Table of Contents
Definition of Decarboxylase Test
Decarboxylase Test Medium base is used to distinguish bacteria based on their ability to decarboxylate amino acids.
- Moeller reported the first practical application of the amino acid decarboxylase testing for the identification of microorganisms.
- Moeller’s work was based upon the experiments by Gale, Gale, and Epps with bacterial amino acid degradation enzymes, decarboxylases.
- Moeller noticed that Enterobacteriaceae members produced lysine, arginine and ornithine decarboxylase. This was an important parameter for other biochemical tests to distinguish bacteria from closely related ones.
- Calquist also developed a medium that uses the lysine-decarboxylase reaction to distinguish Salmonella arizonae and Citrobacter.
- Falkow developed later the lysine-decarboxylase medium to distinguish Salmonellae from Shigellae using the reliable and valid results.
- BIS recommends this medium for the detection of Vibrio Cholerae’s dihydrolase or decarboxylase activity.
- Enteric bacteria ferments dexrose, which results in an acidic pH. Bacteria that produce lysine, ornithine, or arginine will make alkaline products which will increase the pH.
- After 24-96 hours, the result will be a purple-colored alkaline reaction for bacteria producing decarboxylase and an acid pH (yellow), for bacteria not producing it.
- To prevent false alkalinization, inoculated tubes should be shielded from the air by covering the medium with sterile oil. Inoculate control tubes made from basal media.
- Only pure culture isolation should be used for biochemical testing.
- Although the decarboxylase reactions may be indicative of a particular genus or specie, they cannot be used to determine definitive and final identifications of these organisms.
Principle of Decarboxylase Test
- The decarboxylase test is used for determining decarboxylase production and amino acid decarboxylation in bacteria, especially in the family of Enterobacteriaceae species.
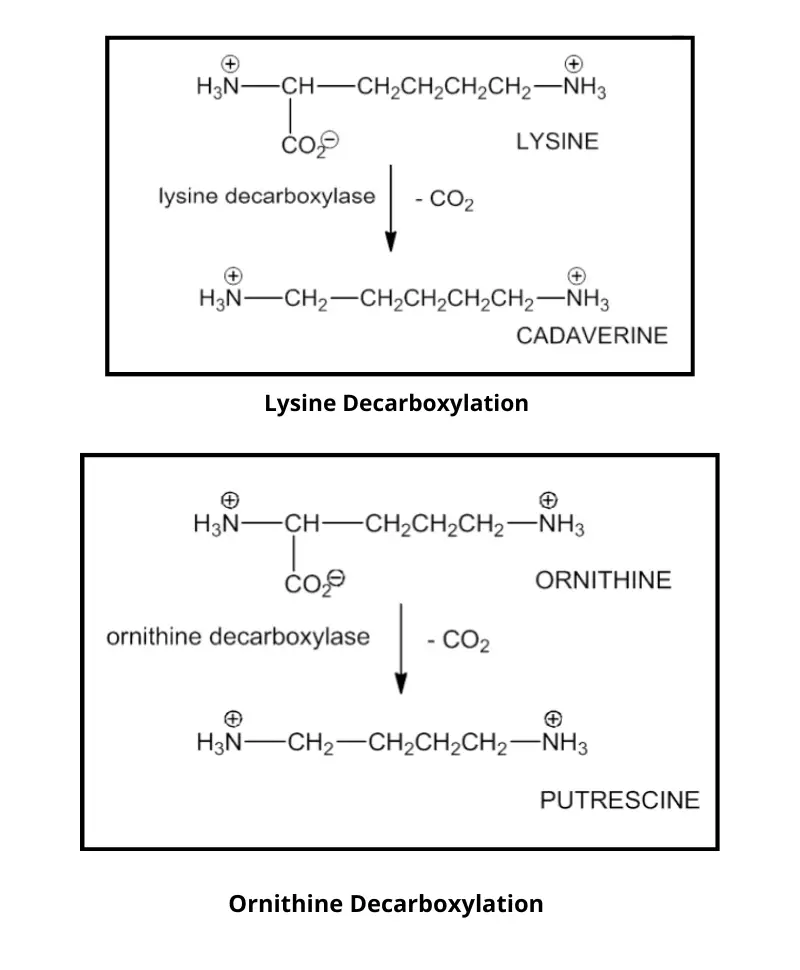
- This simple test can be used to determine if bacteria is capable of producing dihydrolase or decarboxylase. These enzymes are used to remove the carboxyl group of amino acids and form amines.
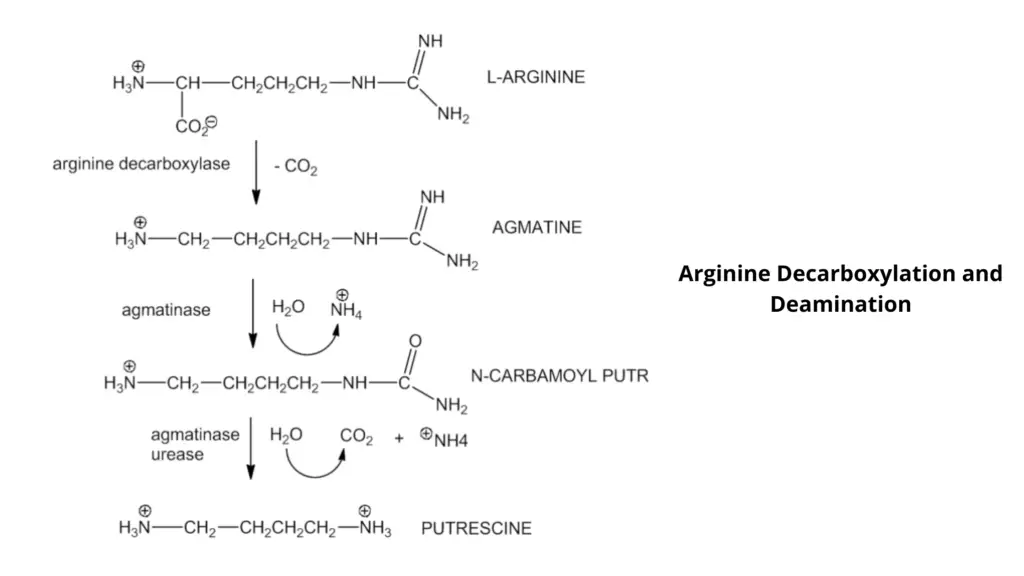
- Decarboxylases are used to remove the carboxyl groups from amino acids such as lysine ornithine, while dihydrolase is used for arginine decarboxylation, in addition to decarboxylase.
- The Decarboxylase medium is made up of beef extract and peptic digestions of animal tissues, which provide nitrogenous nutrients to the organisms.
- Medium contains glucose, a fermentable carbohydrate.
- Bromocresol purple, and cresol red are pH indicators that change color under acidic or alkaline conditions. They help to detect glucose fermentation and amino acid decarboxylation.
- Pyridoxal is the cofactor for decarboxylase.
- To promote fermentation, decarboxylation or dihydrolation are anaerobic processes. Therefore, each tube is coated with sterile mineral oils to protect the medium from oxygen.
- Inoculating medium with a bacterium that can ferment glucose produces acid, which results in a drop in pH. The pH indicator also changes the color of the medium to yellow.
- Decarboxylase is also stimulated by acid production.
- Decarboxylase is a process that decarboxylates an added amino acid, resulting in the formation of amines.
- The pH indicator will change from yellow to purple due to amine formation (results in the increase of the pH).
- The medium will remain yellow or acidic if the organism that is being tested ferments glucose but not decarboxylase.
Objective of Decarboxylase Test
- To determine if an organism can produce a decarboxylase enzyme.
- To distinguish the Enterobacteriaceae members based on their ability to produce decarboxylase enzymes.
Media, Reagents, and Supplies Used
- Media: Decarboxylase Test Medium Base. Other media like Motility-indole-ornithine medium (MIO) and Lysine iron agar can also be used.
- Reagents Used: Mineral oil, Vaspar, liquid paraffin, or petroleum jelly, maintained at 56°C in liquid form
- Other: Sterile sticks or inoculating loops, Incubator at 35°C
Composition of Decarboxylase Test Medium Base
| Base broth | (g/liter) |
| Peptone | 5g |
| Beef Extract | 5g |
| Glucose | 0.5g |
| Bromocresol Purple | 0.01g |
| Cresol Red | 0.005g |
| Pyridoxal | 0.005g |
| Distilled water | 1L |
To prepare different decarboxylase broths with one of the following three L-amino acid
| Arginine | 10g (1%) |
| Lysine | 20g (2%) |
| Ornithine | 10g (1%) |
Preparation of media
- Prepare the base broth first by boiling the first six ingredients in 1 Liter of distilled water.
- The solution should be heated gently until it is completely dissolved.
- To make different decarboxylase broths, you can add L-arginine or L-lysine or Lornithine.
- Mix the ingredients, stirring frequently. Bring to boil.
- Adjust the pH once L-ornithine has been added. The final pH of the medium should reach 6.0 + 0.02 at 25oC.
- For a column height of approximately 3.5 cm, dispense 5 ml in screw-cap tubes (12.5 cmx1.5 cm tubes).
- For 15 minutes at 15 psi, autoclave the medium at 120o C.
- Prepared broth should be light brown in color. It should be kept at 8oC from direct sunlight.
You can also buy pre-mixed, dehydrated powder of decarboxylase broth from commercial suppliers. To prepare the broth, you should follow the manufacturer’s instructions. You can also purchase pre-made broth from bio supply companies. You can also purchase sterilized mineral oil from biological supply businesses.
Procedure of Decarboxylase Test
Inoculation
- As an inoculation source, use a fresh (18-to 24-hour) culture of test bacteria.
- Aseptically transfer one isolated colony to the decarboxylase broth tube along with one of the amino acids.
- Inoculate a tube of control decarboxylase broth base (without any amino acid) and the tube containing decarboxylase broth (as a control).
- Inoculate the tubes with 1 ml of sterile oil. This will separate the medium from oxygen. It will promote fermentation of glucose, decarboxylation reactions, and seal the caps on the test tubes.
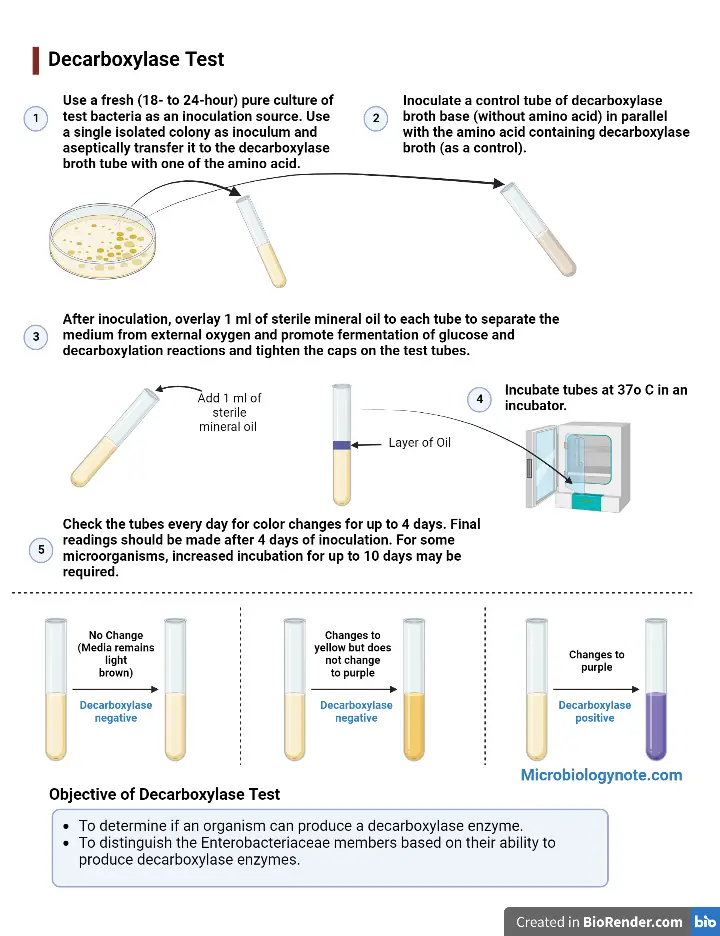
Incubation
- Incubate tubes in an incubator at 37oC.
- For color changes lasting up to four days, make sure you check the tubes each day.
- After 4 days of inoculation, final readings should take place.
- Some microorganisms may require an increased incubation time of up to 10 days.
Result Interpretation of Decarboxylase Test
For signs of fermentation or decarboxylation, check the medium’s color every day for at least 10 days. Uninoculated Moeller’s media base and media with an amino acid are light brown.
- Decarboxylase negative test: If the medium’s color does not change, it means that the organism does not ferment glucose.
- Decarboxylase negative test: A medium that changes to yellow color indicates that it has fermented glucose. However, the organism is not decarboxylase positive (-) for this amino acid. The appearance of yellow broth indicates fermentation of glucose, but it is not indicative of decarboxylation.
- Decarboxylase positive test: A purple color indicates that the media has been decarboxylated and formed amines (alkaline byproducts). This is a sign that the organism is (+) for the amino acid. If the media does not turn purple, it means that the amino acid has not yet been decarboxylated. The organism also did not produce decarboxylase enzymes.
| Media color | Bacterial reaction |
| No Change (Media remains light brown) | Decarboxylase negative (-) |
| Changes to yellow but does not change to purple | Decarboxylase negative (-) |
| Changes to purple | Decarboxylase positive (+) |




Decarboxylase Test Result of Some organisms
| Test organism | Lysine decarboxylase test | Arginine decarboxylation test | Ornithine decarboxylase test |
| Escherichia coli | Positive Result | Positive Result | Positive Result |
| Enterobacter cloacae | Negative Result | Positive Result | Positive Result |
| Klebsiella pneumoniae | Positive Result | Negative Result | Negative Result |
| Klebsiella oxytoca | Positive Result | Negative Result | Negative Result |
| Enterobacter aerogenes | Positive Result | Negative Result | Positive Result |
| Proteus vulgaris | Negative Result | Negative Result | Negative Result |
| Pseudomonas aeruginosa | Negative Result | Positive Result | Negative Result |
| S. serotype typhi | Positive Result | delayed positive reaction or negative reaction,yellow colour | Negative Result |
| Serratia marcescens | Positive Result | Negative Result | Positive Result |
| Shigella flexneri | Negative Result | delayed positive reaction or negative reaction,yellow colour | Negative Result |
| Vibrio cholerae | Negative Result | Positive Result | Positive Result |
Precautions
- Every isolate should be inoculated in a tube containing a basal medium without any amino acid to act as a growth control or negative reference.
- Before incubation, cover the broth with sterile mineral oil. The medium may become alkaline from air exposure, resulting in false positives.
- Different amino acids are not the same in different broths. Be sure to label them so you know which amino acid is in each tube.
- After at least 24 hours incubation, test interpretation should be done.
- Some microorganisms may require a longer incubation time of up to 10 business days.
- Because of incomplete sterility, autoclaving mineral oil should not be done. Sometimes water/condensation can be introduced to the oil and steam doesn’t penetrate as well. This is why sterility is not always achieved. Many recommend drying out small amounts of steam or filtering warmed mineral oil. However, autoclaving is not recommended. There have been reports of steam/oil mixtures explosions.
- There are two media that have been used in differentiating certain members of the Enterobacteriaceae. LIA (Lysine Iron Agar), which is used to distinguish Salmonella species, can be used. It detects hydrogen sulfide and decarboxylation of lysine. MIO (Motility, Indole Ornithine) medium has been recommended for use to test motility, indole production, and ornithine-decarboxylase activity of enteric bacilli.
Control strains
Positive
- Lysine: Klebsiella pneumoniae
- Ornithine: Enterobacter cloacae
- Arginine: Enterobacter cloacae
Negative
- Lysine: Enterobacter cloacae
- Ornithine: Klebsiella pneumoniae
- Arginine: Klebsiella pneumoniae
Uses of Decarboxylase Test
- Plesiomonas has positive results for lysine, ornithine and arginine. This separates it from Vibrio or Aeromonas, which have different results for each species, but not all three decarboxylases positive.
- Staphylococcus lugdunensis is the only Staphylococcus that is pyrrolidinyl-bnaphthylamide (PYR) and ornithine positive.
- Arginine can be used to identify Enterococcus at the species level. Enterococcus aureum is arginine-negative, while Enterococcus flavius and Enterococcus infaecium have arginine-positive.
- Stenotrophomonas maltophilia and Burkholderia cepacia are among the few non-glucose-fermenting, gram-negative rods that are lysine positive. Burkholderia mallei, a polymyxin B-resistant non fermenter, and Burkholderia pseudomallei, are both arginine-positive.
- Lysine Decarboxylase Test: This test is used to identify Salmonellae (positive), and Shigellae, (negative).
Limitations of Decarboxylase Test
- Before incubation, test interpretation should be done within 18 to 24 hours. An earlier interpretation could lead to incorrect results. Incubation for 10-12 hours is sufficient to trigger glucose fermentation. The acidic environment that fermentation creates results in yellow color formation. Once the acidic environment has been established, decarboxylase enzymes cannot be produced.
- Before you interpret the reaction, gently shake the tube if two layers of color appear.
- Non-glucose-fermenting microorganisms may display weak decarboxylase activity, thereby resulting in an insufficient production of amines necessary to convert the pH indicator system. However, some non-fermenters will produce enough amines that the purple color of the basal medium will be deeper than it would be in an uninoculated tube.
- Gray color could indicate a decrease in the indicator rather than alkaline production. Bromocresol purple can be used to aid in interpreting the reaction.
- Nonfermenting bacteria must be arginine-positive and lysine and ornithine negative.
FAQ
Why isn’t a different base broth required for each decarboxylase test?
Base broths do not contain amino acids are therefore unspecific, that’s why there isn’t a different base broth required for each decarboxylase test.
What does lysine decarboxylase test for?
Lysine decarboxylase test assist in the identification of Salmonellae (+ve) and Shigellae (-ve).
why is glucose important for the lysine decarboxylase test to work?
The microbe must first use the glucose present to cause the pH to drop. This is indicated by a change from purple to yellow. Once the medium has been acidified, the enzyme lysine decarboxylase is activated. The culture is incubated an additional 24 hours at 35-37 C to allow the microbe to now use the lysine.
what test is glutamic acid decarboxylase for?
This test is intended for the semiquantitative determination of glutamic acid decarboxylase (GAD) antibody in human serum; it is useful as an aid in the diagnosis of type 1 diabetes mellitus (autoimmune mediated diabetes).
what does a negative lysine decarboxylase test indicate?
Lysine decarboxylation results in cadaverine. These byproducts are sufficient to raise the pH of the media so that the broth turns purple. If the inoculated medium is yellow, or if there is no color change, the organism is decarboxylase-negative for that amino acid.
what ph indicator is used in decarboxylase test?
Decarboxylase broth contains nutrients, dextrose (a fermentable carbohydrate), pyridoxal (an enzyme cofactor for decarboxylase), and the pH indicators bromcresol purple and cresol red. Bromcresol purple turns purple at an alkaline pH and turns yellow at an acidic pH.
what does the lysine decarboxylase test check?
Lysine decarboxylase test assist in the identification of Salmonellae (+ve) and Shigellae (-ve).
References
- Lynae S. Carcia, Second Edition update, Clinical Microbiology Procedures Handbook
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- B.D. Skerman, A guide to the identification of the genera of bacteria, The Williams & Wilkins Co., Baltimore, MD, (1967)
- Cowan and Steel’s, manual for the identification of medical bacteria
- Fay GD, Barry AL. 1972. Rapid ornithine decarboxylase test for the identification of Enterobacteriaceae. Appl. Microbiol 23: 710 – 713.
- Fluka Analytical D2935 Decarboxylase broth base, Moeller.
www.sigma-aldrich.com - Gilardi GL. 1971. Characterization of Pseudomonas species isolated
from clinical specimens. Appl. Microbiol 21: 414 – 419. - Gilardi GL. 1972. Practical scheme for the identification of nonfermentative gram negative bacteria encountered in medical
bacteriology. Amer. J. Med. Technol. 38: 65 -72. - Moeller V. 1954. Activity determination of amino acid
decarboxylases in Enterobacteriaceae. Acta Pathologica et microbiologica
Scandinavica 34: 102 – 114. - https://microbiologyinfo.com/amino-acid-decarboxylase-test/
- https://journals.asm.org/doi/pdf/10.1128/aem.37.2.254-260.1979
- https://www.onlinebiologynotes.com/decarboxylase-test-principle-procedure-and-results/
- https://www.austincc.edu/microbugz/decarboxylation_test.php
- https://himedialabs.com/TD/M912S.pdf
- https://www.vumicro.com/vumie/help/VUMICRO/Ornithine_decarboxylase_Test.htm
- https://microbeonline.com/decarboxylation-test-types-uses-principles-procedure-results/
- https://universe84a.com/decarboxylase-test/
