Table of Contents
What is Synapse?
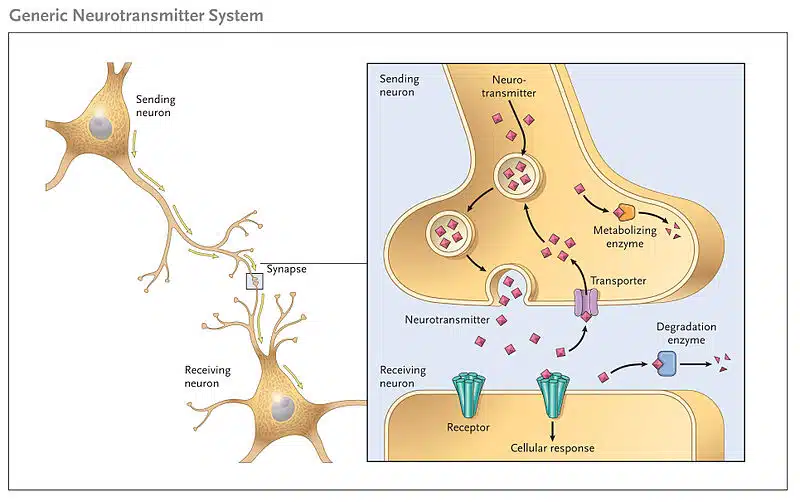
- A synapse is a specialized junction between two neurons or between a neuron and a target cell, such as a muscle cell. It is a fundamental component of the nervous system, enabling the transmission of electrical or chemical signals from one cell to another.
- The synapse consists of two main parts: the presynaptic neuron, which is the signal-sending neuron, and the postsynaptic neuron or target cell, which receives the signal. These two components are separated by a tiny space called the synaptic cleft. The presynaptic neuron releases neurotransmitters into the synaptic cleft, which then bind to specific receptors on the postsynaptic neuron or target cell, initiating a response.
- There are two types of synapses: electrical synapses and chemical synapses. Electrical synapses allow the direct flow of electrical currents between cells through gap junctions, whereas chemical synapses involve the release and binding of neurotransmitters to transmit signals.
- The synapse plays a critical role in the transmission of nerve impulses. When an action potential reaches the axon terminal of the presynaptic neuron, it triggers the release of neurotransmitters into the synaptic cleft. These neurotransmitters diffuse across the cleft and bind to receptors on the postsynaptic neuron or target cell, leading to the generation of a new electrical signal or the modulation of cellular activity.
- The precise structure and function of synapses are complex and involve intricate molecular machinery. Both presynaptic and postsynaptic sites contain specialized proteins and molecules that facilitate the transmission and reception of signals. Additionally, synaptic adhesion molecules help stabilize synapses and contribute to their formation and function.
- The concept of the synapse was proposed by Santiago Ramón y Cajal, who suggested that neurons are discrete units communicating with each other. The term “synapse” was introduced by Charles Sherrington in 1897 and originates from the Greek word “synapsis,” meaning “conjunction” or “together.”
- The development of electron microscopy in the 1950s allowed for the visualization of the synaptic structure in detail, revealing the parallel membranes and the synaptic cleft that separate the presynaptic and postsynaptic elements.
- In summary, a synapse is a specialized connection between neurons or a neuron and a target cell. It enables the transmission of signals within the nervous system, playing a vital role in various processes such as learning, memory, and motor function. The synapse is a remarkable structure that allows for the precise and efficient communication between cells in the complex network of the nervous system.
Definition of Synapse
A synapse is a junction or connection between two neurons or a neuron and a target cell, allowing the transmission of electrical or chemical signals.
Synapse and Neurotransmitters
Synapses and neurotransmitters play a crucial role in neuronal communication. Neurotransmitters are chemicals that are released from neurons in response to an action potential. These neurotransmitters traverse the synapse, the junction between two neurons, and interact with receptors on the target neuron, either exciting or inhibiting its activity.
Neurotransmitters act as messengers, transmitting nerve impulses from the presynaptic membrane to the postsynaptic membrane in the receiving neuron. Different types of neurons utilize distinct neurotransmitters, leading to diverse effects on their target cells.
There are two main categories of neurotransmitters: inhibitory neurotransmitters and excitatory neurotransmitters. Inhibitory neurotransmitters, such as GABA and glycine, suppress the activity of the postsynaptic neuron, making it less likely to generate an action potential. On the other hand, excitatory neurotransmitters, including glutamate, acetylcholine, and aspartate, enhance the activity of the postsynaptic neuron, increasing its chances of firing an action potential.
The balance between inhibitory and excitatory neurotransmission is vital for maintaining proper neuronal function and overall brain activity. Imbalances in neurotransmitter levels or dysregulation of their actions can lead to various neurological disorders and conditions. Understanding the role of synapses and neurotransmitters provides valuable insights into the complex workings of the nervous system.
Excitatory And Inhibitory Postsynaptic Potentials
- Excitatory and inhibitory postsynaptic potentials (EPSP and IPSP) are crucial in determining the response of the postsynaptic neuron to signals from the presynaptic neuron.
- Neurotransmitters, the chemical messengers released by neurons, play a key role in these processes. Inhibitory neurotransmitters, such as serotonin, have a calming effect on the postsynaptic neuron, decreasing its likelihood of firing and promoting relaxation and sleep. On the other hand, excitatory neurotransmitters like adrenaline increase the chances of an excitatory signal being sent to the postsynaptic cell, stimulating activity.
- When the presynaptic neuron releases neurotransmitters that excite the postsynaptic neuron, it leads to an excitatory postsynaptic potential (EPSP). This depolarizes the postsynaptic cell, making it more likely to generate an action potential and propagate signals further. In contrast, inhibitory neurotransmitters binding to the postsynaptic receptors result in an inhibitory postsynaptic potential (IPSP). This hyperpolarizes the postsynaptic cell, reducing its firing likelihood and promoting inhibition.
- The firing rate of the postsynaptic neuron depends on the balance between excitatory and inhibitory inputs received from synapses on its dendrites and soma. If excitatory synapses are more active, the axon of the postsynaptic neuron will fire at a higher rate. Conversely, if inhibitory synapses are dominant, the firing rate may decrease or cease altogether.
- EPSPs contribute to the depolarization of the neuron, increasing the likelihood of action potential generation. IPSPs, on the other hand, have an inhibitory effect by reducing the membrane potential, making it less likely for the neuron to reach the threshold for firing. The interplay between EPSPs and IPSPs determines the net effect on the postsynaptic neuron, allowing for fine-tuned modulation and regulation of neural signaling.
- The balance and integration of excitatory and inhibitory postsynaptic potentials are essential for proper neuronal function and information processing within the nervous system.
Structure of Synapse
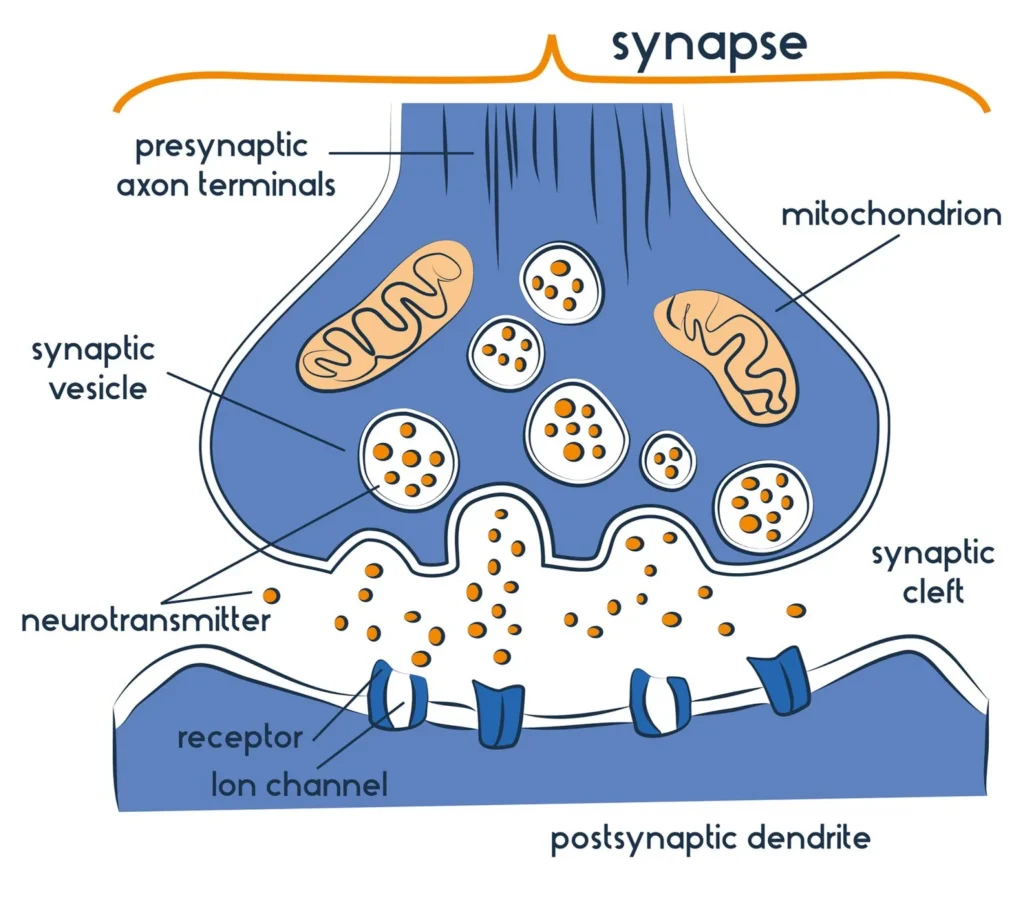
- The structure of a synapse consists of several key components that facilitate the transmission of signals between neurons.
- At the synapse, there is a presynaptic neuron, a postsynaptic neuron, and a small space known as the synaptic cleft that separates them. The presynaptic neuron stores and releases neurotransmitters, which are the chemical messengers involved in transmitting signals. The postsynaptic neuron receives these neurotransmitters.
- Within the presynaptic neuron’s axon terminal, there are various vesicles responsible for neurotransmitter storage and release. Agranular vesicles, also known as synaptic vesicles, contain small molecule neurotransmitters like glycine and glutamate. These vesicles are spherical, typically measuring 35-50 nm in diameter. In contrast, granular vesicles are larger, with a diameter of about 80 nm to 160 nm, and typically contain neuropeptides. The movement of synaptic vesicles towards the presynaptic membrane, where they fuse and release neurotransmitters, relies on actin filaments and regulatory proteins such as synapsin I.
- The presynaptic terminal also contains proteins like fodrin and microtubules, along with F-actin and synapsin. These components contribute to the structural integrity and functional regulation of the presynaptic terminal. The specific region where neurotransmitter release occurs is known as the active zone, characterized by a dense collection of proteins at the membrane.
- On the postsynaptic side, there is a higher concentration of receptor proteins or ligand-gated ion channels. These receptors bind with neurotransmitters released by the presynaptic neuron, leading to the flow of ions into the postsynaptic neuron. This ion flow results in the generation of postsynaptic potentials, either excitatory postsynaptic potentials (EPSP) or inhibitory postsynaptic potentials (IPSP), depending on the neurotransmitter involved.
- For example, in excitatory synapses using glutamate as the neurotransmitter, the binding of glutamate to its corresponding receptors triggers the opening of ion channels, allowing positive ions to flow into the postsynaptic cell. In inhibitory synapses, the binding of inhibitory neurotransmitters to their receptors leads to the opening of ion channels that allow the flow of negative ions or inhibit the flow of positive ions.
- Overall, the structure of the synapse and the interaction between neurotransmitters and receptors play a critical role in facilitating communication between neurons and regulating the flow of signals within the nervous system.
A synapse is a combination of the following
A synapse is a combination of several elements that facilitate communication between neurons. These elements include:
- Presynaptic endings: These are found at the terminal branches of the axon of the presynaptic neuron. They contain neurotransmitters, which are chemical messengers responsible for transmitting signals. When an action potential reaches the presynaptic endings, neurotransmitters are released into the synaptic cleft.
- Synaptic cleft: This refers to the small gap or space between the presynaptic and postsynaptic neurons. It serves as the physical barrier that separates the two neurons.
- Postsynaptic endings: These are located on the dendrites or cell body of the postsynaptic neuron. They contain receptors, which are molecules that receive the signals transmitted by neurotransmitters. The binding of neurotransmitters to these receptors initiates a response in the postsynaptic neuron.
The synapse not only allows the transmission of signals from the presynaptic neuron to the postsynaptic neuron but also enables communication in the reverse direction. Postsynaptic neurons have the ability to send feedback signals back to the presynaptic neuron, influencing the release of neurotransmitters. This bidirectional communication allows for dynamic modulation and regulation of synaptic activity.
For example, if the postsynaptic neuron detects an excessive or insufficient response to neurotransmitters, it can send signals back to the presynaptic neuron, modifying the release of neurotransmitters. This feedback mechanism helps maintain the balance and efficacy of synaptic communication.
In summary, a synapse encompasses presynaptic endings, synaptic clefts, and postsynaptic endings. It facilitates the transmission of signals between neurons and allows for bidirectional communication, enabling modulation and adjustment of neurotransmitter release.
Properties of Synapse
Synapses possess several distinct properties that contribute to their role in neural communication. Here are some key properties of synapses:
- One-way conduction: Synapses allow impulses to travel in one direction only, typically from the presynaptic neuron to the postsynaptic neuron. This ensures the proper flow of information in neural circuits.
- Synaptic delay: There is a slight delay in transmitting impulses across synapses, known as synaptic delay. This delay occurs due to processes such as neurotransmitter release, diffusion, and receptor binding. The synaptic delay ensures that the transmission of signals is coordinated and allows for the integration of information from multiple sources.
- Fatigue: Synapses can experience fatigue during prolonged or intense activity. Similar to Betz cells in the motor area of the cerebral cortex, synapses can become fatigued due to the depletion of neurotransmitters like acetylcholine. Fatigue at synapses can contribute to a decline in neural function during extended periods of activity.
- Electrical properties: Synapses exhibit electrical properties known as inhibitory postsynaptic potentials (IPSPs) and excitatory postsynaptic potentials (EPSPs). IPSPs hyperpolarize the postsynaptic membrane, making it less likely to generate an action potential. EPSPs, on the other hand, depolarize the postsynaptic membrane, increasing the likelihood of an action potential.
- Summation: Synaptic summation refers to the combined effects of multiple EPSPs or IPSPs on the postsynaptic neuron. When multiple presynaptic excitatory terminals are stimulated simultaneously or when a single presynaptic terminal is repeatedly stimulated, the effects of the individual EPSPs can summate. This summation can lead to a gradual increase in the overall EPSP, influencing the likelihood of an action potential being generated in the postsynaptic neuron.
These properties of synapses are essential for the proper transmission and integration of signals within the nervous system. They contribute to the precision, coordination, and modulation of neural communication, allowing for complex information processing and response generation.
Types of Synapse
Types of Synapse Based on anatomy
Synapses can be classified into different types based on their anatomy and function. Let’s explore each classification:
- Axoaxonic synapse: In this type of synapse, the axons of two different neurons come into contact with each other. This interaction allows for the modulation of neurotransmitter release from the presynaptic neuron. Axoaxonic synapses play a role in regulating synaptic transmission by influencing the amount of neurotransmitter released.
- Axodendritic synapse: Here, the axon of one neuron terminates on the dendrite of another neuron. This is the most common type of synapse in the nervous system. Axodendritic synapses are involved in transmitting signals from one neuron to another, allowing for the integration and processing of information.
- Axosomatic synapse: In this type of synapse, the axon of one neuron attaches to the soma or cell body of another neuron. Axosomatic synapses can have a direct impact on the firing of the postsynaptic neuron by influencing its excitability and overall activity.
Types of Synapse Based on function or physiology
Classification based on function or physiology:
- Electrical synapses: These synapses are characterized by the direct flow of electrical current between the cytoplasm of two connected neurons. Gap junctions, specialized protein channels, allow for the passage of ions and small molecules, enabling rapid and synchronized communication between neurons. Electrical synapses facilitate the rapid transmission of signals, particularly in cases where synchronization and coordination of activity are crucial, such as in reflex arcs.
- Chemical synapses: This is the most common type of synapse in the nervous system. Chemical synapses rely on the release of neurotransmitters into the synaptic cleft to transmit signals between neurons. When an action potential reaches the presynaptic terminal, neurotransmitters are released and bind to specific receptors on the postsynaptic neuron, leading to changes in its membrane potential and subsequent signal transmission. Chemical synapses allow for a wide range of modulation and fine-tuning of synaptic communication.
Chemical Synapse and Its Functions
Chemical synaptic transmission is a fundamental process in neural communication, predominantly observed in human beings. It relies on the release and binding of neurotransmitters to facilitate the transmission of signals between neurons.
The process begins with the generation of electrical activity in the presynaptic neuron. This electrical activity triggers the opening of voltage-gated calcium channels in the presynaptic membrane. The influx of calcium ions (Ca² +) into the presynaptic neuron initiates a series of events.
Within the presynaptic neuron, specialized structures called synaptic vesicles, which contain neurotransmitters, undergo fusion with the presynaptic membrane. This fusion allows the neurotransmitters to be released into the synaptic cleft, the small gap between the presynaptic and postsynaptic neurons.
The neurotransmitter molecules diffuse across the synaptic cleft and bind to specific receptors located on the membrane of the postsynaptic neuron. The binding of neurotransmitters to their receptors triggers a cascade of biochemical reactions within the postsynaptic neuron.
Depending on the type of neurotransmitter and receptor involved, the postsynaptic neuron can be either excited or inhibited. Excitation occurs when the binding of neurotransmitters leads to the opening of ion channels in the postsynaptic membrane. This allows the influx of positively charged ions, resulting in depolarization of the postsynaptic neuron and the generation of an action potential.
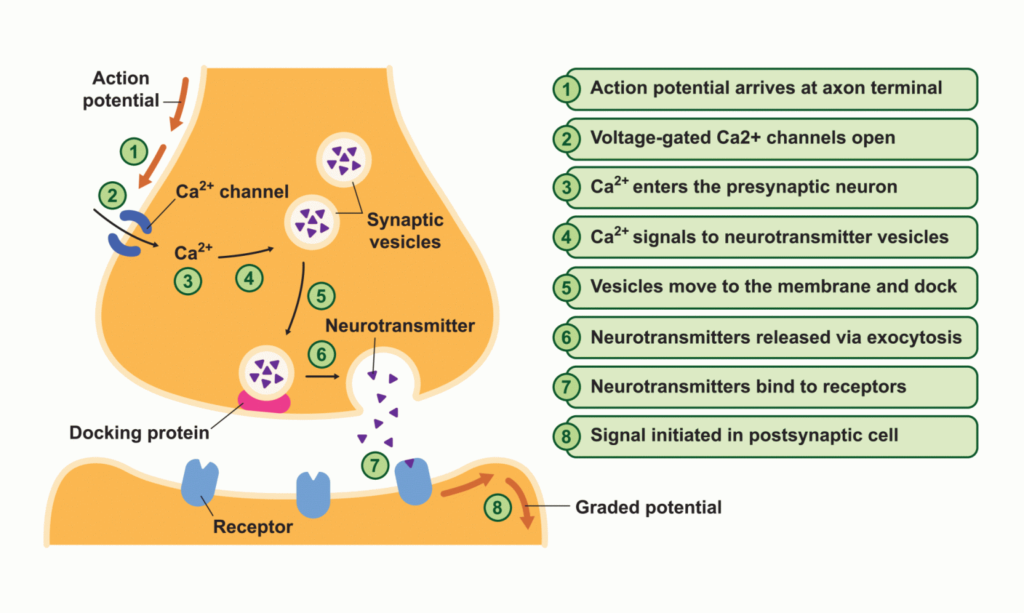
Conversely, inhibition occurs when neurotransmitters cause the opening of ion channels that allow the influx of negatively charged ions or the efflux of positively charged ions. This leads to hyperpolarization of the postsynaptic neuron, making it less likely to generate an action potential.
The balance between excitatory and inhibitory inputs from multiple synapses determines the overall response of the postsynaptic neuron. By integrating and processing these signals, the postsynaptic neuron contributes to the complex network of neural communication.
Chemical synaptic transmission plays a crucial role in various cognitive and physiological processes, including sensory perception, motor coordination, memory formation, and emotional regulation. Its intricate mechanisms enable the precise and dynamic modulation of neural activity, contributing to the complexity of the human nervous system.
A chemical synapse plays a crucial role in transmitting nerve impulses between neurons. Let’s explore the functions and processes involved in a chemical synapse:
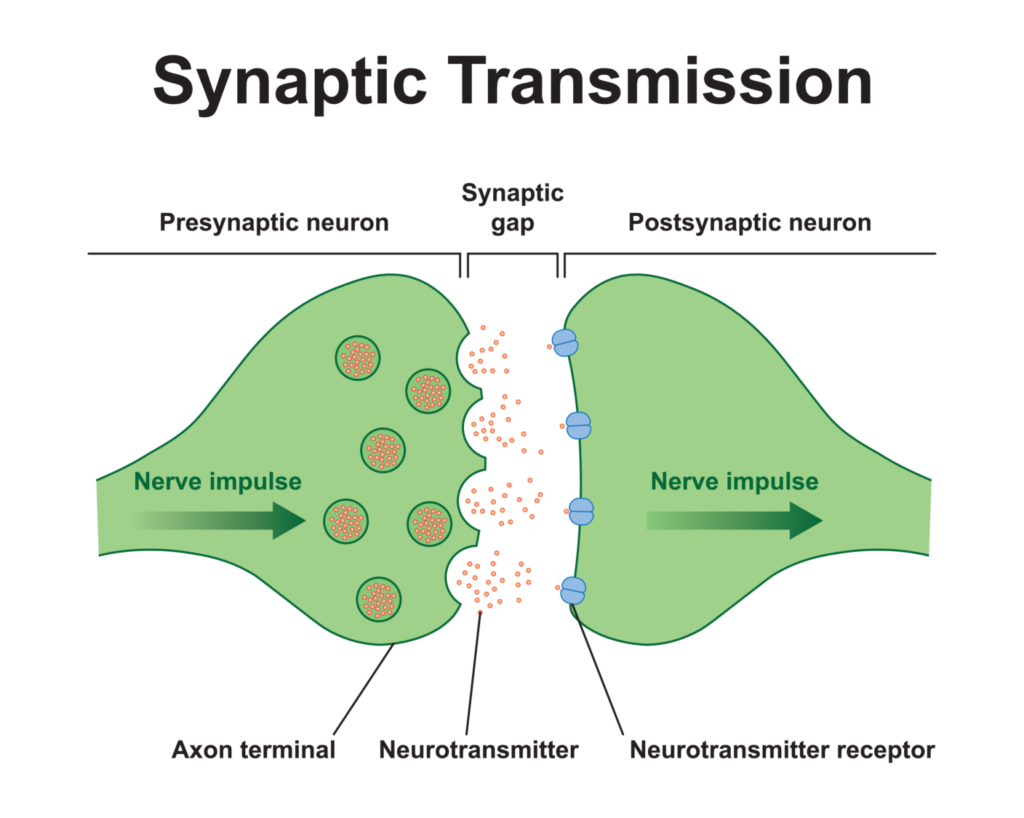
- Transmission of nerve impulses: Chemical synapses are the predominant form of synapses in the nervous system. They enable the transmission of nerve impulses or signals from one neuron to another. Unlike an electrical synapse, the signal transmission in a chemical synapse involves the release and binding of neurotransmitters.
- Synaptic cleft: The synaptic cleft is a fluid-filled space that separates the presynaptic neuron (sending neuron) from the postsynaptic neuron (receiving neuron). The transmission of the nerve impulse cannot occur through direct electrical contact but relies on the chemical messengers.
- Axon terminals and synaptic vesicles: The axon terminals of the presynaptic neuron contain knob-like structures that house synaptic vesicles. These vesicles store and transport neurotransmitters, which are essential for signal transmission.
- Release of neurotransmitters: When an action potential reaches the axon terminals, it triggers the release of synaptic vesicles. These vesicles fuse with the presynaptic membrane, releasing neurotransmitters into the synaptic cleft.
- Binding of neurotransmitters: Neurotransmitters diffuse across the synaptic cleft and bind to specific receptors present on the postsynaptic membrane of the receiving neuron. The binding of neurotransmitters initiates a series of events in the postsynaptic neuron.
- Ion channels and membrane polarization: Depending on the type of neurotransmitter and receptor, the binding can either open or close specific ion channels on the postsynaptic membrane. If the neurotransmitter is excitatory, the opening of ion channels leads to the influx of positively charged ions (e.g., sodium), resulting in depolarization and the generation of an excitatory postsynaptic potential (EPSP). In contrast, if the neurotransmitter is inhibitory, it causes hyperpolarization by increasing the influx of negatively charged ions (e.g., chloride), leading to an inhibitory postsynaptic potential (IPSP).
- Termination of the signal: Once the neurotransmitters have completed their function, they are either broken down by enzymes in the synaptic cleft or taken back up into the presynaptic neuron for recycling. This termination of the signal ensures that the synapse can reset and be ready for subsequent signal transmission.
In summary, chemical synapses facilitate the transmission of nerve impulses through the release and binding of neurotransmitters. The presynaptic neuron releases neurotransmitters into the synaptic cleft, which bind to receptors on the postsynaptic neuron. This binding leads to changes in the postsynaptic membrane potential, generating either excitatory or inhibitory responses. The signal is then terminated through enzymatic breakdown or reuptake of neurotransmitters. Chemical synapses play a crucial role in integrating and modulating neuronal communication in the nervous system.
Electrical Synapse and Its Functions
Electrical synaptic transmission is a distinct mode of communication between neurons that differs from chemical synapses in several ways. Unlike chemical synapses, electrical synapses involve a direct physical connection between the presynaptic and postsynaptic neurons through specialized structures called gap junctions.
Gap junctions consist of paired channels in the membranes of the presynaptic and postsynaptic neurons, forming pores that allow ions and various substances to flow freely between the cells. These channels are larger than the voltage-gated ion channels found in chemical synapses, facilitating rapid and direct transmission of electrical signals.
One notable characteristic of electrical synapses is their remarkable speed. Transmission across electrical synapses occurs almost instantaneously, in contrast to the relatively slower transmission observed in chemical synapses, which can take several milliseconds.
However, one limitation of electrical synapses is that their signal strength diminishes over time. This means that the strength of the electrical signal weakens as it propagates through the synaptic connection. In contrast, chemical synapses do not experience a loss of signal strength, allowing for more reliable and precise signal transmission.
Another distinction is that electrical synapses are exclusively excitatory. This means that they can only transmit signals that have an excitatory effect on the postsynaptic neuron, promoting the generation of action potentials.
Electrical synapses are found in various regions of the nervous system and serve specific functions. They play a crucial role in synchronizing the activity of neurons, facilitating rapid communication, and coordinating the electrical behavior of interconnected neural networks.
In summary, electrical synaptic transmission involves a direct physical connection between neurons through gap junctions, enabling fast and synchronized electrical communication. While electrical synapses offer speed advantages, they have limitations in terms of signal strength and excitatory transmission. Understanding the properties and functions of electrical synapses contributes to our knowledge of neural communication and the complexity of the nervous system.
An electrical synapse is a specialized connection between neurons that allows for the rapid transmission of electrical signals. Here are the main characteristics and functions of electrical synapses:
- Speed: Electrical synapses are faster than chemical synapses. This is because the electrical signal can directly pass from the presynaptic neuron to the postsynaptic neuron through gap junctions, without the need for chemical messengers.
- Gap junctions: In an electrical synapse, the presynaptic and postsynaptic neurons are in close proximity and form gap junctions. Gap junctions are specialized protein channels that physically connect the cytoplasm of the two neurons. These channels facilitate the direct flow of ions between the neurons.
- Direct transmission: Through the gap junctions, electrical synapses allow the transmission of an electric signal to pass from the presynaptic neuron to the postsynaptic neuron. This transmission is similar to the conduction of an impulse along an axon.
- Lack of flexibility: Unlike chemical synapses, electrical synapses are not as flexible in terms of signal modulation. They cannot convert an excitatory signal to an inhibitory signal or vice versa. The electrical signal transmitted through an electrical synapse maintains its original nature.
- Occurrence: Electrical synapses are more commonly found in lower invertebrates compared to mammals. In humans, they are primarily found between certain types of glial cells, which are non-neuronal cells that provide support and insulation to neurons.
- Directionality and prevention of random stimulation: Like chemical synapses, electrical synapses ensure the conduction of nerve impulses in the correct direction. The physical connection between the neurons at the gap junctions allows for efficient signal transmission and helps prevent random or uncontrolled stimulation.
In summary, electrical synapses enable fast and direct transmission of electrical signals between neurons through gap junctions. While they lack the flexibility of chemical synapses, they play a role in rapid and synchronized communication within neural circuits. The presence of electrical synapses contributes to the overall functionality and coordination of the nervous system, particularly in organisms where they are more prevalent.
Differences Between Chemical Synapse and Electrical Synapse – Chemical Synapse vs Electrical Synapse
| Chemical Synapses | Electrical Synapses |
|---|---|
| Gap between cells is about 20 nanometres | Gap between cells is about 3.5 nanometres |
| Speed of transmission is several milliseconds | Speed of transmission is nearly instantaneous |
| Can be excitatory or inhibitory | Excitatory only |
| Signal strength diminishes over time | No loss of signal strength |
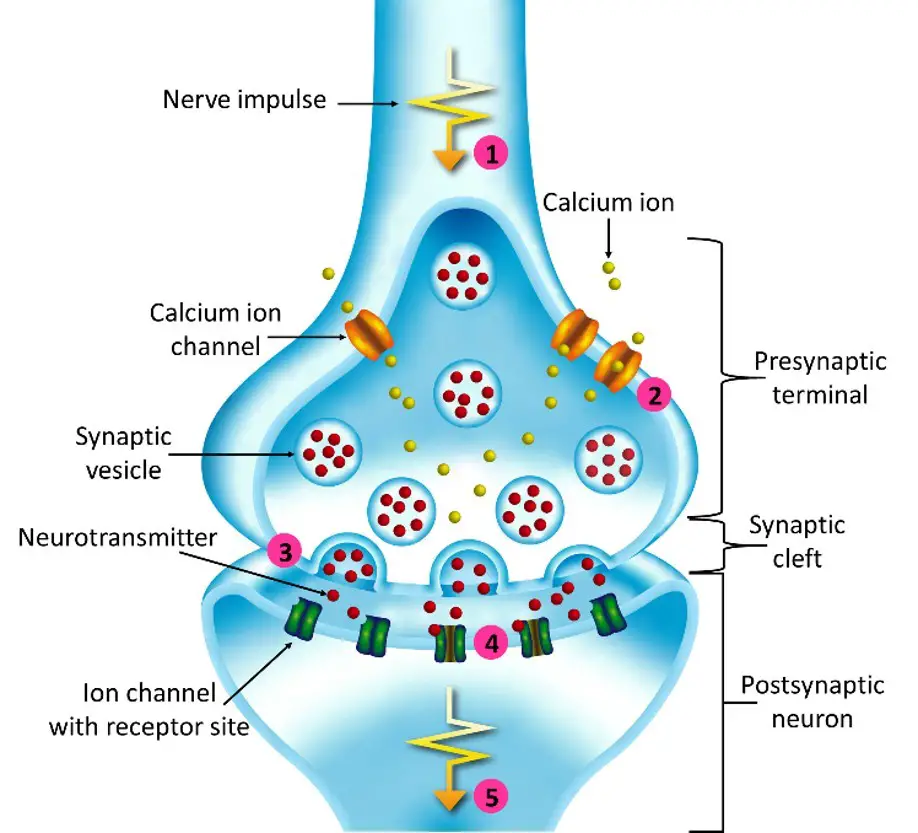
Synaptic transmission
Synaptic transmission is a fundamental process that enables communication between neurons and other target cells. It can occur through either electrical synapses or chemical synapses, each with its own mechanisms.
In electrical synapses, direct and rapid communication occurs through specialized ion channels known as gap junctions. These channels create a low-resistance pathway between the pre-synaptic and post-synaptic cells, allowing the flow of ions and electrical currents. This direct electrical coupling allows for the synchronized activity and rapid transmission of signals between neurons. Electrical synapses are particularly important in situations that require fast and synchronized responses, such as in reflex arcs.
In contrast, chemical synapses involve a more complex process. When an action potential reaches the presynaptic neuron, it triggers the release of neurotransmitters from specialized structures called synaptic vesicles. These neurotransmitters are released into the synaptic cleft, a small gap between the pre-synaptic and post-synaptic cells. The neurotransmitters then diffuse across the cleft and bind to specific receptors on the post-synaptic cell’s membrane.
The binding of neurotransmitters to their receptors initiates a series of events in the post-synaptic cell. Depending on the specific neurotransmitter and receptor combination, this can lead to the opening or closing of ion channels, resulting in changes in the electrical potential of the post-synaptic cell. These changes can either depolarize the cell, increasing the likelihood of generating an action potential (excitatory synapse), or hyperpolarize the cell, decreasing the likelihood of an action potential (inhibitory synapse).
The transmission of signals across chemical synapses is a finely regulated process. After neurotransmitter binding, the neurotransmitters are either cleared from the synaptic cleft through reuptake mechanisms or enzymatically broken down. This termination of the signal allows for precise control and modulation of synaptic transmission.
Overall, synaptic transmission plays a crucial role in information processing, integration, and communication within the nervous system. It allows for the transfer of signals from one neuron to another or to target cells, enabling the coordination of various physiological processes, sensory perception, motor control, and cognitive functions.
4 Steps of Synaptic transmission
1. Synthesis and Storage of Neurotransmitters
- The synthesis and storage of neurotransmitters are crucial steps in the process of synaptic transmission. Neurotransmitters are chemical messengers that allow communication between neurons in the nervous system. There are two main categories of neurotransmitters: small-molecule neurotransmitters and neuropeptides.
- Small-molecule neurotransmitters, such as acetylcholine (ACh), are synthesized locally within the axon terminal. The precursors required for the synthesis of these neurotransmitters are either taken up by selective transporters on the membrane of the terminal or produced as byproducts of cellular processes within the neuron itself. Enzymes necessary for catalyzing the synthesis of small-molecule neurotransmitters are typically produced in the cell body and then transported to the axon terminal through slow axonal transport.
- Taking acetylcholine as an example, it is an excitatory small-molecule neurotransmitter found in various locations throughout the central and peripheral nervous systems, as well as at neuromuscular junctions. The synthesis of acetylcholine involves the enzyme choline acetyltransferase, which combines choline and acetate to produce acetylcholine. This synthesis takes place within the nerve terminal.
- Neuropeptides, on the other hand, are synthesized in the cell body of the neuron. Unlike small-molecule neurotransmitters, neuropeptides are larger in size and require more complex processes for their synthesis. Neuropeptides typically consist of 3 to 36 amino acids. The synthesis of neuropeptides is similar to the synthesis of secretory proteins within the cell. It starts with gene transcription in the cell nucleus, where a specific peptide-coding sequence of DNA is used as a template to construct a corresponding strand of messenger RNA (mRNA). The mRNA then moves to a ribosome, where translation occurs. During translation, the nucleotide sequence of the mRNA acts as a code to assemble a specific sequence of amino acids that will eventually become the neuropeptide needed at the synaptic terminal.
- After synthesis, neuropeptides undergo post-translational processing in the endoplasmic reticulum (ER) and are then packaged in the Golgi apparatus. From there, they are transported in storage vesicles down the axon to the synaptic terminal. One example of neuropeptides is the family of endogenous opioids, which act as natural analgesics. These opioids are produced through selective cleaving and splicing of precursor molecules, resulting in the production of different but related neurotransmitters within this family.
- Once neurotransmitters, both small molecules, and neuropeptides, are synthesized, they are stored in vesicles within the axon terminal until an action potential arrives. Small-molecule neurotransmitters, like acetylcholine, are stored in small vesicles with clear centers, ranging from 40 to 60 nm in diameter. Neuropeptides, however, are stored in larger dense-core vesicles, ranging from 90 to 250 nm in diameter, which appear dark and electron-dense in electron micrographs.
- When an action potential reaches the axon terminal, it triggers the release of neurotransmitters from their storage vesicles into the synaptic cleft, initiating the process of synaptic transmission and allowing for communication between neurons.
2. Neurotransmitter Release
- Neurotransmitter release is a critical step in synaptic transmission, allowing for the communication between neurons. The release of neurotransmitters occurs when an action potential reaches the terminal of a presynaptic neuron, triggering a series of events that lead to the release of neurotransmitters into the synaptic cleft.
- At rest, neurotransmitter-containing vesicles are stored near the pre-synaptic membrane in two main locations. Some vesicles are positioned along the pre-synaptic membrane at specific sites called “active zones,” which are the sites of neurotransmitter release. However, the majority of vesicles are held close to these active zones but slightly further away from the membrane until they are needed. These vesicles are held in place by Ca2+-sensitive vesicle membrane proteins (VAMPs), which bind to elements of the cytoskeleton such as actin filaments and microtubules.
- When an action potential arrives at the presynaptic terminal, voltage-dependent calcium (Ca2+) channels embedded in the pre-synaptic membrane open, allowing an influx of calcium ions (Ca2+). The increase in intracellular calcium concentration sets off a cascade of events that ultimately lead to neurotransmitter release.
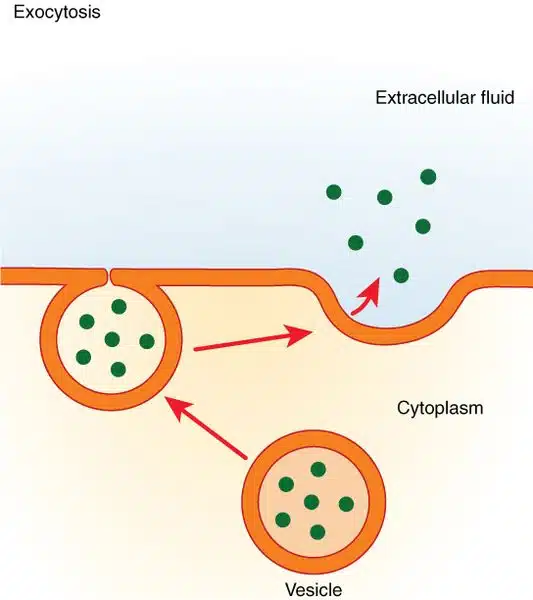
- The first step in this process is the liberation of the neurotransmitter-containing vesicles from their attachments to the cytoskeleton. Once released, these vesicles travel towards the active zones where docking occurs. Docking is the alignment of the vesicle and the pre-synaptic membrane in a position that enables easy fusion. Fusion follows, where the vesicle membrane and the pre-synaptic membrane merge, forming a small pore that connects the lumen of the vesicle with the synaptic cleft. This pore gradually expands until the vesicle membrane collapses into the pre-synaptic membrane, releasing the neurotransmitter into the synaptic cleft. This fusion process is known as exocytosis.
- After exocytosis, the vesicular membrane, which is now a continuation of the pre-synaptic membrane, forms a pit and pinches off to create a new empty vesicle within the terminal. This newly formed vesicle can undergo recycling and be refilled with neurotransmitter for subsequent release. Alternatively, it may be transported to the cell body, where it is broken down, processed into new vesicles, and then transported back to the terminal to be filled with neurotransmitter.
- This recycling and refilling process ensures that neurotransmitters can be stored and released repeatedly, allowing for efficient synaptic transmission and communication between neurons.
3. Neurotransmitter Postsynaptic Receptors
- Once neurotransmitters are released into the synaptic cleft, they interact with specific receptor proteins on the membrane of the postsynaptic cell. These receptors play a crucial role in transmitting the signal from the presynaptic neuron to the postsynaptic neuron or target cell.
- There are two main types of postsynaptic receptors: ionotropic receptors and metabotropic receptors. Ionotropic receptors, also known as ligand-gated ion channels, are composed of multiple protein subunits embedded in the cell membrane. These receptors act rapidly and directly influence the membrane potential of the postsynaptic neuron. When a neurotransmitter binds to the recognition site on the receptor, the protein subunits open, forming a pore through which ions can flow. This opening of the ion channel leads to either depolarization or hyperpolarization of the postsynaptic membrane, depending on the specific neurotransmitter and receptor involved. The depolarization can initiate another action potential if the threshold is reached.
- An example of an ionotropic receptor is the receptor for γ-aminobutyric acid (GABA). GABA is an inhibitory neurotransmitter used in a significant number of synapses in the brain. When GABA binds to the GABA recognition site on the ionotropic receptor, the channel opens, allowing negatively charged chloride ions to enter the cell. This influx of negative ions hyperpolarizes the postsynaptic neuron, inhibiting the generation of an action potential. It’s important to note that while GABA acts as an inhibitory neurotransmitter, there are other ionotropic receptors that recognize excitatory neurotransmitters, leading to the stimulation of action potentials in the postsynaptic cell.
- In contrast to ionotropic receptors, metabotropic receptors, also called G-protein coupled receptors (GPCRs), do not form ion channels themselves. Instead, they rely on an intermediary molecule within the postsynaptic cell called a G-protein. When a neurotransmitter binds to the extracellular recognition site of a metabotropic receptor, it activates the associated G-protein. This activated G-protein, through a series of enzymatic reactions, can modulate the activity of ion channels located elsewhere on the cell membrane. The actions of metabotropic receptors are more complex and slower compared to ionotropic receptors. The depolarization or hyperpolarization they induce takes longer to develop, lasting from hundreds of milliseconds to minutes, hours, or even days.
- Metabotropic receptors are involved in the recognition of various neurotransmitters, including neuropeptides and several small-molecule neurotransmitters. Dopamine (DA) is one example of a small-molecule neurotransmitter that binds to a metabotropic receptor. The binding of DA to the recognition site of a postsynaptic DA receptor triggers a cascade of reactions that ultimately result in the opening of ion channels along the postsynaptic membrane, leading to the generation of an action potential. Dopamine plays a critical role in the regulation of motor and emotional behaviors and is found in major tracts of the brain, such as the nigrostriatal tract, tuberoinfundibular tract, mesolimbic tract, and mesocortical tract.
- In summary, postsynaptic receptors respond to neurotransmitters released into the synaptic cleft, initiating changes in the postsynaptic cell’s membrane potential. Ionotropic receptors directly modulate ion flow, causing rapid depolarization or hyperpolarization. Metabotropic receptors activate G-proteins, which then influence ion channels through a series of biochemical reactions, resulting in more prolonged and diverse effects on the postsynaptic neuron.
A. Ionotropic (ligand-gated) receptors
- GABAa Receptors: a representative family of ligand-gated receptors: GABAa receptors belong to the family of ionotropic receptors, which are ligand-gated ion channels that mediate fast synaptic transmission in the nervous system. They are specifically activated by the neurotransmitter gamma-aminobutyric acid (GABA). GABAa receptors are widely distributed throughout the brain and play a crucial role in inhibitory neurotransmission.
- GABAa receptor function: inhibitory postsynaptic potentials: When GABA binds to the GABAa receptor, it triggers the opening of an ion channel, allowing the influx of chloride ions (Cl-) into the postsynaptic neuron. This leads to hyperpolarization of the postsynaptic membrane, inhibiting the generation of an action potential. Consequently, GABAa receptors mediate inhibitory postsynaptic potentials (IPSPs), which counteract excitatory signals and help regulate the overall excitability of neural circuits.
- Postsynaptic GABA recognition by its receptor: GABA, the neurotransmitter, is released from presynaptic neurons and diffuses across the synaptic cleft to reach the postsynaptic neuron. The GABAa receptor, located on the postsynaptic membrane, recognizes and binds to GABA molecules through specific binding sites.
- Binding of GABA to receptor: When GABA binds to the GABAa receptor, it induces conformational changes in the receptor protein. These changes result in the opening of the ion channel pore within the receptor complex, allowing chloride ions to pass through.
- Structure of the GABAa receptor: The GABAa receptor is composed of multiple protein subunits that come together to form a functional receptor complex. Typically, this receptor is made up of five subunits, consisting of various combinations of different subunit types (α, β, γ, δ, ε, π, θ, and ρ). The subunit composition determines the pharmacological and functional properties of the receptor.
- Ion channel opening: mechanism of action: When GABA binds to the GABAa receptor, it promotes the opening of the ion channel within the receptor complex. This allows chloride ions to flow into the postsynaptic neuron, leading to hyperpolarization and inhibition of neuronal activity.
- Positive neuromodulation of GABAa receptors: tranquilizers: Certain drugs, such as benzodiazepines, can enhance the function of GABAa receptors. They act as positive allosteric modulators by binding to specific sites on the receptor complex, which enhances the effect of GABA binding. These drugs, known as tranquilizers or anxiolytics, have sedative and anxiolytic effects, promoting relaxation and reducing anxiety.
- Negative neuromodulation of GABAa receptors: anxiogenics: Conversely, there are substances that have the opposite effect and reduce the activity of GABAa receptors. These substances, known as anxiogenics, can induce anxiety and increase excitability within neural circuits. They may interfere with GABAergic inhibition, leading to an imbalance between inhibitory and excitatory neurotransmission.
- Clinical Application: GABA and Anxiety: The involvement of GABAa receptors in anxiety regulation has significant clinical implications. Enhancing GABAergic transmission, either through direct activation of GABAa receptors or by increasing the availability of GABA, can be an effective strategy in the treatment of anxiety disorders. Medications such as benzodiazepines and barbiturates that target GABAa receptors are commonly prescribed to alleviate anxiety symptoms.
In summary, GABAa receptors are ligand-gated ion channels that mediate inhibitory neurotransmission in the brain. They play a crucial role in regulating neuronal excitability and maintaining the balance between inhibition and excitation. The binding of GABA to the receptor leads to the opening of an ion channel, resulting in hyperpolarization and inhibition of postsynaptic neuronal activity. Drugs that modulate GABAa receptors can have either anxiolytic or anxiogenic effects, making them important targets for the treatment of anxiety disorders.
B. Metabotropic (G-protein coupled) receptors
- Dopamine Receptors: a representative family of metabotropic receptors: Metabotropic receptors, also known as G-protein coupled receptors (GPCRs), include a diverse family of receptors, with dopamine receptors being one prominent example. These receptors play a crucial role in mediating the effects of dopamine, a neurotransmitter involved in various brain functions such as reward, motivation, and motor control.
- Dopamine D1 Receptor: The dopamine receptor family consists of multiple subtypes, with D1 receptors being a specific subtype. Dopamine D1 receptors are primarily excitatory and are widely distributed in the brain. Activation of D1 receptors can lead to intracellular signaling cascades and modulate neuronal activity.
- Function of the G-protein: Metabotropic receptors, including dopamine receptors, exert their effects through G-proteins. When a ligand, such as dopamine, binds to the receptor, it induces a conformational change that enables the receptor to interact with a G-protein located on the intracellular side of the receptor.
- G-proteins, cAMP, and Ion Channel Opening: Upon activation by the receptor, G-proteins undergo a series of intracellular signaling events. One common pathway involves the activation of adenylyl cyclase, an enzyme that converts ATP into cyclic adenosine monophosphate (cAMP). Increased cAMP levels then trigger downstream signaling cascades, resulting in the modulation of various intracellular processes, including ion channel opening.
- Dopamine Receptor Blockade: Antipsychotic Drugs: Blockade of dopamine receptors, particularly the D2 subtype, is the mechanism of action of antipsychotic drugs used in the treatment of schizophrenia and other psychotic disorders. These drugs, known as antipsychotics or neuroleptics, bind to dopamine receptors, preventing dopamine from binding and reducing dopaminergic activity in certain brain regions, which helps alleviate psychotic symptoms.
- Clinical Application: Dopamine and Schizophrenia: The dysregulation of dopamine signaling has been implicated in the pathophysiology of schizophrenia. Excessive dopaminergic activity, particularly in the mesolimbic pathway, is associated with positive symptoms of schizophrenia such as hallucinations and delusions. Antipsychotic drugs, by blocking dopamine receptors, help restore the balance of dopamine neurotransmission, leading to a reduction in symptoms.
In summary, metabotropic receptors, such as dopamine receptors, utilize G-protein mediated signaling to modulate neuronal activity. Activation of dopamine receptors can lead to various intracellular signaling pathways, influencing processes like cAMP production and ion channel opening. Antipsychotic drugs target dopamine receptors to manage conditions like schizophrenia, highlighting the importance of dopamine signaling in psychiatric disorders.
4. Inactivation of Neurotransmitters
After the release and binding of neurotransmitters at the synapse, it is crucial for their prompt inactivation to maintain proper neuronal signaling and prevent overstimulation. There are various mechanisms involved in the inactivation of neurotransmitters, ensuring their efficient removal from the synaptic cleft. Here are some key aspects of neurotransmitter inactivation:
- Reuptake by Transporter Proteins: Many neurotransmitters are taken back up into the presynaptic neuron through specialized transporter proteins located on the presynaptic membrane. These transporters actively transport the neurotransmitter molecules from the synaptic cleft back into the presynaptic cell. Once inside, the neurotransmitter can be either repackaged into vesicles for future release or undergo enzymatic breakdown. Serotonin is an example of a neurotransmitter that is recycled through this reuptake mechanism.
- Enzymatic Breakdown: Some neurotransmitters are chemically inactivated by specific enzymes in the synaptic cleft. Acetylcholine (ACh) is an important neurotransmitter that undergoes enzymatic inactivation by the enzyme acetylcholinesterase (AChE). AChE is present at cholinergic synapses and breaks down acetylcholine into its components, acetate and choline, through hydrolysis. Acetate diffuses away, while choline is taken up by a high-affinity choline uptake (HACU) system back into the presynaptic cell. Choline can then be recycled to synthesize more acetylcholine.
- Diffusion: Some neurotransmitters, particularly neuropeptides, simply diffuse away from the receptors into the surrounding extracellular fluid. These neurotransmitters do not undergo reuptake or enzymatic breakdown but instead disperse and become diluted in the vicinity of the synapse.
The timely inactivation of neurotransmitters is essential for regulating synaptic transmission and maintaining the balance of neuronal activity. Dysregulation or impairment of these inactivation processes can lead to abnormal neurotransmitter levels and contribute to various neurological and psychiatric disorders.
Understanding the mechanisms of neurotransmitter inactivation provides insights into the development of therapeutic interventions. For example, drugs that target transporter proteins can modulate neurotransmitter reuptake, influencing neurotransmitter availability and synaptic signaling. Similarly, medications that inhibit specific enzymes, such as acetylcholinesterase inhibitors, can increase the levels of neurotransmitters like acetylcholine, benefiting conditions such as Alzheimer’s disease.
In summary, neurotransmitter inactivation involves processes such as reuptake by transporter proteins, enzymatic breakdown, or diffusion. These mechanisms ensure the removal or inactivation of neurotransmitters in the synaptic cleft, preventing excessive stimulation of the post-synaptic cell and maintaining proper neuronal function. The understanding of neurotransmitter inactivation pathways contributes to the development of therapeutic strategies for various neurological and psychiatric disorders.
A. Enzymatic inactivation of neurotransmitters
Enzymatic Inactivation of Neurotransmitters
Enzymatic inactivation plays a crucial role in terminating the action of neurotransmitters at the synaptic cleft. Acetylcholinesterase (AChE) is a representative enzyme involved in the inactivation of the neurotransmitter acetylcholine (ACh). Let’s explore the key aspects of enzymatic inactivation and the specific example of acetylcholinesterase:
- Acetylcholinesterase (AChE): AChE is an enzyme that is widely distributed throughout the nervous system, especially at cholinergic synapses. It is responsible for the rapid breakdown of acetylcholine, an excitatory neurotransmitter, into its components: acetate and choline.
- Location, Structure, and Function of Acetylcholinesterase: Acetylcholinesterase is primarily found in the synaptic cleft, anchored to the extracellular matrix or associated with the postsynaptic membrane. It is a tetrameric enzyme composed of four subunits, with each subunit containing a catalytic site responsible for the hydrolysis of acetylcholine. The active site of AChE accommodates acetylcholine, allowing the enzyme to rapidly catalyze the breakdown reaction.
- Enzyme Inhibition: Anticholinesterases: Certain compounds can interfere with the function of acetylcholinesterase by inhibiting its activity. These compounds are called anticholinesterases. By inhibiting AChE, anticholinesterases prevent the breakdown of acetylcholine, leading to increased acetylcholine levels in the synaptic cleft. This prolonged presence of acetylcholine can enhance cholinergic signaling and stimulate postsynaptic receptors for an extended period. Anticholinesterases are used in various clinical applications, such as the treatment of conditions like myasthenia gravis and Alzheimer’s disease.
- Clinical Application: Acetylcholine, Nerve Gas, and Myasthenia Gravis: Acetylcholine plays a vital role in neuromuscular transmission, cognitive functions, and autonomic nervous system regulation. In conditions like myasthenia gravis, an autoimmune disorder, autoantibodies can target and impair acetylcholine receptors at the neuromuscular junction, leading to muscle weakness and fatigue. The administration of anticholinesterase drugs, which inhibit AChE, can increase the availability of acetylcholine, compensating for the receptor dysfunction and improving muscle strength.
Furthermore, acetylcholinesterase inhibitors have been used as chemical warfare agents, as in the case of nerve gases. These potent inhibitors irreversibly bind to acetylcholinesterase, preventing its normal activity and causing an accumulation of acetylcholine. This excessive acetylcholine release can lead to severe overstimulation of cholinergic pathways, resulting in respiratory distress, paralysis, and potentially fatal effects.
B. Presynaptic transporters: inactivation of neurotransmitters by reuptake
In addition to enzymatic inactivation, neurotransmitters can be rapidly removed from the synaptic cleft through a process called reuptake, which is facilitated by specific presynaptic transporters. One such representative transporter is the serotonin transporter. Let’s explore the key aspects of presynaptic transporters and the example of the serotonin transporter:
- Serotonin Transporter: The serotonin transporter, also known as SERT, is a protein primarily located on the presynaptic membrane of neurons that release serotonin. It is responsible for the reuptake of serotonin from the synaptic cleft back into the presynaptic neuron.
- Location and Structure of the Serotonin Transporter: The serotonin transporter is predominantly found in regions of the brain that are involved in mood regulation, cognition, and emotional processing. Structurally, it consists of a transmembrane protein with multiple domains that span the presynaptic membrane.
- Mechanism of Action of the Serotonin Transporter: The serotonin transporter works by actively transporting serotonin molecules from the synaptic cleft back into the presynaptic neuron. It utilizes energy derived from ATP hydrolysis to move serotonin against its concentration gradient. This reuptake process is crucial for the termination of serotonin signaling and the regulation of its availability in the synaptic cleft.
- Transporter Inhibition: Selective Serotonin Reuptake Inhibitors: Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs commonly used to treat depression and other mood disorders. SSRIs selectively block the reuptake of serotonin by binding to and inhibiting the serotonin transporter. By preventing serotonin reuptake, SSRIs increase the concentration of serotonin in the synaptic cleft, enhancing its effects and promoting neurotransmission.
- Clinical Application: Serotonin and Depression: Serotonin is a neurotransmitter that plays a crucial role in mood regulation and emotional well-being. Imbalances in serotonin levels have been implicated in the development of depression. By inhibiting the reuptake of serotonin, SSRIs increase its availability in the synapse, potentially alleviating symptoms of depression and improving mood.
The reuptake of neurotransmitters through presynaptic transporters is an essential mechanism for regulating neurotransmitter levels and maintaining appropriate synaptic signaling. The serotonin transporter, in particular, is involved in the homeostasis of serotonin, and its dysfunction has been linked to various psychiatric conditions. Understanding the role of presynaptic transporters and their modulation opens avenues for the development of therapeutics targeting neurotransmitter reuptake systems to treat a range of neurological and psychiatric disorders.
FAQ
What is a synapse?
A synapse is a specialized junction between two neurons, where information is transmitted from one neuron to another.
What is synaptic transmission?
Synaptic transmission is the process by which information is transferred from the presynaptic neuron (sending neuron) to the postsynaptic neuron (receiving neuron) across the synapse.
What are neurotransmitters?
Neurotransmitters are chemical messengers that transmit signals between neurons. They are released from the presynaptic neuron and bind to receptors on the postsynaptic neuron.
How does information transfer occur at the synapse?
Information transfer at the synapse occurs through the release of neurotransmitters from the presynaptic neuron, which bind to receptors on the postsynaptic neuron, leading to changes in its electrical activity.
What are excitatory and inhibitory synapses?
Excitatory synapses increase the likelihood of the postsynaptic neuron firing an action potential, while inhibitory synapses decrease the likelihood of firing an action potential.
What is the role of vesicles in synaptic transmission?
Vesicles are small sacs within the presynaptic neuron that store neurotransmitters. They release neurotransmitters into the synaptic cleft upon the arrival of an action potential.
How are neurotransmitters cleared from the synapse?
Neurotransmitters are cleared from the synapse through various mechanisms, including reuptake by presynaptic transporters, enzymatic degradation, and diffusion away from the synaptic cleft.
What is long-term potentiation (LTP)?
Long-term potentiation is a process that strengthens the connection between two neurons, leading to enhanced synaptic transmission and improved neural communication. It is believed to underlie learning and memory formation.
What are the types of synapses?
There are two main types of synapses: chemical synapses, where neurotransmitters are used to transmit signals, and electrical synapses, where electrical currents directly pass between neurons through gap junctions.
How do drugs and medications affect synaptic transmission?
Drugs and medications can affect synaptic transmission by altering the release, uptake, or receptor binding of neurotransmitters. For example, antidepressants can increase the availability of certain neurotransmitters in the synapse, helping to alleviate symptoms of depression.
References
- https://web.williams.edu/imput/introduction_main.html
- https://www.oist.jp/image/diagram-synaptic-transmission
- https://www.simplypsychology.org/synapse.html
- https://www.brainkart.com/article/The-Synapse—Human-Neuroanatomy_18874/
- https://www.brainfacts.org/brain-anatomy-and-function/cells-and-circuits/2022/synapses-and-neurotransmission-113022
- https://www.biotopics.co.uk/A17/Synaptic_transmission.html
- https://teachmephysiology.com/nervous-system/synapses/synaptic-transmission/
- https://en.wikipedia.org/wiki/Synapse
- https://www.khanacademy.org/science/biology/human-biology/neuron-nervous-system/a/overview-of-neuron-structure-and-function


