Table of Contents
What is Carbohydrate?
- Carbohydrates are essential macronutrients found in a wide variety of foods and are a primary source of energy for the human body. They are composed of carbon, hydrogen, and oxygen atoms, typically in a ratio of 1:2:1. Carbohydrates can be classified into three main groups: monosaccharides, disaccharides, and polysaccharides.
- Monosaccharides are the simplest form of carbohydrates and cannot be further hydrolyzed into smaller sugar units. Common examples of monosaccharides include glucose, fructose, and galactose. These sugars are often referred to as single sugars and are easily absorbed by the body for quick energy.
- Disaccharides are formed when two monosaccharide units combine through a dehydration reaction, resulting in the formation of a glycosidic bond. Some well-known disaccharides include sucrose (table sugar), lactose (found in milk), and maltose (found in malted grains). Disaccharides are broken down into their individual monosaccharide units during digestion, allowing for absorption and utilization by the body.
- Polysaccharides are complex carbohydrates consisting of long chains of monosaccharide units. They serve as storage and structural compounds in plants and animals. Examples of polysaccharides include starch, glycogen, and cellulose. Starch is the primary energy storage molecule in plants, while glycogen serves as the storage form of glucose in animals. Cellulose, on the other hand, forms the structural component of plant cell walls and is indigestible by humans.
- Carbohydrates play a crucial role in providing energy for bodily functions. When consumed, carbohydrates are broken down into glucose, which is the preferred energy source for cells. Glucose can be readily utilized by cells or stored as glycogen in the liver and muscles for later use. Adequate carbohydrate intake is important for maintaining proper brain function, physical performance, and overall vitality.
- Furthermore, carbohydrates can have different effects on blood sugar levels based on their chemical structure and how quickly they are digested and absorbed. Carbohydrates with a high glycemic index, such as refined sugars and processed grains, are rapidly digested, causing a rapid rise in blood sugar levels. In contrast, carbohydrates with a low glycemic index, such as whole grains, fruits, and vegetables, are digested more slowly, resulting in a more gradual release of glucose into the bloodstream.
- It is worth noting that while carbohydrates are a vital energy source, excessive consumption of refined carbohydrates and added sugars can contribute to weight gain and various health issues, including type 2 diabetes and heart disease. Therefore, it is important to focus on consuming carbohydrates from whole, unprocessed sources and to maintain a balanced diet that includes a variety of nutrients.
- In conclusion, carbohydrates are organic compounds found in numerous foods and are a fundamental source of energy for the body. They are classified into monosaccharides, disaccharides, and polysaccharides, each with its unique characteristics and functions. Understanding the role of carbohydrates in our diet can help us make informed choices to support our overall health and well-being.
Definition of Carbohydrate
Carbohydrates are organic compounds consisting of carbon, hydrogen, and oxygen atoms, and they serve as a primary source of energy for the body.
Structure of Carbohydrates
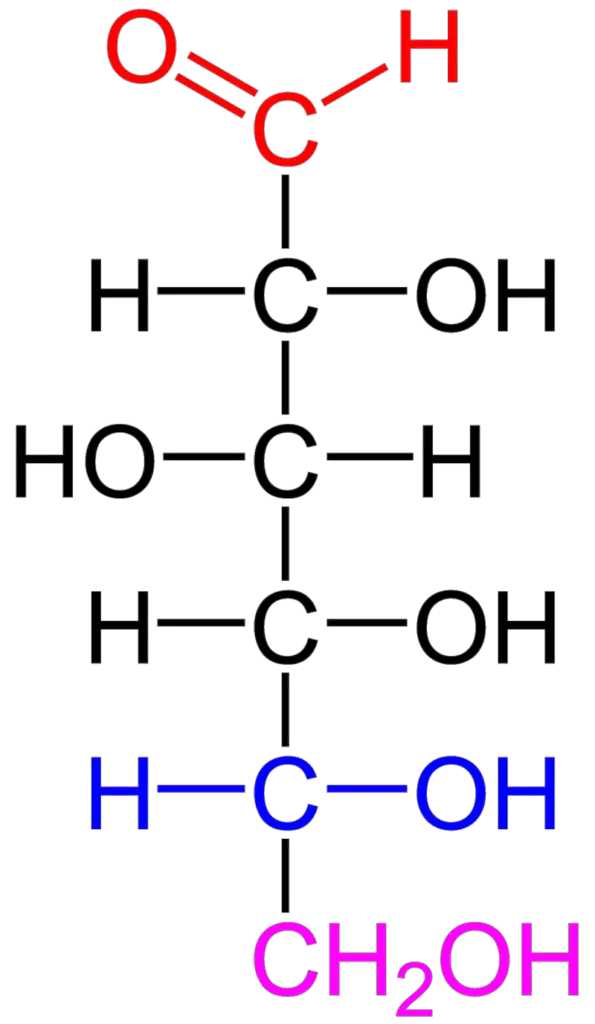
The structure of carbohydrates is based on their composition of carbon, hydrogen, and oxygen atoms. The empirical formula for carbohydrates is typically represented as (CH2O)n, where “n” represents the number of carbon atoms in the molecule.
Carbohydrates are organic compounds that can exist in the form of aldehydes or ketones, with multiple hydroxyl (OH) groups attached to the carbon chain. These hydroxyl groups play a significant role in the chemical properties and reactivity of carbohydrates.
The fundamental units or building blocks of carbohydrates are monosaccharides, which are simple sugars. Monosaccharides can be classified as either aldoses or ketoses, depending on whether they are polyhydroxy aldehydes or polyhydroxy ketones, respectively.
Carbohydrate structures can be represented in different forms:
- Open chain structure: This is the linear or straight-chain form of carbohydrates. It shows the carbon backbone with the various hydroxyl groups attached.
- Hemi-acetal structure: In this form, the first carbon of the monosaccharide condenses with the hydroxyl group of the fifth carbon, forming an intramolecular bond. This results in the formation of a ring structure, with one oxygen atom shared between two carbon atoms.
- Haworth structure: The Haworth structure represents the cyclic or ring form of carbohydrates, specifically the pyranose ring structure. It depicts the monosaccharide molecule folded into a ring shape, with oxygen atoms forming bridges between specific carbon atoms.
These different structural representations help us understand the diverse conformations and spatial arrangements that carbohydrates can adopt. The specific configuration and arrangement of functional groups within a carbohydrate molecule contribute to its biological activity and function.
In summary, carbohydrates exhibit a variety of structures, including open chain, hemi-acetal, and Haworth structures. These structures are determined by the presence of aldehyde or ketone functional groups and the formation of intramolecular bonds. Understanding the structural aspects of carbohydrates is crucial in comprehending their roles in biological processes and their interactions with other molecules.
Properties of Carbohydrates
Carbohydrates exhibit various physical and chemical properties that contribute to their unique characteristics and functions. Here, we will explore the properties of carbohydrates in terms of stereochemistry, optical activity, isomerism, annomerism, and their chemical reactivity.
Physical Properties:
- Stereoisomerism: Carbohydrates can exist as stereoisomers, which have the same structural formula but differ in spatial configuration. For example, glucose has two isomers: D-glucose and L-glucose, based on the arrangement around the penultimate carbon atom.
- Optical Activity: Carbohydrates can rotate the plane of polarized light. Glucose, for instance, can form two optical isomers: (+) glucose and (-) glucose, which differ in their direction of rotation.
- Diastereoisomers: Carbohydrates can also have diastereoisomers, where the configuration changes at specific carbon atoms such as C2, C3, or C4. Examples of diastereoisomers include mannose and galactose.
- Anomerism: Anomerism refers to the spatial configuration around the first carbon atom in aldoses and the second carbon atom in ketoses. This configuration affects the properties and reactivity of carbohydrates.
Chemical Properties:
- Osazone Formation: Carbohydrates can react with an excess of phenylhydrazine to form osazone derivatives. For example, glucose reacts to form glucosazone. This reaction is often used to identify and characterize specific carbohydrates.
- Benedict’s Test: Reducing sugars, when heated in the presence of an alkali, can convert to powerful reducing species known as enediols. Benedict’s reagent, when mixed with reducing sugars and heated, changes color to orange-red or brick red, indicating the presence of reducing sugars.
- Oxidation: Monosaccharides are considered reducing sugars because their carbonyl groups can be oxidized to carboxylic acids. For instance, D-glucose can be oxidized to D-gluconic acid. This property is utilized in tests like Benedict’s test to identify reducing sugars.
- Reduction to Alcohols: The carbonyl groups in open-chain forms of carbohydrates can be reduced to alcohols using reducing agents like sodium borohydride (NaBH4) or catalytic hydrogenation. The resulting products are called alditols.
Properties of Monosaccharides:
- Sweet Taste: Most monosaccharides have a sweet taste, with fructose being the sweetest among them, about 73% sweeter than sucrose (table sugar).
- Solid at Room Temperature: Monosaccharides are typically solid at room temperature, existing as crystalline substances.
- Water Solubility: Despite their relatively high molecular weights, monosaccharides are highly soluble in water due to the presence of multiple hydroxyl (OH) groups. This property allows them to form syrups even in minute amounts of water.
Properties of Disaccharides:
- Sweetness: Disaccharides generally have a sweet taste, although their sweetness can vary. For example, sucrose, which is composed of glucose and fructose, is commonly known as table sugar and has a sweet taste.
- Solubility: Disaccharides, like monosaccharides, are also soluble in water due to the presence of hydroxyl (OH) groups. This solubility allows for their easy dissolution in water, making them suitable for use in various food and beverage applications.
- Reducing Power: Some disaccharides, such as maltose and lactose, are reducing sugars. They have the ability to undergo oxidation reactions, similar to monosaccharides. This property is due to the presence of a free anomeric carbon in their structure.
- Hydrolysis: Disaccharides can be hydrolyzed, meaning they can be broken down into their constituent monosaccharides through a chemical reaction with water. This process is facilitated by enzymes, such as sucrase, maltase, and lactase, which are present in the digestive system.
Properties of Polysaccharides:
- Molecular Weight and Size: Polysaccharides are typically high-molecular-weight compounds composed of numerous monosaccharide units. Their large size and complex structures give them unique physical properties, such as high viscosity and the ability to form gels.
- Insolubility: Many polysaccharides, such as cellulose and chitin, are insoluble in water due to their extensive hydrogen bonding and crystalline nature. This insolubility contributes to their structural roles in organisms, such as providing rigidity to plant cell walls and forming the exoskeleton of arthropods.
- Storage and Energy Reserve: Certain polysaccharides, like starch and glycogen, serve as storage forms of glucose in plants and animals, respectively. They have a branched structure, which facilitates efficient storage and rapid mobilization of glucose when needed.
- Structural Integrity: Polysaccharides such as cellulose, chitin, and pectin provide structural support and integrity to various biological structures. For example, cellulose forms the main component of plant cell walls, providing rigidity and protection.
- Functionality in Food Industry: Some polysaccharides, like pectin and agar, have unique properties that make them useful in the food industry. They can function as thickeners, stabilizers, and gelling agents in food products, contributing to texture and stability.
Classification of Carbohydrates – Types of Carbohydrates
Carbohydrates can be classified into different types based on their molecular structure and composition. The main types of carbohydrates include monosaccharides, oligosaccharides, and polysaccharides.
- Monosaccharides: Monosaccharides are the simplest form of carbohydrates and cannot be further hydrolyzed into smaller units. They are colorless, crystalline solids that are soluble in water and insoluble in non-polar solvents. Monosaccharides have the general formula of Cn(H2O)n or CnH2nOn, where “n” represents the number of carbon atoms in the molecule. They are classified based on the number of carbon atoms and the functional group present. For example, trioses have three carbon atoms, pentoses have five carbon atoms, and hexoses have six carbon atoms. Monosaccharides are further categorized as aldoses or ketoses, depending on whether they contain an aldehyde or ketone group. Examples of monosaccharides include glucose, fructose, erythrulose, and ribulose.
- Oligosaccharides: Oligosaccharides are compound sugars that yield 2 to 10 molecules of monosaccharides upon hydrolysis. They are formed when monosaccharide units are joined together by glycosidic linkages. Based on the number of monosaccharide units, oligosaccharides are further classified as disaccharides, trisaccharides, tetrasaccharides, and so on. Disaccharides yield two monosaccharide units upon hydrolysis, while trisaccharides yield three, and so on. Disaccharides, such as sucrose, lactose, and maltose, are commonly known examples of oligosaccharides.
- Polysaccharides: Polysaccharides are complex carbohydrates composed of more than 10 monosaccharide units, and they can consist of hundreds of sugar units in length. Polysaccharides are also referred to as glycans. They yield more than 10 molecules of monosaccharides upon hydrolysis. Polysaccharides can differ in the identity of their monosaccharide units, the length of their chains, the types of bonds linking the units, and the degree of branching. They serve important functions in the body, including structural support and energy storage. Polysaccharides can be further classified based on the type of molecules produced upon hydrolysis. Homopolysaccharides consist of monosaccharides of the same type, while heteropolysaccharides contain monosaccharides of different types. Examples of homopolysaccharides include starch, glycogen, cellulose, and pectin, while examples of heteropolysaccharides include hyaluronic acid and chondroitin.
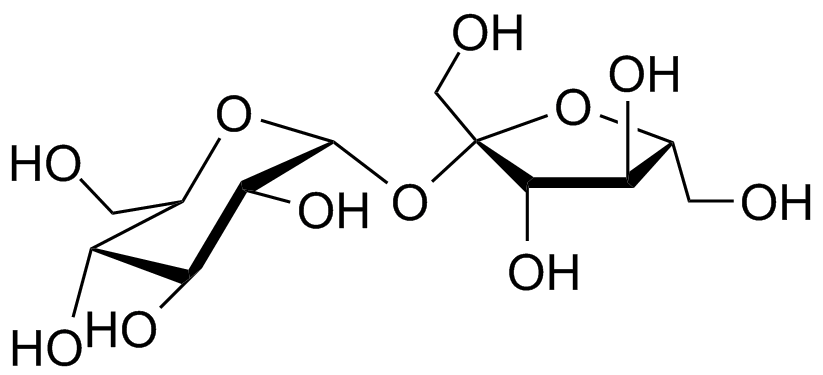
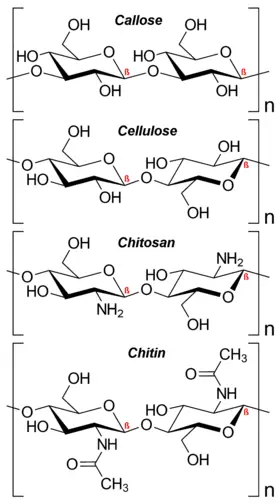
In summary, carbohydrates can be classified into monosaccharides, oligosaccharides, and polysaccharides based on their molecular structure and composition. This classification allows for a better understanding of the diverse nature and functions of carbohydrates in various biological processes.
Functions of Carbohydrates
Carbohydrates play a crucial role in various biological functions and are essential for the proper functioning of living organisms. Some of the key functions of carbohydrates are:
- Energy Source: Carbohydrates are the primary source of energy for the body. When consumed, they are broken down into glucose, which is then used by cells as fuel for various metabolic activities. Glucose is particularly important for the brain and nervous system, as they rely heavily on carbohydrates for energy.
- Energy Storage: Excess glucose is converted into glycogen and stored in the liver and muscles. When energy demand is high, such as during physical activity or fasting, glycogen is broken down into glucose and released into the bloodstream to maintain stable blood sugar levels.
- Structural Support: Carbohydrates play a structural role in organisms. In plants, cellulose forms the cell walls, providing rigidity and support. In animals, chitin is found in the exoskeleton of arthropods, such as insects and crustaceans, providing a protective framework.
- Biosynthesis: Carbohydrates serve as intermediates in the biosynthesis of other important molecules. They are involved in the production of lipids, proteins, and nucleic acids, playing a vital role in cellular growth, repair, and maintenance.
- Cell Communication: Carbohydrates are attached to proteins and lipids on the cell surface, forming glycoproteins and glycolipids. These carbohydrate-rich molecules act as surface antigens, receptor molecules, and signaling molecules, enabling cell-cell communication and interactions with the surrounding environment.
- Immune Modulation: Some carbohydrates, such as certain types of dietary fiber, can modulate the immune system. They help support a healthy gut microbiota, enhance the function of immune cells, and contribute to overall immune system regulation.
- Connective Tissue: Carbohydrates, specifically glycosaminoglycans, are important components of connective tissues. They contribute to the structure, elasticity, and hydration of tissues such as cartilage, tendons, and skin.
- Digestive Health: Carbohydrates that are rich in dietary fiber, such as whole grains, fruits, and vegetables, promote digestive health. They add bulk to the stool, prevent constipation, and support a healthy digestive system.
- Molecular Recognition: Carbohydrates are involved in molecular recognition processes. They participate in the recognition of pathogens by the immune system, assist in cell adhesion and migration, and contribute to the specificity of biological interactions.
Overall, carbohydrates are multifunctional molecules that provide energy, support structure, facilitate communication, and participate in essential biological processes. Including a variety of carbohydrates in the diet is important for maintaining optimal health and well-being.
FAQ
What are carbohydrates?
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen. They are a major source of energy for the body and play important roles in various biological processes.
What are examples of carbohydrates?
Common examples of carbohydrates include sugars (glucose, fructose), starches, fibers, and glycogen.
What is the function of carbohydrates in the body?
Carbohydrates provide energy for bodily functions, support brain function, serve as a structural component in tissues, and play a role in cellular communication and recognition.
Are carbohydrates bad for you?
Carbohydrates are not inherently bad for you. It is important to differentiate between healthy carbohydrates (whole grains, fruits, vegetables) and unhealthy ones (refined sugars, processed foods). A balanced intake of carbohydrates is crucial for overall health.
How many calories do carbohydrates provide?
Carbohydrates provide approximately 4 calories per gram. This is the same energy value as proteins, while fats provide 9 calories per gram.
Are carbohydrates essential in the diet?
Carbohydrates are not considered essential nutrients, as the body can derive energy from other sources. However, they are an important and efficient energy source and provide various health benefits when consumed in appropriate amounts.
What is the recommended daily intake of carbohydrates?
The recommended carbohydrate intake varies based on factors such as age, sex, activity level, and overall health. In general, carbohydrates should contribute 45-65% of total daily caloric intake.
Are all carbohydrates the same?
Carbohydrates can be classified as simple (sugars) or complex (starches, fibers). Complex carbohydrates are generally considered healthier as they provide sustained energy and are accompanied by additional nutrients and dietary fiber.
Can carbohydrates cause weight gain?
Consuming excess calories from any macronutrient, including carbohydrates, can contribute to weight gain. However, weight management is more about overall calorie balance and the quality of the diet, rather than solely blaming carbohydrates.
Should carbohydrates be avoided in a low-carb diet?
Low-carb diets may have specific health benefits for some individuals, such as weight loss or managing certain medical conditions. However, it is important to consult with a healthcare professional or registered dietitian before making significant changes to your diet.
References
- Nelson, D. L., Cox, M. M. Lehninger Principles of Biochemistry. W.H. Freeman and Company, 2017.
- Berg, J. M., Tymoczko, J. L., Gatto, G. J. Stryer, L. Biochemistry. W.H. Freeman and Company, 2018.
- Campbell, M. K., Farrell, S. O. Biochemistry. Cengage Learning, 2017.
- Voet, D., Voet, J. G., Pratt, C. W. Fundamentals of Biochemistry: Life at the Molecular Level. Wiley, 2016.
- Lennarz, W. J., Lane, M. D. Encyclopedia of Biological Chemistry. Elsevier Science, 2013.
