Table of Contents
What is Oxidizing Agent?
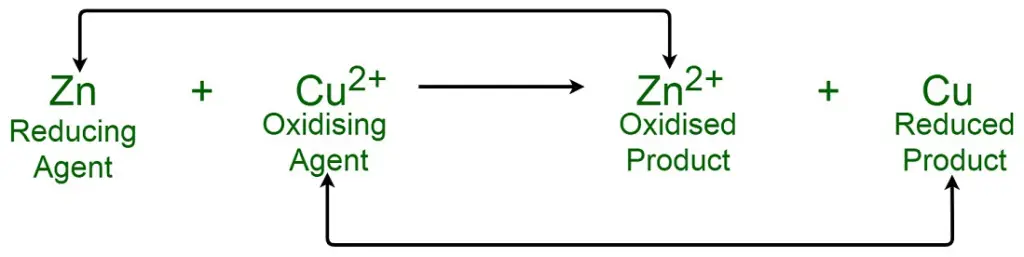
An oxidizing agent, scientifically termed as an oxidant or oxidizer, is a pivotal component in redox (reduction-oxidation) chemical reactions. It functions by accepting or gaining electrons from another substance, known as the reducing agent or reductant. This electron transfer results in the oxidizing agent undergoing a reduction, which is characterized by a decrease in its oxidation state. Conversely, the reducing agent experiences an increase in its oxidation state, signifying its oxidation.
There are two primary definitions that elucidate the role of oxidizing agents:
- Electron Acceptor: In the realm of redox reactions, oxidizing agents are substances that extract one or more electrons from another chemical entity. This electron acceptance leads to the reduction of the oxidizing agent. For instance, when substance ‘A’ is oxidized, its oxidation number amplifies, while substance ‘B’, the oxidizing agent, sees a decrement in its oxidation state due to the gained electrons.
- Atom-Transferring Substance: Oxidizing agents can also be described as substances that confer one or more electronegative atoms, predominantly oxygen, to another chemical species during a reaction. This atom transfer is a cornerstone of many combustion and organic redox reactions. For example, in a given reaction, the molecule Fe2O3 might act as an oxidizing agent by bestowing an electronegative oxygen atom onto a carbon monoxide molecule.
Prominent examples of oxidizing agents encompass halogens like chlorine and fluorine, oxygen, and hydrogen peroxide (H2O2). These agents are quintessential in driving the oxidation of other substances, signified by the loss of electrons from the latter. This electron loss can also be represented by the removal of a hydrogen atom or the incorporation of an oxygen atom.
In summary, an oxidizing agent is a chemical species that plays a dual role: it can either accept electrons or transfer electronegative atoms, primarily oxygen, to other substances. This dual nature underscores its significance in a myriad of chemical reactions, especially in redox processes.
Definition of Oxidizing Agent
An oxidizing agent is a substance that accepts or gains electrons from another substance during a redox (reduction-oxidation) reaction, leading to the oxidation of the latter substance. Common examples include oxygen, halogens, and hydrogen peroxide.
List of Oxidizing Agents
Oxidizing agents are chemical entities characterized by their ability to accept electrons during chemical reactions. These agents play a pivotal role in redox (reduction-oxidation) reactions, facilitating the oxidation of other substances. The inherent electron-accepting nature of certain compounds, including many acids, categorizes them as oxidizing agents. Presented below is a list of notable oxidizing agents:
- Oxygen (O2): A ubiquitous oxidizing agent, oxygen is fundamental to combustion and respiration processes.
- Fluorine (F2): As the most electronegative element, fluorine exhibits a strong tendency to accept electrons, making it a potent oxidizing agent.
- Chlorine (Cl2): Widely used in disinfection and water treatment, chlorine can readily accept electrons, classifying it as an oxidizing agent.
- Nitric Acid (HNO3): A strong acid, nitric acid can act as an oxidizer, especially in reactions where it donates oxygen to other compounds.
- Hydrogen Peroxide (H2O2): This compound is a versatile oxidizing agent, used in various applications from disinfection to bleaching.
It is imperative to note that the realm of chemistry boasts a plethora of oxidizing agents beyond this list, each with its unique properties and applications.
What Factors Affect the Oxidizing Power of an Oxidizing Agent?
Oxidizing agents are chemical entities characterized by their inherent ability to gain electrons, thereby undergoing reduction. The oxidizing power of these agents is influenced by several factors, which determine their efficacy in electron acceptance.
- Oxidation State: Oxidizing agents typically possess the highest feasible oxidation states, which predisposes them to readily accept electrons. The higher the oxidation state, the stronger the oxidizing power.
- Electron Affinity: The propensity of ions, atoms, and molecules to attract and bind with electrons is termed electron affinity. Agents with a pronounced electron affinity are potent oxidizers. The magnitude of this affinity directly correlates with the oxidizing power.
- Electronegativity: Elemental fluorine stands as the epitome of oxidizing agents, largely attributed to its status as the most electronegative element on the periodic table. Its unparalleled electron-attracting capability renders diatomic fluorine (F2) so potent that it can ignite metals like asbestos and quartz upon contact. Similarly, diatomic oxygen (O2) and diatomic chlorine (Cl2), being the elemental forms of other highly electronegative elements, are formidable electron acceptors.
- Standard Electrode Potential: The oxidizing power of a substance can be gauged from its standard electrode potential. A higher positive value indicates a stronger oxidizing agent. This metric offers a comparative measure of the oxidizing prowess of various agents.
- Presence of High Oxidation States: Compounds that can achieve elevated oxidation states tend to be effective oxidizing agents. For instance, ions like permanganate, chromate, and dichromate, as well as acids like nitric acid, perchloric acid, and sulfuric acid, are potent oxidizers. The underlying principle is that as the oxidation state of atoms within these molecules escalates, their electronegativity and, consequently, their oxidizing power also amplify.
In summation, the oxidizing power of an agent is a multifaceted attribute, influenced by its oxidation state, electron affinity, electronegativity, standard electrode potential, and the presence of high oxidation states. Understanding these factors is pivotal in predicting and harnessing the behavior of oxidizing agents in chemical reactions.
Applications of Oxidizing Agents
Oxidizing agents, due to their inherent ability to accept electrons, play a pivotal role in a myriad of commercial, industrial, and biological processes. Their diverse applications are underpinned by their electron-accepting properties, facilitating redox reactions. Herein are some salient applications of oxidizing agents:
- Fabric Bleaching: Oxidizing agents are employed to bleach fabrics, removing unwanted colors and stains, thereby enhancing the fabric’s appearance and quality.
- Water Purification: In water treatment processes, oxidizing agents are utilized to purify water by eliminating contaminants, pathogens, and organic impurities, ensuring safe consumption and usage.
- Fuel Combustion: The combustion of fuels necessitates the presence of an oxidizing agent, typically oxygen, to facilitate the release of energy in the form of heat and light.
- Energy Storage in Batteries: Batteries harness redox reactions, where oxidizing agents play a crucial role in storing and releasing energy, ensuring the efficient functioning of electronic devices.
- Vulcanization of Rubber: Oxidizing agents are instrumental in the vulcanization process, which augments the strength and elasticity of rubber, making it more durable and resilient.
- Biological Processes:
- Metabolism: Oxidizing agents are integral to metabolic processes, where they facilitate the breakdown of nutrients, releasing energy essential for cellular functions.
- Photosynthesis: In plants, oxidizing agents play a role in photosynthesis, aiding in the conversion of light energy into chemical energy.
- Energy Harvesting in Organisms: Many organisms leverage electron acceptors or oxidizers to extract energy from redox reactions, such as during the hydrolysis of glucose.
In summation, oxidizing agents, with their multifaceted applications, are indispensable in various sectors, from industries to biological systems. Their electron-accepting nature underpins their significance, driving processes that are fundamental to modern life and natural ecosystems.
Examples of Oxidizing Agents
Oxidizing agents are chemical entities that exhibit a pronounced propensity to gain electrons, facilitating the oxidation of other substances. Several elements and compounds, due to their inherent chemical properties, serve as potent oxidizing agents. Presented below are some quintessential examples:
- Halogens: Belonging to Group 17 of the periodic table, halogens are renowned for their robust electron-gaining capabilities. This characteristic stems from their elevated electronegativities relative to other elemental groups. Notable halogens that function as effective oxidizing agents encompass:
- Iodine (I2)
- Bromine (Br2)
- Chlorine (Cl2)
- Fluorine (F2): Owing to its unparalleled electronegativity, fluorine is acclaimed as the most potent elemental oxidizing agent.
- Oxygen (O2): Classified under the chalcogen group with the atomic symbol ‘O’, oxygen is a highly reactive non-metal renowned for its oxidizing prowess. Its ubiquity in combustion reactions and its ability to form metal oxides upon reaction with metals underscore its oxidizing potential.
- Hydrogen Peroxide (H2O2): This chemical compound, characterized by its oxygen-oxygen single bond, manifests as a colorless liquid with a viscosity surpassing that of water. Apart from its roles as a disinfectant and bleaching agent, hydrogen peroxide serves as a mild oxidizing agent.
- Additional Oxidizing Agents:
- Household Bleach (NaClO): Commonly utilized for cleaning and disinfection.
- Potassium Nitrate (KNO3): Often employed in the production of fertilizers and fireworks.
- Sulfuric Acid (H2SO4): A strong acid with diverse industrial applications, it also exhibits oxidizing properties under specific conditions.
In essence, the realm of chemistry is replete with a myriad of oxidizing agents, each with distinct properties and applications. Their pivotal role in driving redox reactions underscores their significance in both industrial processes and everyday human activities.
Quiz
What is the primary function of an oxidizing agent in a redox reaction?
a) Donate electrons
b) Accept electrons
c) Release energy
d) Absorb energy
Which of the following is considered the strongest elemental oxidizing agent?
a) Chlorine
b) Oxygen
c) Bromine
d) Fluorine
In the context of oxidizing agents, what does the term ‘reduction’ imply?
a) Loss of electrons
b) Gain of electrons
c) Loss of protons
d) Gain of protons
Which of the following is NOT an application of oxidizing agents?
a) Fabric bleaching
b) Water purification
c) Fuel combustion
d) Sound amplification
Which compound is commonly used as a mild oxidizing agent and also as a disinfectant?
a) Sodium chloride
b) Hydrogen peroxide
c) Methane
d) Nitrogen gas
Which group in the periodic table consists of elements that are commonly known as strong oxidizing agents?
a) Alkali metals
b) Noble gases
c) Halogens
d) Transition metals
In a redox reaction, if the oxidizing agent undergoes reduction, the reducing agent will:
a) Gain electrons
b) Lose electrons
c) Remain unchanged
d) Donate protons
Which of the following is NOT a property of a good oxidizing agent?
a) High electronegativity
b) Ability to accept electrons
c) Tendency to donate electrons
d) Existence in a high oxidation state
Which of the following compounds is used in batteries due to its oxidizing properties?
a) Methanol
b) Sulfuric acid
c) Ethanol
d) Helium gas
In the vulcanization of rubber, which property of rubber is enhanced using oxidizing agents?
a) Color
b) Elasticity
c) Solubility
d) Density
Frequently Asked Questions on Oxidizing Agent
What is an oxidizing agent?
An oxidizing agent is a substance that can accept electrons from another substance during a redox (reduction-oxidation) reaction.
How do oxidizing agents function in redox reactions?
In redox reactions, oxidizing agents undergo reduction by gaining electrons, while causing another substance to lose electrons (oxidation).
Why is fluorine considered the strongest elemental oxidizing agent?
Fluorine is the most electronegative element in the periodic table, giving it a strong tendency to attract and accept electrons, making it a potent oxidizing agent.
Are all halogens good oxidizing agents?
Yes, all halogens are oxidizing agents due to their high electronegativities, but their strength decreases down the group from fluorine to iodine.
What is the role of oxidizing agents in combustion reactions?
In combustion reactions, oxidizing agents, typically oxygen, facilitate the burning process by accepting electrons from the fuel.
Can oxidizing agents be harmful?
Yes, some strong oxidizing agents can be hazardous as they can cause burns, explosions, or support combustion. Proper handling and storage are essential.
How are oxidizing agents used in water purification?
Oxidizing agents like chlorine and ozone are used to disinfect water, killing harmful pathogens and removing impurities.
What is the difference between an oxidizing agent and a reducing agent?
An oxidizing agent gains electrons (undergoes reduction) during a redox reaction, while a reducing agent loses electrons (undergoes oxidation).
Are oxidizing agents always in their highest oxidation states?
Typically, strong oxidizing agents exist in their highest possible oxidation states, making them eager to gain electrons. However, not all oxidizing agents are in their highest oxidation states.
Can hydrogen peroxide act as both an oxidizing and reducing agent?
Yes, hydrogen peroxide (H2O2) can act as both. It can donate electrons, acting as a reducing agent, or accept electrons, acting as an oxidizing agent, depending on the reaction conditions.
