Table of Contents
What is Electroporator (Electroporation machine)?
- An electroporator, also known as an electroporation machine, is a device widely used in the field of molecular biology and genetic engineering. Its main purpose is to facilitate the introduction of foreign DNA, RNA, or other molecules into cells through the application of electric pulses.
- The concept of electroporation dates back to the 1960s when researchers observed that applying an external electric field to a cell resulted in the development of a larger membrane potential at the two poles of the cell. Subsequently, in the 1970s, it was discovered that the cell membrane could break and recover once the membrane potential reached a critical level. Building upon these findings, Dr. Thomas C. Weaver and his colleagues made a significant breakthrough in the 1980s by utilizing electric fields to create transient pores in the cell membrane, thus enabling the entry of molecules. This process was named electroporation.
- An electroporator typically consists of a power supply unit, a capacitor bank, and an electrode chamber or cuvette. The power supply unit generates the electrical pulses required for electroporation. The capacitor bank stores and releases electrical energy in the form of pulses, which are delivered to the cells. The electrode chamber or cuvette holds the cell suspension and electrodes through which the electric pulses are applied.
- Electroporation has become an essential tool in molecular biology and genetic engineering research. It allows scientists to efficiently introduce genetic material into cells, enabling the study of gene function, protein expression, and the development of new therapies and biotechnological applications. Electroporation has found applications in various fields, including medicine, agriculture, and biopharmaceutical production, contributing to advancements in genetic research and biotechnology.
What is Electroporation?
- Electroporation, also known as electropermeabilization, is a microbiology technique used to increase the permeability of cell membranes by applying an electrical field. This technique allows the introduction of various substances, including chemicals, drugs, electrode arrays, or DNA, into the cells. Electroporation is commonly used in microbiology to transform bacteria, yeast, or plant protoplasts by introducing new coding DNA. The process involves applying thousands of volts across suspended cells in an electroporation cuvette, leading to the temporary formation of pores in the cell membrane.
- In the field of genetic engineering, electroporation is highly efficient for introducing foreign genes into tissue culture cells, particularly mammalian cells. It is widely used in the production of knockout mice, as well as in tumor treatment, gene therapy, and cell-based therapies. The introduction of foreign DNA into eukaryotic cells through electroporation is known as transfection. Electroporation is particularly effective for transfecting cells in suspension using electroporation cuvettes. It has also been successfully employed for in vivo applications, such as in utero or in ovo transfection. Additionally, adherent cells can be transfected using electroporation as an alternative to trypsinizing cells prior to transfection.
- Despite its advantages, electroporation has some limitations. One drawback is the potential impact on gene expression, as over 7,000 genes can be affected after the process. This can pose challenges in studies where precise and controlled gene expression is crucial for accurate results. However, efforts have been made to miniaturize electroporation techniques, leading to microelectroporation and nanotransfection approaches. These advancements utilize electroporation-based techniques via nanochannels to deliver cargo to cells in a minimally invasive manner, enhancing the efficiency and viability of the process.
- Moreover, electroporation has been utilized as a means to induce cell fusion. Artificially induced cell fusion has various applications, including investigating and treating diseases like diabetes and regenerating axons in the central nervous system. It is also employed in producing cells with desired properties, such as in cell vaccines for cancer immunotherapy. The production of monoclonal antibodies in hybridoma technology is a well-known application of cell fusion, where specific antibody-producing B lymphocytes are fused with a myeloma cell line, resulting in hybrid cell lines called hybridomas.
- In summary, electroporation is a versatile technique that enables the introduction of substances into cells by increasing the permeability of the cell membrane through the application of an electrical field. It has revolutionized the fields of genetic engineering, microbiology, and cell biology, playing a critical role in various applications, ranging from basic research to therapeutic advancements.
Definition of Electroporator (Electroporation machine)
An electroporator is a scientific instrument or device used in molecular biology and genetic engineering to facilitate the process of electroporation. Electroporation involves the application of electric pulses to cells in order to create temporary pores in their cell membranes, allowing the introduction of foreign molecules such as DNA, RNA, or other substances. The electroporator is responsible for delivering precise and controlled electric pulses to the cells, ensuring the creation of transient pores without causing permanent damage to the cell membranes. It typically consists of a pulse generator, electrodes, and a control system that allows researchers to adjust the pulse parameters to suit their specific experimental requirements. The electroporator has become an essential tool in the field, enabling efficient and targeted delivery of molecules into cells and opening up new possibilities for genetic manipulation, gene therapy, and various biotechnological applications.
Principle of Electroporation
The principle of electroporation involves the application of an optimized electrical pulse to a cell suspension containing host cells and desired molecules, such as DNA. The electrical pulse typically lasts for a few microseconds and is delivered at an optimized voltage.
When the electrical pulse is discharged, it disrupts the phospholipid bilayer of the cell membrane. This disruption results in the formation of temporary pores in the membrane. These pores allow for the exchange of molecules between the extracellular environment and the interior of the cell.
As the electrical pulse is applied, the electrical potential across the membrane increases. This increased potential facilitates the entry of charged molecules, such as DNA, into the cell through the temporary pores. This process is analogous to electrophoresis, where charged particles move in an electric field.
The temporary pores formed during electroporation allow molecules to pass through the cell membrane, which under normal circumstances would not be able to enter. This property of electroporation makes it a powerful tool for introducing foreign molecules, such as DNA, into cells for various applications in molecular biology and genetic engineering.
By carefully optimizing the voltage, duration, and other parameters of the electrical pulse, researchers can achieve efficient and controlled electroporation. This enables the introduction of desired molecules into cells, opening up possibilities for gene transfer, genetic modification, and other molecular manipulations.
In summary, the principle of electroporation involves the application of an optimized electrical pulse to disrupt the cell membrane, leading to the formation of temporary pores. The increased electrical potential across the membrane allows charged molecules, such as DNA, to enter the cells through these pores. This process enables the efficient introduction of foreign molecules into cells and plays a vital role in various fields of research and applications in molecular biology and genetic engineering.
Types of Electroporation
Electroporation can be categorized into different types based on the cells involved, the configuration of the electroporator, and the mode of operation. Let’s explore each type in more detail:
Types of Electroporation based on Cells Involved:
- Bulk Electroporation: This type involves the electroporation of a bulk population of cells. A homogeneous electric field is applied to a suspension of cells, and the electrical pulses are delivered to the entire population simultaneously. Bulk electroporation is commonly used when a large number of cells need to be treated simultaneously, such as in transfection experiments or cell-based assays.
- Single-Cell Electroporation: In this type, individual cells are electroporated. It is particularly useful for single-cell studies or when precise targeting of specific cells is required. Single-cell electroporation involves creating a local electric field near a single cell to deliver the electrical pulses.
Types of Electroporators based on Configuration:
- Open Electroporators: These electroporators allow users to configure various settings such as pulse length, pulse intensity, waveform shape, and other parameters. Researchers have more flexibility in customizing the electroporation conditions to suit their specific experimental needs.
- Closed Electroporators: In closed electroporators, the user selects pre-designed configurations or predefined protocols for electroporation. These configurations are optimized and set by the manufacturer, providing a simpler and user-friendly experience. Closed electroporators are often preferred when standardized and reproducible electroporation protocols are required.
Types of Electroporators based on Mode of Operation:
- Exponential Decay Electroporators: These electroporators generate electrical pulses with an exponential decay waveform. The voltage and capacitance settings can be adjusted to optimize the pulse parameters, allowing the development of a pulse gradient. Exponential decay electroporators are commonly used for various electroporation applications, including mammalian cell transfection.
- Square Wave Electroporators: Square wave electroporators generate electrical pulses with a square waveform. The pulse characteristics include voltage, pulse duration, pulse frequency, and pulse timing. Square wave electroporators are frequently used in mammalian cell electroporation experiments, offering precise control over the pulse parameters.
Additionally, there are electroporators known as time-constant electroporators. These electroporators deliver a single voltage sustained for a designated period of time, characterized by the time constant pulses. Time-constant electroporators are suitable for specific applications where a constant voltage is required.
In summary, electroporation can be classified into different types based on the cells involved (bulk and single-cell electroporation), the configuration of the electroporator (open and closed electroporators), and the mode of operation (exponential decay and square wave electroporators). Understanding these types allows researchers to choose the most appropriate electroporation method and electroporator for their specific experimental requirements.
Parts of Electroporator
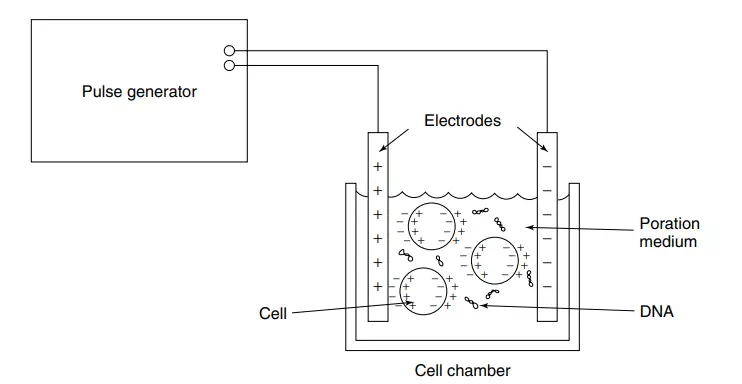
An electroporator, which is used for electroporation, consists of several key parts that work together to facilitate the process. These components include:
- Power supply: The power supply is the central component of an electroporator. It provides the electrical energy required to generate the electric fields necessary for electroporation. The voltage output of the power supply can range from a few volts to kilovolts, depending on the specific application. It can also deliver short pulses of energy.
- Pulse generator: The pulse generator is responsible for controlling the characteristics of the electrical pulses used in electroporation. It regulates the amplitude, duration, and frequency of the pulses. These parameters play a crucial role in achieving efficient electroporation, as they determine the magnitude and timing of the electric field applied to the cells.
- Cuvettes: Cuvettes are specialized containers, typically made of disposable plastic or glass, used for holding the cell suspension and the molecules to be introduced into the cells. The size and design of the cuvettes may vary to accommodate different sample volumes. Cuvettes have two metal electrodes positioned opposite each other, which enable the delivery of the electric pulse to the cells.
- Electrodes: Electrodes are the components that deliver the electric pulses from the electroporator to the cells. They are typically metal plates or wires that make direct contact with the sample inside the cuvette. Proper alignment of the electrodes is crucial to ensure a uniform distribution of the electric field across the cells, which enhances the efficiency and consistency of the electroporation process.
In addition to these core components, modern electroporators may also include additional features and controls for enhanced functionality and user convenience. These can include user-friendly interfaces, programmable settings, and safety mechanisms to ensure the precise and controlled delivery of electric pulses for optimal electroporation results.
Steps for performing electroporation – electroporation protocol
Performing electroporation typically involves a series of steps to ensure successful delivery of nucleic acids into cells. Let’s go through these steps:
- Cell Preparation: The first step is to prepare the cells for electroporation. Cells are typically grown and cultured according to standard laboratory protocols. Prior to electroporation, cells are collected and suspended in a recommended electroporation buffer. The buffer composition may vary depending on the cell type and specific experimental requirements. The cell suspension should be prepared in a way that ensures optimal cell viability and health during the electroporation process.
- Electric Pulse Application: Once the cells are prepared, the next step is to apply the electric pulse. The cell suspension, along with the desired nucleic acids or other molecules, is placed in a suitable electroporation cuvette or chamber. The cuvette contains electrodes that allow the electric pulse to be delivered to the cell suspension. The electric pulse is generated by the electroporator and is applied to the cells in the presence of the electroporation buffer and nucleic acids. During this step, the electric pulse causes potential differences across the cell membrane, leading to the creation of transient pores or openings in the membrane. These transient pores facilitate the entry of the nucleic acids or other molecules into the cells. The parameters of the electric pulse, such as voltage, duration, and number of pulses, are carefully optimized based on the cell type and specific experimental requirements to ensure efficient and successful electroporation.
- Cells Return to Growing Conditions: After the electric pulse application, the cells are returned to their appropriate growth conditions. This involves transferring the electroporated cells back to their culture medium or growth medium under suitable incubation conditions. The cells are allowed to recover and adapt to their normal growth environment. The recovery period may vary depending on the cell type and specific experimental considerations.
- Cells Assay: The final step involves assaying the cells for the desired gene expression or other outcomes. This step typically involves analyzing the cells to determine if the introduced nucleic acids have successfully expressed genes of interest or brought about specific changes in cellular functions. Assays may include techniques such as PCR, gene expression analysis, fluorescence microscopy, or functional assays, depending on the experimental goals.
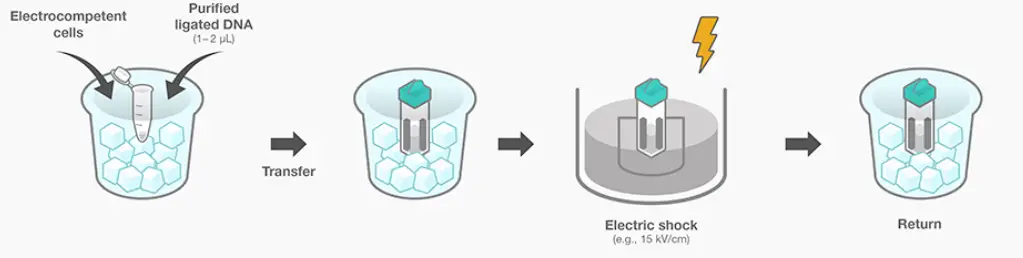
By following these steps, researchers can efficiently deliver nucleic acids or other molecules into cells using electroporation and subsequently study the effects or manipulate cellular processes for various applications in molecular biology and genetic engineering.
How to Prepare Cells for Electroporation?
Preparing cells for electroporation involves several steps to ensure the cells are in an optimal state for successful gene transfer. Here is a detailed procedure for preparing cells for electroporation:
- Inoculate 10 mL of 2× YT medium with the desired E. coli host strain from an LB or 2× YT medium plate. Incubate the culture overnight at 37 °C with shaking.
- Inoculate 1 L of 2× YT medium with 10 mL of the overnight culture of host cells. Incubate this culture for 2 to 2.5 hours at 37 °C with shaking at 250 rpm until the cells reach an optical density (OD) of 0.5 to 0.7 at A600.
- Place the flask containing the culture on ice and let it cool for 15 to 30 minutes. This step helps to arrest cell growth and prepares the cells for subsequent handling.
- Centrifuge the culture at 4,000 × g for 20 minutes at 4 °C. This step separates the cells from the culture medium.
- Carefully pour off and discard the supernatant, and resuspend the cell pellet in 1 L of ice-cold sterile 1 mM HEPES (pH 7.0). HEPES is used as a buffer to maintain the pH and osmolarity of the cell suspension.
- Centrifuge the resuspended cells as described in step 4. Remove and discard the supernatant.
- Repeat step 6, resuspending the cell pellet in 500 mL of ice-cold sterile 1 mM HEPES (pH 7.0) this time.
- Repeat step 6 once again, but this time wash the cells in 20 mL of sterile 1 mM HEPES (pH 7.0) containing 10% glycerol. Glycerol is added to protect the cells during the electroporation process.
- Centrifuge the cells as described in step 4. Discard the supernatant, and resuspend the cell pellet in a total volume of 2 to 3 mL of sterile 10% glycerol in distilled water. This step further protects the cells and prepares them for electroporation.
- Divide the resuspended cells into 50 to 100 µL aliquots. These aliquots can be directly used for the electroporation procedure or frozen on dry ice and stored at -70 °C for future use.
- If you are working with a plasmid vector, extract the ligated pGEX vector (along with the uncut vector) by performing phenol/chloroform and chloroform/isoamyl alcohol extractions.
- Remove the aqueous phase after the extractions and add 1/10 volume of 3 M sodium acetate (pH 5.4) and 2.5 volumes of 95% ethanol to the DNA solution.
- Place the DNA solution on dry ice for 15 minutes and then spin it in a microcentrifuge for 5 minutes to pellet the DNA.
- Carefully remove the supernatant and wash the DNA pellet with 1 mL of 70% ethanol. Centrifuge for 5 minutes, discard the supernatant, and dry the DNA pellet.
- Resuspend each DNA pellet in 20 µL of sterile distilled water. Alternatively, if necessary, the DNA can be purified further by gel electrophoresis.
Note: It is important to ensure that the DNA used for electroporation is free of salt, as the presence of salt can interfere with the electroporation process.
By following these steps, cells can be properly prepared for electroporation, maximizing the efficiency of gene transfer.
Applications of Electroporator
Electroporation, facilitated by an electroporator, is a versatile technique with numerous applications in various fields of science and research. Here are some notable applications of electroporators:
- Genetic Engineering and Gene Delivery: Electroporation is widely used for the delivery of foreign DNA, RNA, or other genetic material into cells. It allows the introduction of exogenous genes or genetic modifications, enabling researchers to study gene function, manipulate gene expression, and create genetically modified organisms. Electroporation is a key tool in genetic engineering and has applications in fields such as biotechnology, agriculture, and medicine.
- Transfection and Transient Gene Expression: Electroporation is employed for efficient transfection of cells with plasmids or other genetic constructs. It enables the transient expression of genes of interest, allowing researchers to investigate gene function, protein production, signaling pathways, and cellular processes. Transient gene expression is particularly useful when long-term genetic modifications are unnecessary or impractical.
- Gene Editing and CRISPR-Cas9 Technology: Electroporation plays a crucial role in gene editing techniques such as CRISPR-Cas9. By delivering CRISPR components (guide RNA and Cas9 protein) into cells through electroporation, precise modifications can be made to the genome. Electroporation enables targeted gene editing, including gene knockout, knock-in, or precise nucleotide substitutions, and contributes to advancements in precision medicine and biotechnology.
- Cell-Based Assays and Functional Studies: Electroporation allows the delivery of molecules into cells for functional studies. It is used to introduce fluorescent dyes, proteins, siRNA, antisense oligonucleotides, or small molecules into cells, enabling the investigation of cellular processes, protein function, signal transduction pathways, and drug screening assays.
- Electroporation-Mediated Drug and Vaccine Delivery: Electroporation enhances the delivery of drugs, vaccines, and therapeutic agents into cells and tissues. It improves the uptake and intracellular delivery of various compounds, including chemotherapeutic drugs, nucleic acid-based drugs, protein-based therapeutics, and vaccines. Electroporation-based drug delivery systems offer targeted and efficient delivery, reducing dosage requirements and enhancing treatment efficacy.
- Tissue Engineering and Regenerative Medicine: Electroporation has applications in tissue engineering and regenerative medicine. It enables the delivery of growth factors, genes, or specific signaling molecules into cells and tissues, promoting cellular reprogramming, differentiation, and tissue regeneration. Electroporation is employed in the development of engineered tissues, organoids, and cell-based therapies.
- Microbial Transformation: Electroporation is utilized for the transformation of bacteria, yeast, and other microbial cells. It allows the introduction of plasmids, genomic DNA, or other genetic material into microorganisms, enabling genetic modifications, recombinant protein expression, or metabolic engineering for industrial, pharmaceutical, or research purposes.
These applications highlight the wide-ranging utility of electroporators in molecular biology, genetic engineering, medicine, agriculture, and biotechnology. Electroporation offers precise and efficient delivery of molecules into cells, driving advancements in scientific research, therapeutic development, and the understanding of cellular processes.
Advantages of Electroporator (Electroporation machine)
Electroporators offer several advantages in the context of molecular biology, genetic engineering, and cell manipulation. Here are some key advantages of using an electroporator:
- Time Efficiency: Electroporation is a rapid method, typically requiring only a few minutes for transfection or transformation experiments. The efficient delivery of molecules into cells through electroporation enables quick and time-saving experiments, enhancing overall research productivity.
- Versatility: Electroporation is a versatile technique that can be used to introduce foreign components such as DNA, RNA, or proteins into various types of cells, including bacteria, yeast, plant cells, and animal cells. This versatility allows researchers to work with different cell types and perform a wide range of experiments.
- High Transfection/Transformation Efficiency: Electroporation often achieves high transfection or transformation efficiency, resulting in a greater number of cells successfully incorporating the desired molecules. This high efficiency is advantageous for downstream applications, such as gene expression studies, protein production, or the creation of genetically modified organisms.
- Chemical-Free and Cell Viability: Electroporation is a chemical-free method, eliminating the need for potentially toxic chemicals that could damage cells or affect cell viability. This aspect ensures the preservation of cell integrity and improves the reliability of experimental results.
- Scalability: Electroporators can be used for both large-scale and small-scale experiments. Bulk electroporators are designed for handling larger volumes of cell suspensions, facilitating large-scale transfection or transformation experiments. This scalability allows researchers to adapt the technique to their specific experimental needs.
- Adjustable Electroporation Conditions: Electroporators provide users with the flexibility to adjust the electroporation conditions according to their specific requirements. Parameters such as voltage, pulse duration, and number of pulses can be optimized to achieve the desired transfection or transformation efficiency for different cell types and experimental setups.
- Vector Independence: Unlike some other transfection methods, electroporation does not necessarily require the use of vectors (e.g., viral vectors or lipid-based carriers). This advantage simplifies the experimental procedure and reduces the reliance on specific vectors, broadening the range of molecules that can be delivered into cells.
- Reproducible Results: Electroporation offers reproducible results, allowing researchers to obtain consistent outcomes across different experiments. This reproducibility is crucial for the reliability and validity of scientific findings.
- Effective with Difficult-to-Transfect Cell Types: Electroporation has proven to be effective in delivering molecules into cell types that are traditionally challenging to transfect, such as primary cells, stem cells, or certain mammalian cell lines. This advantage expands the scope of research possibilities and enables the investigation of various cell types.
- Simplicity and User-Friendliness: Electroporation is a relatively straightforward and user-friendly technique. With appropriate training, researchers can quickly master the technique and perform electroporation experiments with ease. This simplicity contributes to its widespread adoption and popularity in laboratories.
Overall, the advantages of electroporators make them a valuable tool for efficient and versatile cell manipulation, genetic engineering, and molecular biology research.
Disadvantages of Electroporator (Electroporation machine)
While electroporation offers numerous advantages, it also has some disadvantages that should be considered. Here are some notable drawbacks of using an electroporator:
- Optimization of Parameters: Electroporation requires careful optimization of parameters such as voltage, pulse duration, and number of pulses to achieve optimal transfection or transformation efficiency. Finding the optimal conditions for specific cell types or experimental setups may require time-consuming and iterative optimization processes, adding complexity to the experimental design.
- Cost: Electroporators, especially those with advanced features and capabilities, can be expensive to acquire and maintain. The cost of purchasing the electroporator equipment, electrodes, cuvettes, and other associated supplies can be a limiting factor for researchers with budget constraints.
- Complex Setups: Electroporation requires the use of specific setups, including electroporators, electrodes, and cuvettes or chambers for holding the cell suspension. These components need to be properly aligned and maintained to ensure efficient and uniform electric field distribution across the cells. The complexity of the setup may pose challenges for inexperienced users or require additional training and expertise.
- Impact on Cell Viability: The electrical pulses used in electroporation can have an impact on cell viability. High voltages or incorrect pulse parameters can cause cellular damage, leading to decreased cell viability or even cell death. It is crucial to optimize the parameters to minimize potential detrimental effects on cell health and ensure successful experimentation.
- Optimization for Different Cell Types: Optimizing electroporation parameters for different cell types can be a challenging and time-consuming process. Cell types may vary in their sensitivity to electrical pulses, requiring adjustments in voltage, pulse duration, and other parameters. This optimization can prolong experimental setup and increase the complexity of working with diverse cell lines or primary cells.
Despite these disadvantages, electroporation remains a widely used and valuable technique in various research fields. By carefully considering and addressing these drawbacks, researchers can effectively utilize electroporation for their specific experimental needs, taking advantage of its strengths while mitigating its limitations.
Precautions for Electroporator
When working with an electroporator, it is important to follow certain precautions to ensure successful and efficient electroporation experiments. Here are some key precautions to consider:
- Pre-Chilling of Cuvettes and Centrifuges: Prior to use, cuvettes and centrifuges should be pre-chilled in ice to maintain the desired temperature during the electroporation process. Cold temperatures help preserve cell viability and enhance the efficiency of the electroporation procedure.
- Thawing and Suspension of Electrocompetent Cells: Electrocompetent cells should be thawed on ice and thoroughly suspended to ensure a homogeneous cell suspension. Proper thawing and suspension help maintain cell integrity and optimize the efficiency of the electroporation process.
- Avoiding Large Volumes of DNA: Adding excessively large volumes of DNA can decrease the transformation efficiency. It is advisable to use the optimal amount of DNA for the specific experimental requirements to maximize the efficiency of DNA uptake by the cells.
- DNA Quality and Preparation: DNA used for electroporation should be free from salts and proteins. Salts and proteins in the DNA sample can interfere with the electroporation process and reduce transformation efficiency. Purifying the DNA and ensuring its integrity is essential for successful electroporation.
- Optimization of Electroporation Parameters: It is crucial to optimize the electroporation parameters according to the specific cuvettes and electroporators being used. Parameters such as voltage, pulse duration, and number of pulses should be optimized for each setup to achieve optimal transformation efficiency and cell viability.
- Avoiding High Salts and Air Bubbles: High concentrations of salts in the electroporation buffer or the presence of air bubbles in the cuvettes can lead to arcing, which may damage the cells or affect the electroporation process. Care should be taken to minimize salt concentrations and eliminate air bubbles to ensure a reliable and efficient electroporation process.
- Timely Addition of Recovery Medium: Immediately after electroporation, it is crucial to add a recovery medium to the cells. Delays in adding the recovery medium can decrease the transformation efficiency and cell viability. Timely addition of the recovery medium helps provide the necessary nutrients and conditions for cell recovery and successful transformation.
- Pre-Warming of Petri Plates: Pre-warming Petri plates at 37°C for about an hour before spreading transformed cells helps create optimal conditions for cell growth and higher transformation efficiency. This precaution promotes cell recovery and facilitates the successful growth of transformed cells on the agar plates.
By following these precautions, researchers can enhance the reliability, efficiency, and success of their electroporation experiments, ensuring optimal transformation efficiency and maintaining cell viability throughout the process.
Examples of Electroporator
There are several examples of electroporators available in the market, each with its own unique features and capabilities. Here are some examples:
- Cell electroporator Eporator® (Eppendorf SE): This electroporator offers fast sample handling with a single button operation. It features a USB interface for data transfer and GLP (Good Laboratory Practice) for documentation purposes. Its compact design optimizes space utilization, and it includes an integrated cuvette holder for enhanced safety. The electroporator is equipped with display indicators that facilitate intuitive operation.
- Cell electroporator NEPA21 (Bulldog Bio, Inc.): The NEPA21 electroporator operates at low voltage and offers short and long pulses with reversing polarities, delivering precise square waves with minimal cytotoxic effects. It supports high-efficiency transfection of a wide variety of cells, tissues, and organisms. This electroporator utilizes a unique non-capacitor-driven pulsing mechanism.
- Micropulser Electroporator (BioRad): The Micropulser Electroporator includes an arc quenching (ARQ) system, which prevents sample loss due to arcing. The device provides reproducibility through displayed time constant and volts. It offers a wide range of manually optimized parameters, and the pulse indicators are both audible and visible.
- BTX™ ECM™ 399 Exponential Decay Wave Electroporator (fisherscientific): These electroporators are portable and user-friendly, equipped with the ECM 399 Generator and optional PEP™ (Personal Electroporation Pak) Stand. They come with cuvettes and cuvette racks. The electroporator produces precise field strength and pulse lengths, and it features a single-dial control, low and high voltage options, a 16-character LCD readout, and pulse length and peak voltage feedback.
- Gemini Twin Wave Electroporators (BTX): The Gemini Twin Wave Electroporator combines both square wave and exponential decay electroporation capabilities in a single unit. It is suitable for various applications such as CRISPR, in vivo, in vitro, and in ovo experiments. The electroporator supports cuvettes/plates and offers pre-designed protocols for common eukaryotic and prokaryotic cell types, with the ability for users to modify and create their own protocols.
These are just a few examples of electroporators available on the market, each designed to cater to different research needs and experimental requirements. Researchers can choose the electroporator that best suits their specific applications, considering factors such as voltage range, pulse characteristics, user interface, and additional features provided by the manufacturer.
FAQ
What is an electroporator?
An electroporator is a device used in molecular biology and genetic engineering to introduce foreign DNA, RNA, or other molecules into cells using electric pulses.
What are the advantages of using an electroporator?
Electroporation offers several advantages, including high transfection efficiency, versatility in cell types, reproducibility of results, and the ability to work with difficult-to-transfect cells. It is also a time-efficient and toxic chemical-free method.
What are the main types of electroporation?
There are two main types of electroporation: bulk electroporation, which involves electroporating a population of cells simultaneously, and single-cell electroporation, which targets individual cells.
Are there any disadvantages to using an electroporator?
Some disadvantages of electroporation include the need for careful optimization of parameters, the potential for cell damage and reduced viability, and the cost of advanced electroporation devices.
How does an electroporator work?
An electroporator works by applying electrical pulses to cells, creating transient pores in the cell membrane. These pores allow molecules, such as DNA, to enter the cells.
What precautions should be taken when using an electroporator?
Precautions include pre-chilling cuvettes and centrifuges, ensuring proper thawing and suspension of electrocompetent cells, avoiding high concentrations of salts or air bubbles, and adding a recovery medium to cells immediately after electroporation.
What are some examples of electroporators available on the market?
Examples of electroporators include the Eporator® by Eppendorf SE, NEPA21 by Bulldog Bio, Micropulser Electroporator by BioRad, and BTX™ ECM™ 399 Exponential Decay Wave Electroporator by fisherscientific.
Can electroporation be used for mammalian cell transfection?
Yes, electroporation is highly effective for introducing foreign genes into mammalian cells. It is commonly used in producing knockout mice, gene therapy, and cell-based therapies.
What other applications does electroporation have?
Electroporation has applications in tumor treatment, tissue culture, in utero and in ovo transfection, and cell fusion. It is also used for the production of monoclonal antibodies in hybridoma technology.
Is electroporation a widely used technique in research?
Yes, electroporation is a widely used technique in molecular biology and genetic engineering. It is a valuable tool for introducing genetic material into cells, enabling a wide range of studies and applications in various fields of research.
References
- Chang, Donald. (2006). Electroporation and Electrofusion. 10.1002/3527600906.mcb.200300026.
- https://www.bio-rad.com/en-us/category/electroporation?ID=c1ae4cd3-ef42-4734-8d6c-7fce69992ccb
- https://www.bio-rad.com/en-us/product/micropulser-electroporator?ID=83527990-34fb-4b33-b955-ca53b57bf8b9
- https://www.fishersci.com/us/en/browse/90222047/electroporators?page=1
- https://us.vwr.com/store/category/electroporators/2993634
- https://www.thermofisher.com/search/browse/category/us/en/90222047
- https://www.thomassci.com/scientific-supplies/Electroporator
- https://www.biocompare.com/Immunochemicals/11987-Electroporation-Cell-Fusion-Instruments/
- https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000589
- https://www.agarscientific.com/calibre-scientific-eporator
- https://www.nepagene.jp/e_products_nepagene_0001.html
- https://maxcyte.com/electroporation-systems/
- https://www.btxonline.com/gemini-twin-wave-electroporators-2285.html
- https://www.excedr.com/biotech-life-sciences/electroporators/
- https://www.ddbiolab.com/product/0O-60-01?language=en
