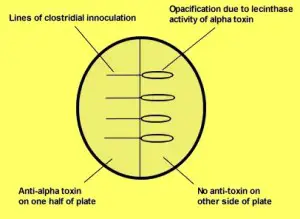
The ingredients in the media neutralize the growth-inhibiting effects of peroxide as well as others reactive oxygen species that could be created when the medium exposes to Oxygen following sterilization, and before being packaged in an Oxygen an oxygen-free environment. (Reducing agents like L- Cysteine). Naglers test is crucial to determine the alpha toxins of Clostridium perfringens. The application of antitoxin in one portion of the egg yolk solution eliminates apparent opacity. This is because of lecithinase activity, which is usually seen in colonies. Other species of Clostridium exhibit negative Naglers reaction (Fig 2.)
To carry in the Naglers reaction, anaerobic and strict conditions must be observed. The Clostridial species to be identified is kept in Thioglycolate broth which is a water-based medium that encourages the growth of oxygen- and anaerobic bacterial species. It is a source of glucose, cystine and sodium thioglycollate, which helps decrease its oxidation-reduction (O/R) capability. It also contains the color known as resazurine which acts as an indicator to detect the presence or absence of oxygen. When oxygen is present, the dye turns pink. Because the oxygen concentration is greater close to the top of the tube the medium will turn pink on top, and colorless at the middle and the bottom. The medium also has an agar-like substance which helps in locating the organisms and promotes anaerobiosis at the lower part in the tube.
Requirements for Nagler Reaction or Lecithinase Test
For Bacillus Species Identification
- Two Nutrient broth tubes are subcultured with Bacillus bacteria.
- Inoculating loop.
- Incubators, (35 ± 1°c).
- Cultures: Bacillus cereus, Bacillus subtilis.
- Medium: Mannitol-egg yolk-Polymyxin (MYP) agar.
For differentiating Clostridium species: Nagler’s reaction
- Thioglycolate broth with inoculated Clostridia species to be differentiated.
- Clostridium perfringens Type A antitoxin.
- Inoculating loop.
- Hockey stick spreader.
- Incubator.
- Medium: AnaeroGRO™ Egg Yolk Agar.
- Cultures: Positive control: Clostridium perfringens, Negative control: Clostridium sporogenes
- Equipment used: Anaerobic gas pak system
Procedure of Nagler Reaction or Lecithinase Test
For Bacillus Species identification
- Mannitol Egg Yolk Polymixin (MYP) Agar is prepared and then sterilized.
- Transfer the MYP Agar into petriplate.
- Dividing the Agar Plate in the three parts equally.
- Transfer a loopful of the culture from the initial nutrient broth of the Bacillus strain suspected. Innoculate a portion of the MYP surface of the agar with an extremely visible circular amount of the organism. Continue to streak the medium until you get isolated colonies.
- Repeat the process for the second Bacillus cultivation in the Nutrient broth. Innoculate the bacteria using a piece of MYP Agar.
- Innoculate broth that is not inoculated (control) on the other side of MYP Agar.
- The plates should be incubated at (35 +/- 1degc) for 24 – 48 hours.
- After 24 to 48 hours, examine the color change within the opacity zone and the medium surrounding colonies. (use the light transmitted to look at the halos)
For differentiating Clostridium species: Nagler’s reaction
- Egg yolk agar plates were made, sterilized, and then aseptically transferred to a sterilized petriplate.
- The plate was split in two parts.
- On one side of the plate, add 60 uls of Clostridium Perfringens antitoxin type A and spread evenly using the spreader of a hockey.
- Allow to dry and absorb and then mark the surface of the plate that has been inoculated with antitoxin.
- Use a test organisms of the Thioglycolate broth, and then streak it in an unidirectional line starting from the antitoxin-free half-way across to the antitoxin portion on the plate.
- Inoculate the organisms that control you using the same dish using the same way.
- Incubate the anaerobes in a gas pak jar as soon as you notice streaking, and then transfer to the incubator at 35-37o C for 24 to 48 hours.
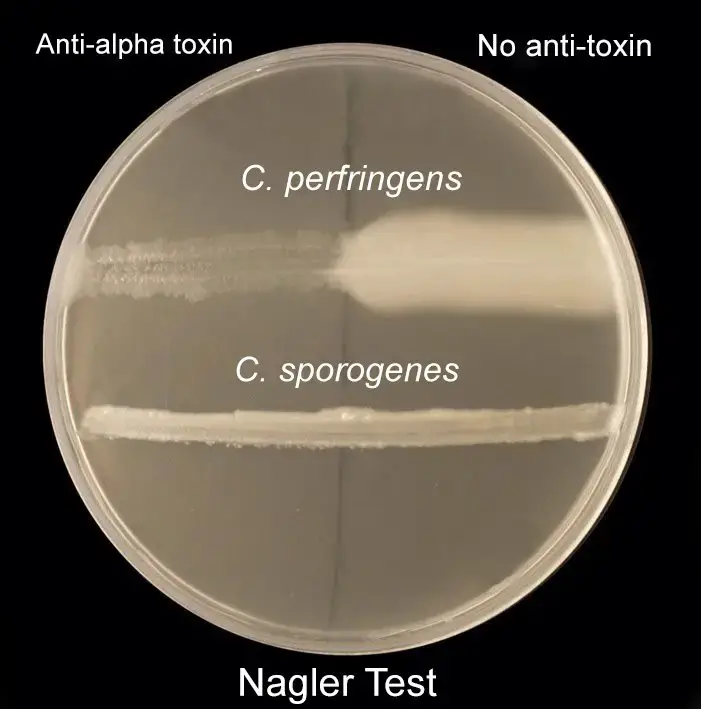
- Check the plate for an Opalescent halo around the inoculum. Also, observe the disappearance of the halo zones on the antitoxin portion on the plate.
Result
For Bacillus Species identification
- Positive test: A positive test shows that the Bacillus colony found on the first plate show pink red without mannitol fermentation. They also show transparent zones of opacity, confirming the activity of lecithinase.
- Negative test: The colonies on another plate, which ferments mannitol and appear yellow and lack Opacity zones.
- Control: No color change.
Gram positive rods with large zones of opacity that are lecithinase positive belong to Bacillus cereus group was confirmed in the first half of the plate and the second half of the plate confirmed the presence of lecithinase negative , mannitol fermenting Bacillus subtilis group.
For differentiating Clostridium species: Nagler’s reaction
- Positive result: Marked decrease in the opacity of the antitoxin portion of the plate as a result of neutralization of lecithin due to the antitoxin.
- Negative result: There is no loss of opacity zones on the antitoxin part on the plate.
Limitations of Lecithinase Test
- Maintaining anaerobic conditions is mandatory.
- A lecithinase test that is negative must be compared with the uninoculated control plate because lecithinase could spread across the entire agar dish and makes interpretation difficult.
- C. perfringens Type A antitoxin isn’t specifically formulated for C. perfringens. However, a positive Nagler reaction may be caused by C. bifermentans, C. sordelli , and C. baratti, if a the use of a large amount of inoculum.
- Non-glucose fermenting rods can give tiny areas of opaqueness.
Quality control
Examine egg yolks for freeze cracks, contamination, cracks and dehydration prior storage and prior to use. Remove the media that is transparent. Conduct QC on each new batch of media before making use of them.
Following strains can be utilized for quality control tests in Nagler’s reaction
- Clostridium perfringens ATCC 13124 – Lecithinase positive
- Clostridium sporogenes ATCC 11437 – Lecithinase negative
- Bacteroides fragilis ATCC 25285 – No activity on agar


