Table of Contents
SARS-CoV-2 is the virus that causes the disease COVID-19 (coronavirus disease 2019). It is a highly infectious disease that can spread from person to person through respiratory droplets or close contact. The virus was first identified in Wuhan, China in December 2019 and has since spread to become a global pandemic. Symptoms of COVID-19 can range from mild to severe and may include fever, cough, and difficulty breathing. The virus can be severe or even deadly, particularly for older adults or people with underlying health conditions. Governments and health agencies around the world have implemented measures such as lockdowns, travel restrictions, and mask-wearing to slow the spread of the virus. There is currently no specific treatment for COVID-19, but researchers are working on developing vaccines and treatments.
SARS-CoV-2 in the conducting airways
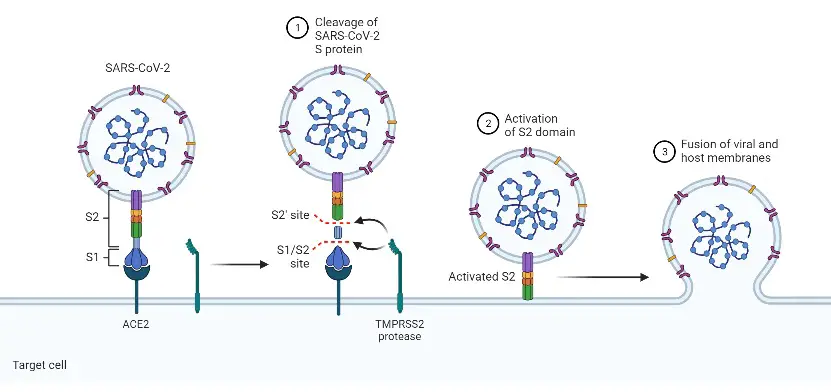
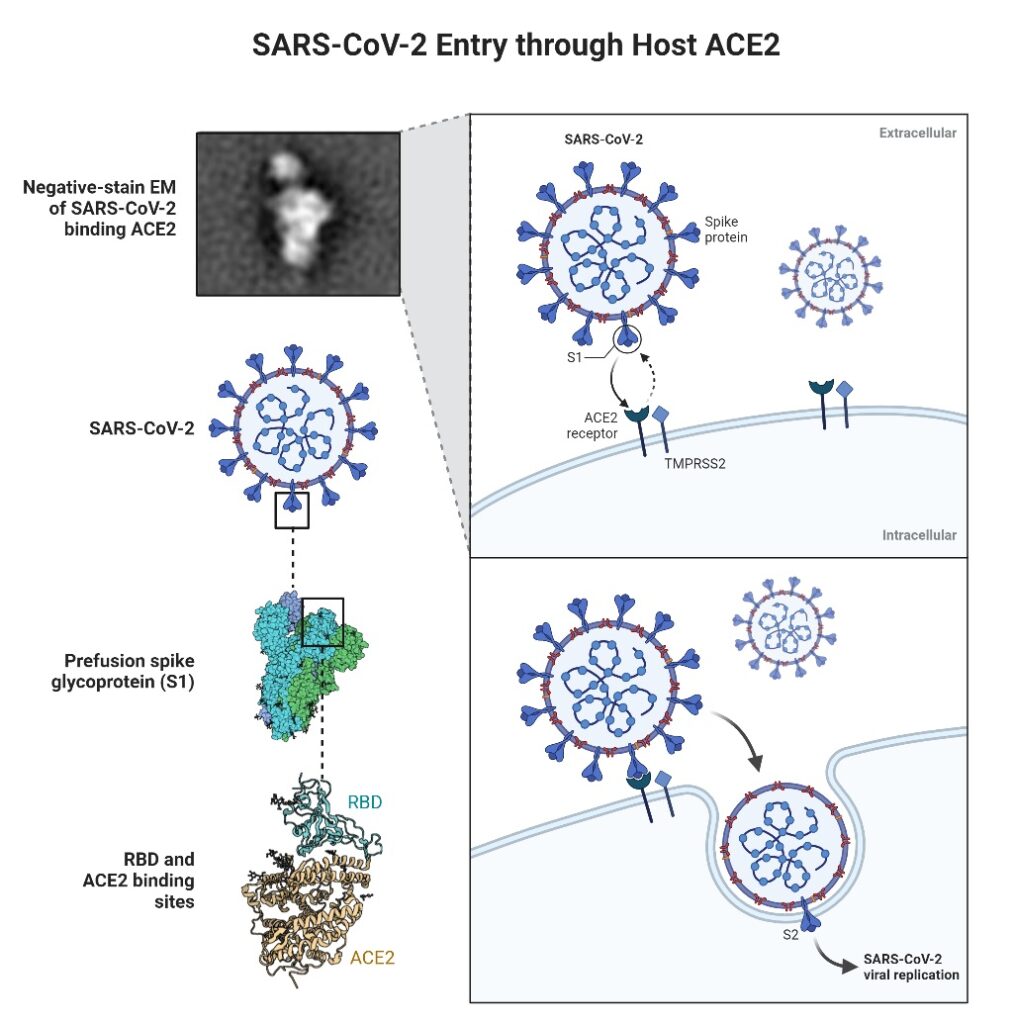
For entry into host cells, SARS-CoV-2 initially attaches its spike (S) protein to the angiotensin-converting enzyme 2 (ACE2) receptor, which is found on the cell surface. ACE2 is found on the surface of type I and type II alveolar epithelial cells, as well as in several additional places, such as the nasal and oral mucosa and nasopharynx.
Notably, ACE2 expression is extremely variable between cells and frequently detected at low levels. TMPRSS2 is a serine protease that cleaves the S protein to initiate viral fusion with the cell membrane.
Initial SARS-CoV-2 infection occurs in the sinonasal airway epithelium. The mucociliary clearance mediated by these cells is the primary innate defence of the lungs. Within six hours of the initial infection, SARS-CoV-2 infects ACE2-expressing ciliated cells, multiplies, and then infects nearby cells following apical release.
Through the secretion of interferons and other cytokines, these infected ciliated cells trigger the innate immune response. In infected tissues, viral replication and activation of the immune system occur practically simultaneously.
When SARS-CoV-2 delays or dampens the cytokine response, infected ciliated cells lose their cilia, resulting in decreased mucociliary clearance. Damaged ciliated and mucous cells render the epithelial barrier ineffective. The progenitor-basal cells, which are normally immune to infection, can proliferate and repair the injured epithelium.
SARS-CoV-2 penetrates the lower conducting airways at the subsequent stage, where there are more ciliated cells, mucus-secreting cells, and club cells, all of which express ACE2 and TMPRSS2. As a result of weaker cough reflexes and decreased mucociliary clearance, this step of virus entry is significantly facilitated in the aged population.
During these phases of infection, the innate immune response to SARS-CoV-2 is inhibited or only partially triggered. At this infection stage, which is critical for cellular and adaptive immune responses, only a small cytokine response and little production of type I and type III interferons are seen. This reaction in the airways against SARS-CoV-2 is comparable to the mild interferon and cytokine response observed in the nose.
For future activation of the adaptive immune response, an early innate immune response is necessary. Immunoglobulin M (IgM), immunoglobulin G (IgG), and immunoglobulin A (IgA) are all required for protection against SARS-CoV-2; however, IgA is essential for mucosal immunity. Consequently, IgA efficiently neutralises SARS-CoV-2 in the first several weeks following the onset of symptoms.
The diminished innate immune response seen in asymptomatic patients may result in diminished antibody and T-cell responses. In addition, this restricted response may prevent acquired immunity from staying in these individuals, resulting in recurring outbreaks of illness.
Therapeutic methods for early infection in the upper airways include monoclonal antibodies targeted to disrupt virus docking on host ACE2 and anti-viral medicines such as remdesivir that impede subsequent viral reproduction. However, these treatment methods have obstacles, such as the requirement for early administration to ensure efficacy, logistical concerns, and the possibility of inactivity against developing variations.
A further therapeutic drug currently being studied in Phase II clinical trials is inhaled beta interferon, which stimulates the innate immune response. Also of interest are systemic and inhaled steroids; nevertheless, definitive findings from clinical trials have not yet been obtained.
SARS-CoV-2 in the gas exchange units of lungs
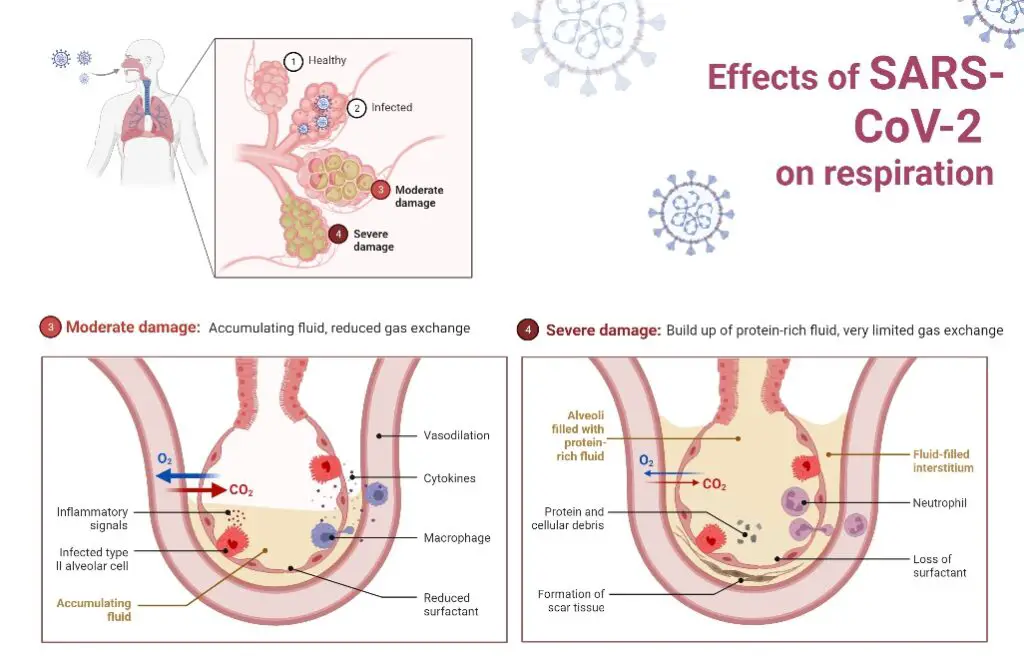
The impact of SARS-CoV-2 on the parts of the lung responsible for gas exchange is the primary cause of COVID-19 severity and mortality. Infection with SARS-CoV-2 in this location is linked with increasing hypoxia, pulmonary infiltrates, alveolar flooding, and a robust influx of inflammatory cells.
Alveolar damage with loss of functional surfactant, hyaline membranes, epithelial and microvascular inflammation, and damage to type I and endothelial cells accelerate the progression of ARDS. In certain instances, thrombi can form in these vessels, resulting in disastrous outcomes.
Alveolar type II cells in this location express both ACE-2 and TMPRSS2. When SARS-CoV-2 penetrates the alveoli, it infects the ACE2-expressing alveolar type II cells, which are the initial cells to activate antiviral immunity.
SARS-CoV-2 propagates to infect type II bystander cells. The innate immune response is initiated by type I and type III interferons, whereas inflammatory cytokines and chemokines recruit and activate immune cells. Notably, type II alveolar cells also function as antigen-presenting cells (APC) to initiate T-cell responses at this time in the infection.
The fact that alveolar type II cells are also progenitor cells for alveolar epithelium raises concerns about the long-term effects of scarring in these tissues. Damage to the parts of the lungs responsible for gas exchange leads to ARDS.
Some of the therapeutic strategies designed to combat alveolar infection aim to restrict viral replication, preserve the survival and function of type II cells, and reduce the inflammatory response in order to preserve the functional integrity of the gas exchange units. At this point in the infection, preventing endothelium damage and microvascular thrombi is also essential.
Some drugs, including dexamethasone, anti-cytokines (Baricitinib, Tocilizumab, and Sarilumab), corticosteroids, cyclic Cyclic adenosine monophosphate (cyclic AMP), and mitogenic growth factors, such as keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and transforming growth factor-alpha (TGFa), are used to treat hospitalised CO However, the effects of these many interventions are highly context-dependent and case-specific.
Sever illness with advancing age
Although the level of ACE2 expression on cells varies and is dependent on the cell type, its expression in alveolar type II cells of the elderly is high. Several studies have found that COVID-19 paediatric patients have fewer interferon responses in their epithelial cells and more innate lymphoid and naive T-cells in their peripheral blood when compared to adult patients.
