Table of Contents
What is a Spectrometer?
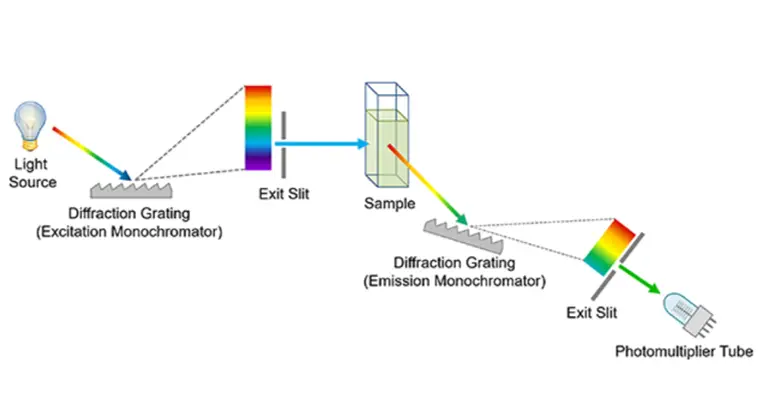
- A spectrometer is a sophisticated scientific instrument designed to detect and analyze light waves, thereby measuring the physical attributes of a substance across a specific spectrum. This device operates by first introducing a sample of the substance in question, which is then vaporized. As light traverses through this vaporized sample, the spectrometer meticulously divides the light into its individual wavelengths, producing a spectrum akin to the colors seen in a rainbow when light refracts through a prism. Each distinct wavelength or color provides invaluable insights into the inherent properties of the substance being examined.
- Incorporated within many spectrometers, particularly those integrated into telescopes, is a collimator. This component ensures the alignment of the various wavelengths into a parallel arrangement. Subsequently, the light encounters a detector that identifies and records the distinct wavelengths.
- Spectrometers serve a dual purpose: they are instrumental in analyzing both light and material samples. For instance, astronomers frequently employ spectrometers to scrutinize extraterrestrial light, facilitating the determination of the elemental composition of celestial bodies such as stars.
- Furthermore, spectrometers are pivotal in discerning variations in physical characteristics across a spectrum through a method known as spectrometry. They are adept at gathering data about a material based on its emission or absorption of specific light bands, be it infrared, visible, or ultraviolet. In the realm of astronomy, spectrometers are indispensable tools, aiding in ascertaining the mass, velocity, and thermal conditions of cosmic entities.
- Beyond astronomical applications, spectrometers have found utility in diverse fields. For example, in medical science, they are employed to detect toxins and impurities in the bloodstream, verify the presence of performance-enhancing substances in athletes, and even identify markers indicative of certain diseases.
- In summary, a spectrometer is an essential tool in the scientific arsenal, offering precise and technical insights into the properties of substances by analyzing their interaction with light across various spectra. Its applications span from the vast expanse of space to the intricate complexities of the human body, underscoring its versatility and significance in advancing our understanding of the universe.
Types of Spectrometers
Spectrometers, pivotal instruments in the realm of scientific research, are designed to detect and analyze various properties of substances. These devices are categorized based on their specific functionalities and the type of measurements they are engineered to perform. Herein, we delve into the primary types of spectrometers and their distinct applications:
- Optical Spectrometers: These are primarily employed to detect optical absorption or emission, facilitating the measurement of light intensity. As a testament to their versatility and efficacy, optical spectrometers are ubiquitously utilized in research settings. Their primary function revolves around gauging the intensity of light across different wavelengths, providing insights into the optical properties of materials.
- Mass Spectrometers: Often referred to informally as “mass spec,” these instruments are adept at measuring the mass-to-charge ratios present within a chemical specimen. By doing so, they ascertain the identity of the sample under investigation. Their precision and sensitivity make them invaluable in forensic science, where even minuscule traces of evidence can be pivotal. Through mass spectrometry, investigators can glean crucial information from limited samples, aiding in their analytical endeavors.
- Nuclear Magnetic Resonance (NMR) Spectrometers: NMR spectrometers operate by employing a magnetic field to induce magnetization in certain atomic nuclei. Post magnetization, these instruments measure the magnetic resonance frequencies emanating from the nuclei. Subsequently, these frequencies are transmuted into a spectrum that can be analyzed. The data procured from NMR spectrometry offers profound insights into the molecular structure and dynamics of compounds.
- Electron Spectrometers: While not as prevalent as the aforementioned types, electron spectrometers are designed to gauge the energy encapsulated within electron beams. These instruments employ either electric or magnetic fields to deviate the electron beam, segregating electrons based on their inherent energy levels. Through this process, electron spectrometers provide a detailed energy spectrum of the sample.
In summation, spectrometers, with their diverse types and functionalities, serve as indispensable tools in scientific research. Each variant, tailored for specific measurements, contributes uniquely to our understanding of the intricate properties of substances, reaffirming the significance of spectrometry in advancing scientific knowledge.
Applications for Spectrometers
Spectrometers, with their capability to measure emitted electromagnetic radiation across various spectra, have carved a niche for themselves across a multitude of scientific domains. Their precision and versatility have rendered them indispensable in diverse research and practical applications. Herein, we explore the salient applications of spectrometers across various scientific disciplines:
- Chemistry: In the realm of chemistry, particularly within the subfields of physical and analytical chemistry, spectrometers are paramount. Their prowess lies in detecting and quantifying molecular compositions. Whether it’s discerning the atomic structure of a sample, elucidating the metabolic intricacies, or characterizing proteins, spectrometers play a pivotal role.
- Pharmaceuticals: The pharmaceutical industry leverages spectrometers for a myriad of applications. These instruments aid in scrutinizing and modifying the molecular structure of drugs, thereby enhancing their therapeutic efficacy. Furthermore, in clinical settings such as hospitals and clinics, spectrometers find utility in respiratory gas analysis, providing critical insights into a patient’s respiratory health.
- Ecology: Ecologists employ spectrometers to meticulously monitor and identify various biological specimens, encompassing vegetation, fungi, and other mycobiota. Contrary to the subjective nature of morphological characterizations, spectrometers offer objective and consistent insights. Portable handheld spectrometers have emerged as invaluable tools for gauging the dissolved oxygen content in both freshwater and marine ecosystems, ensuring the health and balance of aquatic life.
- Astronomy: The vast expanse of the cosmos, with its myriad celestial entities, remains largely inaccessible for direct sampling. Astronomers, therefore, rely heavily on spectrometry to glean insights into the chemical composition of stars and other celestial bodies. Beyond mere compositional analysis, spectrometers empower astronomers to ascertain the temperature of distant cosmic objects, providing a comprehensive understanding of their physical properties.
In essence, spectrometers, with their multifaceted applications, have become an integral part of modern scientific endeavors. Their ability to provide precise and objective data across various spectra underscores their significance in advancing knowledge and innovation across diverse scientific fields.
What is a Spectrophotometer?
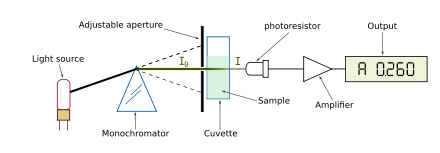
A spectrophotometer is a specialized scientific instrument designed to quantitatively assess the intensity of light across various wavelengths. Integral to its function is a spectrometer, which aids in determining both the magnitude and the specific type of light waves traversing the apparatus.
The operational mechanism of a spectrophotometer can be elucidated through the following sequential steps:
- Light Intake: The spectrophotometer initiates its process by capturing light, which is directed towards a sample cell within the device.
- Wavelength Separation: Post light intake, the instrument employs a diffraction grating mechanism to segregate the light into its constituent wavelengths. This ensures that each wavelength is distinctly isolated for accurate measurement.
- Interaction with Sample: As the separated light waves traverse the sample cell, multiple interactions can occur. The light might be absorbed by specific molecules present in the sample, leading to its attenuation. Alternatively, the light can scatter, being re-emitted in diverse directions. In certain instances, the light might even navigate through the sample cell without undergoing any molecular interactions. The degree of light absorption is contingent upon both the concentration of the sample and the specific wavelength of the incident light. This relationship between sample absorbance, its path length, and concentration is encapsulated by the Beer-Lambert Law.
- Detection and Quantification: Subsequent to its passage through the sample cell, the light reaches a detector. This component is tasked with quantifying the amount of light that has successfully traversed the sample. The resultant data is then articulated in specific units, either as absorbance (A) or as percent transmission (%T).
In essence, a spectrophotometer offers a meticulous and precise method to measure light intensity across different wavelengths. By leveraging the principles of the Beer-Lambert Law, it provides invaluable insights into the properties of various samples, making it an indispensable tool in numerous scientific disciplines.
Types of Spectrophotometers
Spectrophotometers, integral to various scientific applications, are primarily categorized based on their beam configuration: single-beam and double-beam. Each type possesses distinct attributes tailored for specific analytical requirements.
- Single-Beam Spectrophotometers: These instruments operate by assessing the relative light intensity before and after the introduction of a test sample. Their design is inherently compact, and they boast a superior dynamic range. However, their sensitivity to external fluctuations might be a limiting factor in certain applications.
- Double-Beam Spectrophotometers: Contrary to their single-beam counterparts, double-beam spectrophotometers are engineered to juxtapose the light from reference paths with that passing through the substance under examination. Their primary advantage lies in their reduced sensitivity to external perturbations, making them ideal for environments demanding consistent and stable measurements.
Delving deeper, spectrophotometers can be further classified into five predominant subcategories, each tailored for specific analytical applications:
- Atomic Absorption Spectrophotometer: This variant is adept at measuring the absorption of light by free metallic ions in gaseous states. It provides insights into the concentration of specific metal ions in samples.
- Fluorescence Spectrophotometer: Employed to gauge the intensity of fluorescence emitted by a sample upon exposure to light, this instrument is pivotal in studies involving luminescent materials.
- Infrared Spectrophotometers: These instruments operate in the infrared spectrum and are instrumental in identifying and quantifying compounds based on their infrared absorption patterns. They offer insights into molecular vibrations and rotations, facilitating the study of molecular structures.
- UV-VIS Spectrophotometer: Operating in the ultraviolet (UV) and visible (VIS) light spectra, these spectrophotometers are employed to analyze the absorption patterns of samples when exposed to UV and visible light. They are widely used in fields like biochemistry and pharmaceuticals.
- VIS Spectrophotometer: Exclusively functioning in the visible spectrum, VIS spectrophotometers assess the absorption of visible light by samples. They find applications in colorimetry and the study of pigments and dyes.
In summation, the diverse types of spectrophotometers, each with its unique attributes and functionalities, cater to a wide array of scientific applications. Their precision and versatility underscore their significance in advancing analytical research across multiple disciplines.
Applications for Spectrophotometers
Spectrophotometers, with their precision in measuring light diffusivity across a broad spectrum ranging from 200-2500nm, have become an indispensable tool in various scientific fields. Their ability to deliver accurate wavelength results, even with minuscule samples as tiny as 1uL, underscores their significance in advancing research and industrial applications. Here, we delve into the primary applications of spectrophotometers across distinct scientific disciplines:
- Biochemistry: In the domain of biochemistry, spectrophotometers hold paramount importance. Their capabilities extend to the meticulous analysis of DNA and RNA samples, facilitating genetic research and diagnostics. Furthermore, they play a pivotal role in the isolation of proteins, enabling researchers to study enzyme kinetics and conduct comprehensive analyses of various biochemical entities.
- Colorimetry: Beyond the realm of biochemistry, spectrophotometry finds profound application in the field of colorimetry. This science of measuring colors is integral to several industries. For instance, ink manufacturing units utilize spectrophotometers to ensure the consistency and quality of their products. Similarly, the printing industry relies on these instruments to achieve accurate color reproduction. In the textile manufacturing sector, spectrophotometry aids in ensuring that dyes and pigments adhere to the desired specifications. Moreover, businesses leverage colorimetry to compare batches of colorants against established standards, ensuring that the final product aligns seamlessly with production criteria.
In summation, spectrophotometers, with their multifaceted applications, play a crucial role in both research and industrial settings. Their precision, coupled with their ability to analyze minute samples, makes them an invaluable asset in driving innovation and ensuring quality across diverse scientific and industrial domains.
Differences Between Spectrometer vs Spectrophotometer
Spectrometers and spectrophotometers are both instrumental in the study and analysis of light and matter interactions. However, they serve distinct functions and have specific applications. Here’s a detailed comparison between the two, elucidating their differences:
- Basic Function:
- Spectrometer: It is an instrument that measures the properties of light over a specific portion of the electromagnetic spectrum, typically producing a spectrum which can be used to determine the material’s properties, such as concentration or composition.
- Spectrophotometer: It is a type of spectrometer that measures the intensity of light as a function of its wavelength. It quantifies how much a substance absorbs or transmits light at each wavelength.
- Components:
- Spectrometer: Typically consists of a device that disperses light into its different colors (wavelengths) and a detector to measure the intensity at each wavelength.
- Spectrophotometer: Contains a spectrometer within its system, along with a light source and a means to measure the intensity of light before and after it passes through a sample.
- Applications:
- Spectrometer: Used in various scientific fields such as physics, chemistry, and astronomy to study the properties of light and the information it can provide about a substance or celestial body.
- Spectrophotometer: Predominantly used in biochemistry for DNA, RNA, and protein analysis. It’s also used in colorimetry for industries like ink manufacturing, printing, and textile manufacturing.
- Measurement:
- Spectrometer: Measures the spectrum of light emitted or absorbed by a sample.
- Spectrophotometer: Measures the amount of light absorbed by a sample at each wavelength, often resulting in a graph of absorbance versus wavelength.
- Usage:
- Spectrometer: Can be used to study a wide range of electromagnetic spectra, including visible light, infrared, and ultraviolet.
- Spectrophotometer: Primarily used for studying substances in the ultraviolet and visible regions of the electromagnetic spectrum.
- Output:
- Spectrometer: Produces a spectrum which is a graphical representation of the relationship between a specific property of light and its wavelength.
- Spectrophotometer: Outputs data in terms of absorbance or transmittance, which can be used to determine concentration or purity of a sample.
In essence, while both instruments are rooted in the study of light-matter interactions, a spectrophotometer is a specialized type of spectrometer with specific applications in quantifying light absorption or transmission by a sample.
Spectrometer vs Spectrophotometer Chart
| Parameter | Spectrometer | Spectrophotometer |
|---|---|---|
| Basic Function | Measures properties of light over a specific portion of the electromagnetic spectrum. | Measures intensity of light as a function of its wavelength. Quantifies light absorption or transmission. |
| Components | Device that disperses light into different wavelengths and a detector. | Contains a spectrometer, light source, and means to measure light intensity before and after a sample. |
| Applications | Physics, chemistry, astronomy. | Biochemistry (DNA, RNA, protein analysis), colorimetry (ink, printing, textiles). |
| Measurement | Measures the spectrum of light emitted or absorbed by a sample. | Measures light absorbed by a sample at each wavelength. |
| Usage | Studies a wide range of electromagnetic spectra (visible, infrared, UV). | Primarily for ultraviolet and visible regions of the electromagnetic spectrum. |
| Output | Produces a spectrum (graphical representation of light property vs wavelength). | Outputs data in terms of absorbance or transmittance (absorbance vs wavelength graph). |


