Table of Contents
What is Atomic Absorption Spectrophotometer?
- Atomic Absorption Spectrophotometry (AAS) stands as a paramount technique in the realm of spectroanalytical procedures, specifically designed for the quantitative elucidation of chemical elements in their gaseous atomic state. Rooted in the principle of light absorption by free metallic ions, AAS serves as a pivotal tool in analytical chemistry, aiming to ascertain the concentration of a specific element, termed the analyte, within a given sample.
- The essence of AAS lies in its ability to determine the presence of over 70 distinct elements in liquid solutions or even directly in solid matrices through a process known as electrothermal vaporization. This versatility has rendered AAS indispensable in diverse scientific domains, including pharmacology, biophysics, archaeology, and toxicology.
- Historically, the foundational concepts of atomic emission spectroscopy, a precursor to AAS, were pioneered in the latter half of the 19th century by eminent scientists Robert Wilhelm Bunsen and Gustav Robert Kirchhoff from the University of Heidelberg, Germany.
- However, the contemporary version of AAS that we recognize today was meticulously crafted in the 1950s by an adept team of Australian chemists, spearheaded by Sir Alan Walsh, at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) in Melbourne.
- AAS operates on a fundamental principle: the detection of elements through the emission or absorption of characteristic wavelengths of electromagnetic radiation. Each element possesses a unique wavelength absorption profile, and by measuring these absorbances against established standards, precise elemental identification and quantification can be achieved.
- The procedure commences with the atomization of analytes, post which their characteristic wavelengths are emitted and subsequently documented. During the excitation phase, atomic electrons elevate to a higher energy level upon absorbing specific energy, which corresponds to a distinct wavelength intrinsic to that element. The differential absorption of these wavelengths by various atoms facilitates the detection and measurement of specific elements.
- In the broader context of applications, AAS is revered for its myriad uses, especially in the sectors of quality control, toxicology, and environmental testing. Two prominent variants of AAS, Flame Atomic Absorption Spectrometry (FAAS) and Graphite Furnace Atomic Absorption Spectrometry (GFAAS), have been instrumental in offering unparalleled accuracy in elemental analysis.
- While FAAS is celebrated for its widespread acceptance across multiple industries, GFAAS is renowned for its capability to detect elements at minuscule concentrations, even in the parts per billion range, utilizing exceptionally low sample volumes.
- In conclusion, the Atomic Absorption Spectrophotometer stands as a testament to the confluence of scientific rigor and technological advancement, offering unparalleled precision and versatility in the domain of elemental analysis. Its enduring relevance in modern analytical chemistry underscores its pivotal role in advancing scientific inquiry and ensuring the safety and quality of myriad products and environments.
Definition of Atomic Absorption Spectrophotometer
The Atomic Absorption Spectrophotometer (AAS) is a scientific instrument used to quantitatively determine the presence and concentration of specific chemical elements in a sample by measuring the absorbed wavelengths of light by free metallic ions in their gaseous state.
Principle of Atomic Absorption Spectrophotometer
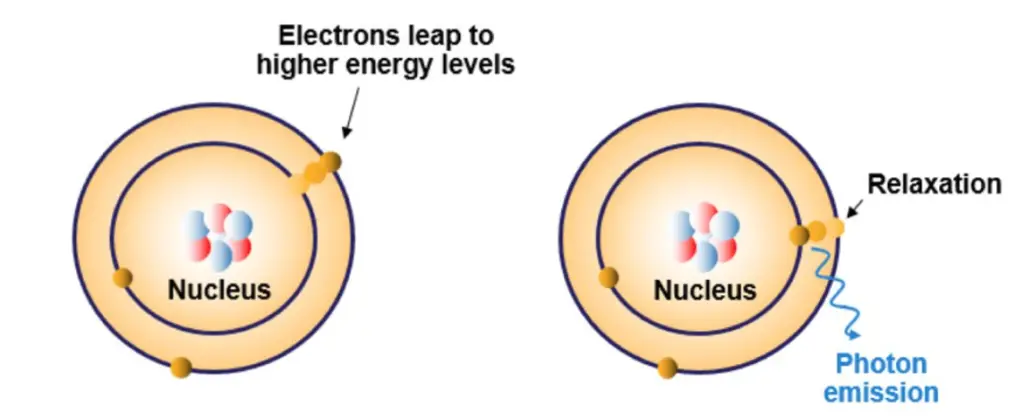
The Atomic Absorption Spectrophotometer (AAS) operates on foundational principles rooted in atomic physics and spectroscopy. At its core, AAS is predicated on the fact that atoms, when in their ground state, have the capability to absorb light at specific and unique wavelengths. This absorption is element-specific, meaning that each chemical element will absorb light at its own characteristic wavelength.
When a sample is introduced into the AAS, it undergoes atomization, converting the sample into free atoms. These atoms are then illuminated by a light source, typically emanating from a hollow-cathode lamp. This light source produces radiation that corresponds to the unique wavelength absorbed by the atomized sample. As atoms absorb this light, their electrons transition from a ground state to higher energy excited states. The energy absorbed during this transition is directly tied to the specific electronic structure of the element in question, making this absorption a distinctive property of each individual element.
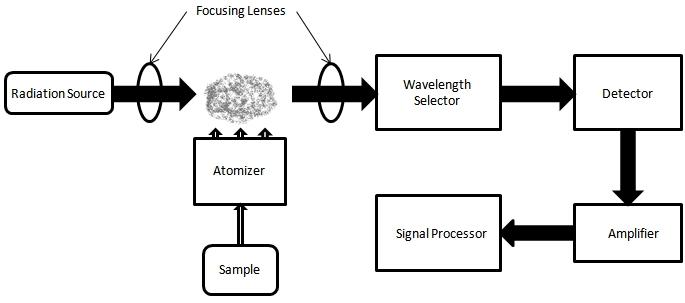
The AAS instrument is composed of four primary components:
- Light Source: Typically a hollow-cathode lamp that emits radiation at specific wavelengths.
- Atomization System: This system, which can be a flame or a graphite furnace, converts the sample into free atoms.
- Monochromator: Positioned between the sample and the detector, the monochromator filters out unwanted wavelengths, ensuring that only the specific wavelength of interest reaches the detector.
- Detection System: This system measures the intensity of the light beam and translates it into absorption data.
The process of atomization can be achieved through various techniques. Flame atomic absorption spectroscopy (FAAS) is a prevalent method where the sample is nebulized into a flame, converting the metal ions into free atoms. Another technique, graphite furnace atomic absorption spectroscopy (GFAAS), involves placing the sample in a graphite tube and heating it to vaporize the sample. GFAAS offers higher sensitivity compared to FAAS.
Additionally, there are specialized atomizing techniques like glow-discharge systems, hydride-generating atomizers, and cold-vapor atomization, each tailored for specific types of samples or elements.
The radiation sources in AAS can be broadly categorized into line sources (LS) and continuum sources (CS). While LS emits radiation at specific wavelengths, CS emits light over a broad spectrum. High-resolution-CS AAS (HR-CS AAS) is a modern advancement that uses a xenon short-arc lamp, a high-resolution double monochromator, and a CCD array detector.
In summary, the principle of AAS revolves around the absorption of light by free atoms in their ground state. This absorption, unique to each element, allows for the quantitative determination of specific elements in a sample. The technique’s specificity, combined with its precision, makes AAS a valuable tool in various scientific and industrial applications.
Instrumentation of Atomic Absorption Spectrophotometer
The Atomic Absorption Spectrophotometer (AAS) is a sophisticated analytical instrument designed for the quantitative determination of specific elements in a sample. Its operation is based on the principle of atomic absorption, where atoms in the gaseous state absorb light of specific wavelengths. The instrumentation of AAS is intricate, comprising several key components, each playing a pivotal role in its functionality.
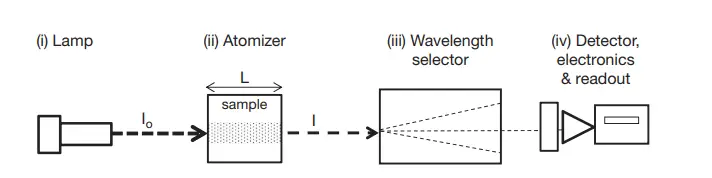
1. Main Components of AAS: The AAS is composed of four primary components:
- Light Source: Typically, this is a lamp emitting spectral lines specific to the element of interest. Hollow Cathode Lamps (HCL) and Electrodeless Discharge Lamps (EDL) are the most prevalent types. These lamps produce narrow atomic lines, ensuring high specificity and sensitivity.
- Atomizer: This component is responsible for converting the sample into gaseous atoms. Two primary atomizers are used: a flame (using chemical combustion energy) and an electrothermal atomizer (using electrical energy). The choice of atomizer depends on the nature of the sample and the specific requirements of the analysis.
- Wavelength Selector: This component isolates the specific light wavelength that the atoms absorb. Monochromators, based on various designs, are commonly used as wavelength selectors. The introduction of echelle optics has further enhanced the precision of this component.
- Detector: The detector quantifies the intensity of light passing through the wavelength selector. Photomultiplier tubes (PMT) are commonly used due to their wide wavelength coverage and high amplification gain. However, advancements have led to the adoption of multichannel detectors for enhanced performance.
2. Supplementary Components:
- Light Modulator: This modulates the light source, ensuring only the modulated signal from the lamp is measured, thereby eliminating continuous emission from the atomizer.
- Background Corrector: This corrects for unspecific background absorption, ensuring only the specific absorption of the analyte is measured. Various methods, including the use of a deuterium lamp and the Zeeman effect, are employed for background correction.
3. Spectrometer Configurations: To ensure accurate measurements, AAS instruments often employ a double-beam spectrometer configuration. This configuration uses a beam splitter to create two light paths: one passing through the sample cell (sample beam) and the other serving as a reference. This continuous comparison between the two beams ensures accurate and reliable measurements.
4. Additional Accessories: Modern AAS instruments come equipped with a range of accessories to enhance their functionality. These include autosamplers, modular systems for specific operations, direct solid samplers, and automatic burner rotation systems, among others.
In conclusion, the Atomic Absorption Spectrophotometer is a culmination of advanced scientific and engineering principles. Its intricate design and precise components ensure accurate, reliable, and specific measurements, making it an indispensable tool in various scientific and industrial applications.
Light sources of Atomic Absorption Spectrophotometer
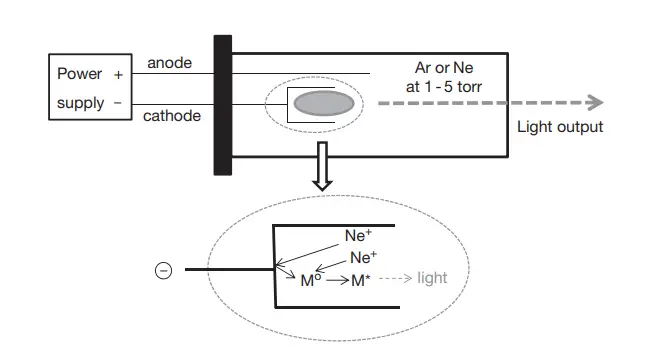
Atomic Absorption Spectrophotometry (AAS) is a widely used analytical technique that relies on the absorption of light by free metallic ions. Central to the efficacy of this method is the choice of light source, which determines the precision and accuracy of the measurements. This article delves into the primary light sources utilized in AAS, emphasizing their scientific principles and applications.
- Line Sources in AAS: Line sources are predominant in AAS, producing a narrow-line spectrum specific to the element under investigation. The two primary types of line sources are the Hollow Cathode Lamp (HCL) and the Electrodeless Discharge Lamp (EDL). Additionally, there have been propositions to use laser diodes as alternative light sources in AAS.
- Hollow Cathode Lamp (HCL): The HCL is a prominent light source in AAS, known for its bright and stable line emission. Structurally, an HCL comprises a hollow cylindrical cathode containing the target element and an anode, both enclosed within a glass tube filled with an inert gas like neon or argon at low pressures. When a potential difference is applied, gas ionization occurs, leading to the formation of a plasma between the electrodes. This plasma, in turn, causes metal atoms to be ejected from the cathode, which upon excitation, emit radiation at wavelengths characteristic of the element. There are advanced versions of HCLs, such as boosted HCLs and multi-element HCLs, designed to enhance the lamp’s output and versatility.
- Electrodeless Discharge Lamp (EDL): EDLs are preferred for elements that present challenges with HCLs, especially volatile ones like arsenic, selenium, and mercury. An EDL consists of a bulb containing the metal or its salt, surrounded by a radiofrequency or microwave coil. The low gas pressure and temperature in EDLs minimize broadening effects, allowing for the production of exceptionally narrow atomic lines.
- Continuum Emitting Light Sources: Recent advancements in AAS have introduced instruments that combine a high-intensity continuum-emitting light source, such as a xenon short-arc lamp, with a high-resolution spectrometer. This combination offers several advantages, including multi-element detection, enhanced background correction, and an extended dynamic range. Moreover, it allows for the analysis of elements that lack a specific radiation source.
In conclusion, the choice of light source in AAS plays a pivotal role in determining the accuracy, precision, and versatility of the analysis. Both line sources and continuum-emitting sources have their unique advantages, and their selection is contingent upon the specific requirements of the analysis. As with all scientific instruments, continuous research and technological advancements promise to further refine and expand the capabilities of AAS in the future.
Atomizer of Atomic Absorption Spectrophotometer
In the realm of Atomic Absorption Spectrophotometry (AAS), the atomizer stands as a pivotal component, tasked with the conversion of the analyte into a gaseous atomic state. This scientific discussion seeks to elucidate the functionalities and characteristics of the predominant atomizers utilized in AAS, namely, the flame and the electrothermal atomizer, while adhering to a formal, scientific, and informative tone.
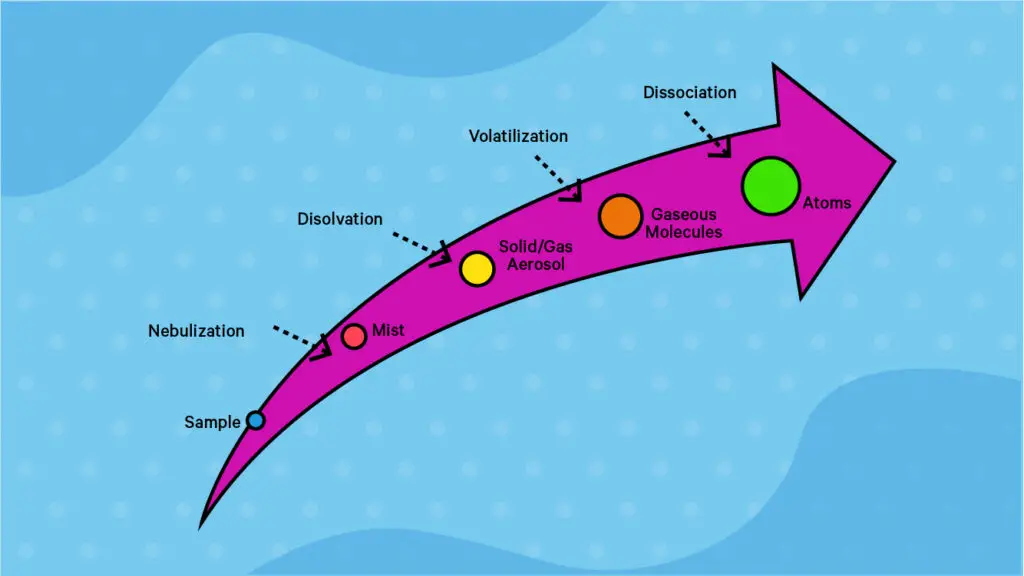
- Flame Atomizer: The flame atomizer in AAS is instrumental in the analysis of liquid samples through Flame Atomic Absorption Spectrophotometry (FAAS). The process commences with the aspiration and nebulization of the solution, followed by the elimination of larger droplets and the introduction of finer droplets into the burner. Pneumatic nebulizers, which utilize a compressed gas jet, are commonly employed, generating droplets with diameters ranging between 1 and 50 micrometers. Subsequently, a premix chamber facilitates the condensation of larger droplets, while the finer ones merge with the fuel and oxidant gases, eventually reaching the burner head. The flame, characterized by its stable structure and defined cones, undergoes a series of complex processes, including desolvation, vaporization, dissociation, and potential ionization of atoms. The flame’s temperature and oxidizing or reducing characteristics are pivotal, with popular gas combinations being air-acetylene and nitrous oxide-acetylene, each catering to different analytical needs.
- Electrothermal Atomizer: The electrothermal atomizer, often constructed from small-size graphite tubes, is renowned for its high vaporization and atomization efficiencies, controlled thermal and chemical environment, and the requirement of minimal sample volume. In Electrothermal Atomic Absorption Spectrophotometry (ETAAS), a well-defined volume of the sample is introduced into the atomizer and subjected to a multi-step temperature program, sequentially undergoing drying, pyrolysis, and atomization. The concentration of the analyte atoms during atomization is contingent upon the analyte concentration and the sample volume introduced. Following atomization, a high-temperature clean-out step is typically executed before the furnace cools down for subsequent analyses. The integration of various components and technologies, such as an autosampler, a L’Vov platform, and pyrolytically coated graphite tubes, has facilitated the development of precise and highly sensitive ETAAS analytical methods.
In summary, both flame and electrothermal atomizers play crucial roles in AAS, each offering unique advantages and being suitable for different analytical applications. The flame atomizer excels in the stable and consistent analysis of liquid samples, while the electrothermal atomizer is celebrated for its efficiency and precision, especially in scenarios requiring low sample volumes and high sensitivity. The meticulous selection and understanding of these atomizers are paramount in ensuring the accuracy and reliability of AAS analyses, thereby contributing to the advancement of scientific research and analysis.
Types of Atomic Absorption Spectroscopy
Atomic Absorption Spectrometry (AAS) is a sophisticated analytical technique that has gained prominence in various scientific domains due to its precision in detecting and quantifying elemental compositions. Over the years, advancements in technology have made AAS systems more affordable, and some modern variants even offer multi-element detection capabilities. Delving into the diverse types of AAS, we can categorize them into the following primary systems:
- Flame Atomic Absorption Spectrometry (FAAS): FAAS is one of the most common and widely used types of AAS. In this method, a sample solution is nebulized and introduced into a flame, where the analyte atoms are desolvated, vaporized, and atomized. The flame serves as both the atomization source and the heat source, facilitating the production of free atoms that can absorb light at a specific wavelength corresponding to the element of interest.
- Cold Vapour Atomic Absorption Spectrometry (CV AAS): CV AAS is specifically tailored for the analysis of mercury, given its unique properties. In this technique, mercury in the sample is reduced to its elemental form, which is then vaporized at room temperature. The resultant cold mercury vapor is subjected to a light source, and the absorption is measured. This method offers high sensitivity and is particularly effective for trace-level mercury detection.
- Hydride-Generating Atomic Absorption Spectrometry (HG AAS): HG AAS is employed for the analysis of elements that form volatile hydrides, such as arsenic, selenium, and antimony. The sample undergoes a chemical reaction with a reducing agent, leading to the formation of volatile hydrides. These hydrides are then introduced into a flame or a heated quartz cell, where they decompose to produce free atoms. The atomic absorption of these atoms is subsequently measured.
- Graphite Furnace Atomic Absorption Spectrometry (GF-AAS): GF-AAS, also known as Electrothermal AAS, utilizes a graphite furnace as the atomization source. The sample is directly introduced into a graphite tube, which is then electrically heated in a stepwise manner. This controlled heating process facilitates the evaporation of solvents, ashing of the sample matrix, and atomization of the analyte. GF-AAS offers the advantage of analyzing minute sample volumes and provides enhanced sensitivity, especially for trace element analysis.
In summary, Atomic Absorption Spectrometry, with its diverse types, stands as a versatile and powerful tool in the realm of analytical chemistry. Each type of AAS is optimized for specific applications, ensuring precise and accurate elemental analysis across a wide range of samples and concentrations.
Atomic Absorption Spectrophotometer Applications
Atomic Absorption Spectrophotometry (AAS) is a powerful analytical technique that offers precise quantification of trace metals in diverse samples. This method has found extensive applications in various scientific and industrial domains. Herein, we delve into the multifaceted applications of AAS, elucidating its significance in each sector.
- Mining and Geology: In the realm of geology and mining, AAS aids in determining the elemental composition of minerals and rocks. Such analyses are pivotal in assessing the viability of mining operations in explored regions. Post-mining, the composition of ores and minerals is evaluated to optimize refining processes. Furthermore, trace metal analysis is indispensable in prospecting for oil and water deposits. In the gemological sector, the grading of gemstones is influenced by the presence of specific trace metals. Additionally, the elemental composition of archaeological artifacts can provide insights into their origins.
- Environmental Monitoring: AAS plays a crucial role in monitoring environmental samples for trace metal contamination. Assessing the metal content in industrial effluents, oceans, rivers, and lakes ensures the safety of water for consumption and commercial applications. Such evaluations are essential to ascertain compliance with regulatory standards. Moreover, environmental assessments are integral to determining the suitability of sites for commercial ventures.
- Materials Development: Material properties, including hardness, brittleness, grain size, and crystallinity, are influenced by their elemental and trace metal composition. Through AAS, insights into the performance attributes of materials can be gleaned.
- Pharmaceuticals: In the pharmaceutical sector, trace metal analysis is vital for formulation development, assessing catalyst efficiency, and establishing dosage limits. While many elements are beneficial within specific thresholds, exceeding these limits can have detrimental effects.
- Foods and Beverages: In the food industry, especially with processed foods, metal contamination can occur due to contact with processing equipment and catalytic reactions. With rising consumer awareness regarding food safety, manufacturers must ensure that trace metal levels remain within permissible limits, necessitating rigorous quality control through AAS.
- Oil and Petroleum: Both edible and mineral oils undergo refining processes, which can introduce metal contaminants. Analyzing these oils for trace metals is essential to prevent performance degradation or potential health risks. Moreover, trace metal analysis of engine oil can offer insights into engine wear and tear.
- Agriculture: The trace metal composition of soils, coupled with their pH levels, determines their fertility and nutrient content. Analyzing the trace metal distribution in plants under varying growth conditions provides insights into mineral uptake patterns.
- Forensics: In forensic science, AAS is invaluable for analyzing specimens like stomach contents in cases of food poisoning, paint residues, fibers, and hair strands collected from crime scenes. Such analyses can provide crucial evidence in criminal investigations.
In conclusion, Atomic Absorption Spectrophotometry stands as a cornerstone in various scientific and industrial sectors, offering precise and reliable analyses of trace metals in diverse samples. Its versatility and accuracy make it an indispensable tool in modern analytical science.
Advantages Atomic Absorption Spectrophotometer
- Cost-Effective Analysis: One of the primary benefits of Atomic Absorption Spectrophotometer (AAS) is its low cost per analysis. This makes it a preferred choice for routine elemental analysis in various laboratories.
- User-Friendly Operation: AAS instruments are designed to be straightforward and easy to operate, even for individuals with minimal training in the field.
- High Sensitivity: AAS boasts a remarkable sensitivity, with the capability to detect elements at concentrations as low as parts per billion (ppb).
- Precision and Accuracy: The technique offers high accuracy in elemental quantification, ensuring reliable results that can be trusted for various applications.
- Minimal Inter-Element Interference: AAS is mostly free from interference caused by other elements present in the sample, ensuring that the readings obtained are specific to the element of interest.
- Broad Industrial Applications: Due to its versatility, AAS finds applications across a wide range of industries, from environmental monitoring to pharmaceuticals and mining.
Limitations Atomic Absorption Spectrophotometer
- Inability to Detect Non-Metals: AAS is primarily designed for metal analysis. It cannot be used to detect non-metallic elements, which limits its scope in certain applications.
- Initial Equipment Cost: While the cost per analysis is low, the initial investment required for new AAS equipment can be relatively high.
- Primarily for Liquid Analysis: AAS is more suited for the analysis of liquid samples. While it can analyze other forms, such as solids, they often need to be converted into a liquid form, which can be a limitation in certain scenarios.
- Destructive Analysis: One of the drawbacks of AAS is that the sample being analyzed is destroyed in the process. This means that post-analysis, the sample cannot be recovered or used for further testing.
Quiz
What is the primary purpose of an Atomic Absorption Spectrophotometer (AAS)?
a) To measure the concentration of non-metallic elements in a sample.
b) To measure the concentration of metallic elements in a sample.
c) To measure the pH of a sample.
d) To measure the viscosity of a sample.
Which of the following is NOT a type of AAS?
a) Flame AAS
b) Cold vapour AAS
c) Hydride-generating AAS
d) Ultraviolet AAS
In AAS, what is primarily detected?
a) Atoms
b) Molecules
c) Ions
d) Electrons
Which of the following gases is commonly used in Flame AAS?
a) Oxygen
b) Nitrogen
c) Acetylene
d) Hydrogen
Which component of AAS is responsible for converting the sample into gaseous atoms?
a) Atomizer
b) Detector
c) Monochromator
d) Light source
Which of the following is a limitation of AAS?
a) Cannot detect metals
b) Sample is destroyed during analysis
c) High cost per analysis
d) Difficult to operate
In which industry is AAS NOT commonly used?
a) Mining
b) Environmental monitoring
c) Food processing
d) Textile manufacturing
What is the role of the light source in AAS?
a) To detect the concentration of the element
b) To convert the sample into gaseous atoms
c) To emit radiation that is absorbed by the sample atoms
d) To separate the wavelengths of light
Which of the following elements might require a hotter flame in AAS due to its refractory nature?
a) Sodium
b) Potassium
c) Aluminum
d) Lithium
Which of the following is an advantage of using Graphite Furnace AAS over Flame AAS?
a) Faster analysis time
b) Suitable for gas samples
c) Requires larger sample volume
d) Higher sensitivity and lower detection limits
FAQ
What is an Atomic Absorption Spectrophotometer (AAS)?
An Atomic Absorption Spectrophotometer (AAS) is an analytical instrument used to measure the concentration of specific metallic elements in a sample by determining the amount of light absorbed by free atoms in a gaseous state.
How does AAS work?
AAS works by introducing a sample into a flame or graphite furnace, where it is atomized. A light source then emits radiation through the atomized sample, and the amount of light absorbed by the sample is measured, indicating the concentration of the element in question.
What types of samples can be analyzed using AAS?
AAS is primarily used for analyzing liquid samples, but with certain attachments, it can also analyze solid samples.
Why is the sample destroyed in AAS?
The sample is atomized, meaning it is converted into free atoms in a gaseous state, in order to measure the absorption of light. This process destroys the original form of the sample.
What are the main types of AAS?
The main types of AAS include Flame AAS (FAAS), Cold Vapour AAS (CV AAS), Hydride-generating AAS (HG AAS), and Graphite Furnace AAS (GF-AAS).
Is AAS suitable for non-metal detection?
No, AAS is specifically designed for the detection and quantification of metallic elements.
How sensitive is AAS?
AAS is highly sensitive and can detect concentrations up to parts per billion (ppb) levels for certain elements.
What gases are used in Flame AAS?
Commonly used gases in Flame AAS include acetylene and nitrous oxide, among others.
Are there any interferences in AAS?
While AAS is mostly free from inter-element interference, certain conditions or elements might cause interference, affecting the accuracy of results.
Can AAS be used for routine analysis in laboratories?
Yes, due to its ease of operation, accuracy, and sensitivity, AAS is widely used in various industries and research laboratories for routine elemental analysis.
