Table of Contents
What is Immunodeficiency?
- Immune system integrity is necessary for protection against pathogenic organisms and their harmful byproducts, and thus for the survival of all persons. Defects in one or more immune system components can result in life-threatening and frequently deadly conditions known together as immunodeficiency diseases.
- Immunodeficiency illnesses and syndromes are a major cause of death and morbidity, as well as a rich source of information regarding the physiology of the human immune system.
- Immunodeficiency can affect T cells, B cells, complement, and phagocytes, the immune system’s primary components.
- A functional immune system deficiency is suspected when a patient:
- Has an unusually high incidence of illnesses caused by common or opportunistic bacteria.
- Having uncommonly severe infections.
- Incapable of eliminating illnesses with microorganisms-susceptible antibiotics. Recurrent infections with some viruses, protozoa, and fungi indicate a shortage in T-cells, but recurrent infections with pyogenic bacteria (such as staphylococci) suggest a deficiency in B-cells.
General Features Of Immunodeficiency Diseases
Prior to beginning our examination of specific disorders, it is necessary to review some common characteristics of immunodeficiencies.
- Immunodeficiency mostly results in an increased vulnerability to infection. The type of an infection in a specific patient is primarily determined by the deficient immune system component. Deficiencies in humoral immunity enhance susceptibility to infection by encapsulated, pus-forming bacteria and certain viruses, whereas deficiencies in cell-mediated immunity result in infection by viruses and other intracellular microorganisms. Patients with combined deficits in humoral and cell-mediated immunity are susceptible to infection by all pathogen types. Such infections are termed opportunistic. Immunocompromised patients frequently appear with illnesses caused by microorganisms that are routinely encountered but effectively removed by healthy individuals.
- Certain kinds of cancer are also prone to patients with immunodeficiencies. It appears that oncogenic viruses, such as the Epstein-Barr virus, are responsible for a significant proportion of these malignancies. T cells play a crucial role in monitoring against oncogenic viruses and the tumours they produce, which explains why T cell immunodeficiency is the most common cause of increased cancer risk.
- Certain immunodeficiencies are paradoxically associated with a higher risk of autoimmunity. Unknown is the mechanism underlying this connection.
- Immunodeficiency can be caused by problems in lymphocyte formation or activation, as well as by defects in the effector mechanisms of innate and adaptive immunity. Immunodeficiency illnesses are clinically and pathologically varied, in part due to the fact that different diseases engage distinct immune system components.
Classification of Immunodeficiency
Immunodeficiency diseases are categorised as
- Primary immunodeficiencies
- secondary immunodeficiencies.
A. Congenital or Primary Immunodeficiencies
- Primary immunodeficiency refers to a disorder resulting from a genetic or developmental abnormality in the immune system.
- In this case, the defect is present at birth but may not emerge until later in life.
- Most major immunodeficiencies are passed down from parents to children.
- Adaptive or innate immune functions may be affected by primary immunodeficiency.
- The majority of immunodeficiency-causing mutations affect either myeloid or lymphoid cell lineages.
- Lymphoid cell problems can influence T cells, B cells, or both B and T cells, whereas myeloid cell illnesses can impact phagocytic function.
- Primary immunodeficiency illnesses can be classed as: (a) B-cell immunodeficiencies, (b) T-cell immunodeficiencies, (c) mixed B-cell and T-cell deficiencies, (d) complement immunodeficiencies, and (e) phagocyte deficiencies.
1. B-Cell Immunodeficiencies
(a) X-linked hypogammaglobulinemia, (b) selective immunoglobulin deficiencies, (c) hyperIgM syndrome, and (d) interleukin-12 receptor deficiency are B-cell deficiencies.
X-linked hypogammaglobulinemia
- The prototypical “pure” B-cell deficit is X-linked hypogammaglobulinemia, also known as infantile agammaglobulinemia or X-linked agammaglobulinemia (XLA).
- In most instances, the disease is transmitted through a sex-linked feature. The faulty gene is found on Xq21.2–22, the B-cell progenitor kinase or Bruton’s tyrosine kinase coding region (Btk).
- Btk plays a crucial function in B-cell differentiation and maturation and is a member of the tyrosine kinase family involved in adult B-cell signalling.
- Most cases of infantile agammaglobulinemia are attributable to Btk mutations.
- X-linked hypogammaglobulinemia exhibits the subsequent characteristics:
- It is characterised by an absence of other immunoglobulin types and exceedingly low IgG levels.
- Individuals affected by XLA lack peripheral B cells and develop recurrent bacterial infections at 9 months of age. Patients suffer from recurrent infections caused by common pyogenic organisms (e.g., Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae, Staphylococcus aureus, etc.), resulting in pyoderma, purulent conjunctivitis, pharyngitis, otitis media, sinusitis, bronchitis, pneumonia, empyema, pur Chronic obstructive pulmonary disease and bronchiectasis result from frequent bronchopulmonary infections. Infections with Giardia lamblia are identified more frequently in this patient population, and may result in persistent diarrhoea and malabsorption.
- After immunisation with the attenuated virus, patients with agammaglobulinemia are at risk of getting paralytic polio and chronic viral meningoencephalitis, which is typically caused by echoviruses.
- Arthritis of the major joints is assumed to be infectious and caused by Ureaplasma urealyticum in 30–35% of patients.
- Intravenous administration of gamma globulin (a plasma fraction containing primarily IgG, taken from normal, healthy donors) is the most effective treatment for this illness.
Selective immunoglobulin deficiencies
- In this condition, serum immunoglobulin levels are normal or increased except for one or more immunoglobulins.
- The most prevalent example of selective immunoglobulin deficits is IgA deficiency. IgA deficit is marked by almost nonexistent serum and secretory IgA.
- The IgA level is below 5 ng/dL, but all other immunoglobulin class levels are either normal or high.
- The disorder may be inherited or acquired as a result of measles, other types of viral infection, or toxoplasmosis.
- It is unknown what causes IgA insufficiency, however it is considered to be the result of halted B-cell development.
- IgA B-cell differentiation seems to be the primary issue. Adults with selective IgA deficiency typically exhibit an immature phenotype, of which only a few can develop into IgA-producing plasma cells.
- Despite the fact that IgA cells are created, these cells do not secrete IgA. IgA is the predominant immunoglobulin in secretions and an essential component of mucosal surface defence.
- Consequently, IgA-deficient patients are more susceptible to respiratory, gastrointestinal, and urogenital infections.
- In addition, they have a higher frequency of autoimmune illnesses such systemic lupus erythematosus and rheumatoid arthritis.
- Certain atopic individuals exhibit an elevated prevalence of the condition. Some patients with selective IgA deficiency develop high titers of anti-IgA antibodies.
- They may experience anaphylactic reactions after getting blood transfusions containing IgA.
- A selective IgA deficit is identified when serum IgA levels are fewer than 5 mg/dL. They have normal amounts of IgG and IgM antibodies, though.
- Some individuals acquire anti-IgG, anti-IgM, and anti-IgA antibodies.
Hyper-IgM syndrome
- This condition is characterised by a high blood IgM concentration and a very low serum IgG, IgA, and IgE content.
- They have normal levels of T and B lymphocytes. Several of these immunodeficiencies are X-linked, whereas others are autosomal recessive.
- This syndrome makes patients susceptible to repeated microbial infections and numerous autoimmune illnesses, such as thrombocytopenia, neutropenia, and hemolytic anaemia.
Interleukin-12 receptor deficiency
- Patients with interleukin-12 receptor deficiency are extremely sensitive to mycobacterial infections that have spread throughout the body.
- The absence of interleukin-12 receptor hinders IL-12 from generating a Th-1 response, which is necessary for preventing mycobacterial infections.
2. T-Cell Immunodeficiencies
Among the T-cell deficiencies are (a) DiGeorge syndrome, (b) chronic mucocutaneous candidiasis, (c) transitory hypogammaglobulinemia of infancy, and (d) common, variable, undefined immunodeficiency.
Thymic aplasia (DiGeorge syndrome)
- The DiGeorge syndrome or thymic aplasia is a classic example of a T-cell deficiency that is limited to T cells alone.
- Despite the fact that DiGeorge syndrome is a congenital immunodeficiency, it is not inherited.
- It is hypothesised that the illness is caused by an intrauterine infection before the eighth week of life, potentially of viral origin. It is connected with microdeletions of the 22q11 region of the chromosome.
- Immunologically, it is the result of abnormal embryogenesis of the third and fourth pharyngeal clefts during the first 6–8 weeks of foetal life, resulting in inadequate development of the thymus and parathyroids.
- Tetany and hypocalcemia, which are also symptoms of hypoparathyroidism, are present in DiGeorge syndrome in addition to T-cell immunity abnormalities.
- In regions that are dependent on the thymus, peripheral lymphoid tissues lack lymphocytes.
- A problem in delayed-type hypersensitivity is indicated by the inability of affected patients to generate positive skin tests to routinely used antigens, such as candidin or streptokinase, as well as the inability to develop an allograft reaction.
- In vitro reactivity to T-cell antigens or mitogens is limited or nonexistent. Defective cell-mediated immunity can increase a patient’s vulnerability to opportunistic infections and make them susceptible to graft-versus-host reactions in blood transfusion recipients.
- In patients with DiGeorge syndrome, the B or bursa equivalent-dependent regions, such as lymphoid follicles, contain normal numbers of B cells and plasma cells.
- Serum immunoglobulin levels are within the usual range, and the immunological response to immunisation with routinely used immunogens is normal.
- With foetal thymic transplants and passive administration of thymic humoral factors, DiGeorge syndrome has been successfully treated with a high degree of efficacy.
Chronic mucocutaneous candidiasis
- Some patients with persistent Candida albicans infection of the skin and mucosa revealed a specific impairment of cell-mediated immunity.
- Individuals with this condition have severe and widespread forms of candidal infections. Skin tests with Candida antigens and in vitro lymphocyte proliferation responses to C. albicans demonstrate an absence of reaction.
- When tested with additional antigens and mitogens, T-lymphocyte functionality is normal.
- Normal is also the humoral reaction to C. albicans.
- Symptomatic treatment with antimycotic drugs is frequently ineffective.
Transient hypogammaglobulinemia of infancy
- Hypogammaglobulinemia in babies due to the gradual degradation of maternal IgG throughout the second and third months of life characterises this syndrome.
- This syndrome may remain until 2–3 years of age and intensify over time.
- Immunoglobulin synthesis appears to be delayed in the majority of instances due to a dysfunctional helper T-cell population.
- This condition is characterised by low-for-age levels of circulating immunoglobulins.
- Typically, peripheral blood B cells are normal. Gamma globulin should be administered intravenously until the child’s immunoglobulin levels stabilise.
- Most children acquire normal immunological function over time.
Common, variable, unclassified immunodeficiency
- This syndrome, also known as late onset hypogammaglobulinemia, encompasses a large number of cases of primary immunodeficiency with a variety of clinical manifestations.
- These disorders have variable ages of onset and inheritance patterns, and their clinical picture is comparable to that of XLA, but their clinical manifestations are less severe.
- In the majority of cases, T-cell function looks weak, with abnormally low proliferative responses to T-cell mitogens.
- Patients with common, variable, unclassified immunodeficiency are mostly affected by sinusitis and bacterial pneumonia. Giardiasis of the intestines is prevalent.
- These individuals are also more susceptible to opportunistic infections caused by Pneumocystis jiroveci, mycobacteria, viruses, and other fungi.
- Typically, intravenous gamma globulin is administered for treatment.
3. Combined B-Cell and T-Cell Deficiencies
Combination B-cell and T-cell deficits are characterised by (a) severe combined immunodeficiency, (b) Wiskott–Aldrich syndrome, (c) ataxia telangiectasia, and (d) MHC class II deficiency.
Severe combined immunodeficiency
SCID comprises numerous syndromes characterised by severe deficiencies in both humoral and cell-mediated immune responses. All of these are inherited diseases in which early stem cells fail to differentiate. Two types exist: X-linked and autosomal.
(i) X-linked SCID
- SCID is associated with a gene defect that codes for a polypeptide chain shared by multiple interleukin receptors (IL-2, IL-4, IL-7, IL-11, and IL-15).
- This chain is involved in the signalling of second messages; therefore, T-cell precursors cannot receive the signals required for their proliferation and differentiation in its absence.
- T- and B-cell lymphopenia and decreased IL-2 production are present.
- Following an immunogenic challenge, there is a lack of delayed-type hypersensitivity, cellular immunity, and normal antibody synthesis.
(ii) Autosomal SCID
- This is the result of a mutation in the gene encoding ZAP-70, a tyrosine kinase that plays an essential role in signal transduction in T cells.
- Other SCID patients exhibit mutations in other genes, such as RAG-1 or RAG-2, which are required for the production of the T-cell antigen receptor and the antigen-carrying IgM monomer on the B cell.
- SCID is an infant disease characterised by failure to thrive. Affected individuals typically pass away within the first two years of life.
- They may develop a rash similar to measles, hyperpigmentation, and severe recurrent (particularly pulmonary) infections.
- These individuals are more susceptible to Pneumocystis carinii, Candida albicans, and other pathogens.
- SCID patients are susceptible to infection by even attenuated microorganisms, such as those used for immunisation, e.g., attenuated poliomyelitis viruses.
- All of these types of SCID are treatable with a bone marrow transplant from HLA-DR-matched sibling donors.
- The graft is typically successful, but there is a high risk of graft-versus-host disease developing.
(iii) Graft-versus-host disease (GVHD)
- It is an issue with SCID individuals receiving unirradiated blood transfusions. It can also arise after transfusion of any fresh blood component contaminated with live T cells.
- It is characterised by fever, maculopapular rash including the volar surfaces, diarrhoea and protein-losing enteropathy, Coombs’ positive hemolytic anaemia, thrombocytopenia, and splenomegaly.
- In full-blown instances, the outcome is often bad, with death occurring within 10–14 days from the onset of symptomatology.
- The response may be prevented in the case of transfusion by utilising frozen or irradiated blood products.
- Current attempts at removing all cells except stem cells from bone marrow grafts show promising.
Wiskott–Aldrich syndrome
- Infants with Wiskott–Aldrich syndrome have an X-linked recessive immunodeficiency.
- It is distinguished by thrombocytopenia, eczema, and elevated levels of IgA and IgE.
- Reduced cell-mediated immunity is observed. The most significant deficiency is the inability to mount an IgM response to capsular polysaccharide of bacteria.
- IgA and IgE are elevated, whereas IgM is decreased, whereas IgG serum values are typically normal.
- Electron microscopy reveals that T cells lack the characteristically fimbriated surface of normal T cells. T cell sialophorin is aberrant.
- It appears that the deficiency is caused by T cells’ failure to aid B cells. Transplantation of bone marrow corrects the deficit.
Ataxia telangiectasia
- It is an autosomal recessive disorder caused by mutations in the DNA repair enzyme-encoding gene.
- This syndrome is characterised by ataxia, telangiectasia, and repeated infections in infants less than 2 years.
- IgA deficiency and lymphopenia occur frequently.
MHC class II deficiency
- It is an autosomal recessive illness in which antigen-presenting cells, such as macrophages and B lymphocytes, fail to express MHC molecules on their surface. This leads to a shortage of CD4 T cells.
- Absence of these helper T cells results in inadequate antibody synthesis.
4. Complement Deficiencies
Inadequate complement includes the following conditions:
Recurrent infections
- This is a condition caused by a deficiency of C1, C3, or C5, or even C6, C7, or C8 components of the complement.
- Patients with C3 deficiency are highly susceptible to infection with S. aureus and other pyogenic bacteria.
- Similarly, patients with C6, C7, or C8 deficiency are more susceptible to bacteremia with N. meningitidis or Neisseria gonorrhoeae.
Autoimmune diseases
- Patients with deficits in C2 and C4 components have autoimmune disorders similar to systemic lupus erythematosus.
- The majority of patients with C2 deficiency are asymptomatic, and C2 deficiency is the most prevalent complement abnormality.
Paroxysmal nocturnal hemoglobinuria
- It is a disorder characterised by nighttime hemoglobinuria in sleeping patients.
- The nighttime occurrence of hemoglobinuria is related to complement-mediated hemolysis. This is because the lower oxygen concentration in the circulation during sleep makes red blood cells more susceptible to lysis.
- Patients with a mutation in the gene encoding the molecules that attach decay-accelerating factor (DAF) and other proteins to the cell membrane have this condition.
- This results in a lack of DAF on the surface of blood cell precursors, which increases complement activation and hemolysis.
Hereditary angioedema
- Hereditary angioedema is a disorder characterised by a lack of the complement component C1 inhibitor.
- This insufficiency leads in the continuous action of C1 on C4 to generate more C4a, followed by additional C3a and C5a complement components.
- An increase in the production of vasoactive components, such as C3a and C5a, causes capillary permeability and edoema in the larynx and other organs.
- Steroids (such as oxymetholone and danazol) are used to treat the disorder because they raise the concentration of C1 inhibitors, preventing a rise in C3a and C5a synthesis.
5. Phagocyte Deficiencies
Phagocyte deficiencies include (a) chronic granulomatous disease, (b) Chediak–Higashi syndrome, (c) Job’s syndrome, (d) leukocyte adhesion deficiency, (e) myeloperoxidase deficiency, and ( f ) cyclic neutropenia.
Chronic granulomatous disease
- Chronic granulomatous disease (CGD) is an illness that is inherited in two-thirds of cases as an X-linked trait and in the remaining one-third as an autosomal recessive trait.
- Typically, clinical signs appear before the end of the second year of life. This disorder is caused by a lack of the enzyme NADPH oxidase.
- This enzyme deficit leads to impaired oxygen consumption and glucose utilisation through the hexose monophosphate shunt in neutrophils and monocytes.
- In spite of the fact that neutrophils phagocytose bacteria, they do not produce superoxide and other oxygen intermediates that are typically part of the respiratory burst.
- All of these factors limit intracellular bacterial and fungal death. Therefore, these individuals are more susceptible to infection with bacteria that are typically of low virulence.
- Aspergillus, Serratia marcescens, and Staphylococcus epidermidis are examples.
- Patients with CGD may exhibit hepatosplenomegaly, pneumonia, osteomyelitis, abscesses, and lymph nodes that are draining.
- CGD is confirmed using both the quantitative nitroblue tetrazolium (NBT) test and the quantitative killing curve.
- Abscesses are treated with interferon gamma, antibiotics, and surgical drainage.
Chediak–Higashi syndrome
- This childhood disease is inherited in an autosomal recessive manner. The disease is characterised by the presence of enormous, highly persistent lysozomal granules in leukocytes that undergo sluggish degranulation.
- Large cytoplasmic granular inclusions, which are detected in white blood cells, can also be observed in blood platelets and are visible in peripheral blood smears under standard light microscopy.
- The disorder is characterised by a deficiency in neutrophil chemotaxis and a change in the cells’ ability to destroy ingested microbes.
- The majority of affected individuals pass away during childhood; however, a few cases may live longer.
- Antibiotics are the only medicine that is effective against the bacteria that cause infection.
- It has been demonstrated that high dosages of ascorbic acid restore normal chemotaxis, bactericidal action, and degranulation.
Job’s syndrome
- Job’s syndrome is caused by the inability of helper T cells to create gamma interferon, which limits macrophages’ ability to fight bacteria.
- This results in an increase in Th-2 production, which in turn increases IgE synthesis.
- All of these factors result in an increase in histamine synthesis, which limits inflammatory response and chemotaxis.
- Consequently, the patient with this disease suffers from recurrent staphylococcal abscesses and eczema with a high IgE level.
Leukocyte adhesion deficiency
- It is an autosomal recessive disorder caused by a mutation in the gene encoding the B chain of an integrin that mediates leukocyte attachment to microorganisms.
- This causes poor adherence of neutrophils to endothelium surfaces, resulting in insufficient phagocytosis of microorganisms.
Cyclic neutropenia
- In this autosomal dominant condition, the gene for neutrophil esterase, an enzyme generated by neutrophils, is mutated.
- The condition is defined by a neutrophil count of fewer than 200/L for three to six days over a 21-day cycle.
- During these 3–6 days of low neutrophil count, patients are susceptible to life-threatening bacterial infections, but not when neutrophil levels are normal.
Myeloperoxidase deficiency
- It is a disorder caused by a lack of the enzyme myeloperoxidase, which is necessary for the synthesis of hypochlorite, a microbicide.
- This enzyme shortage occurs often but has minimal clinical significance.
- This is because other intracellular leukocyte killing mechanisms remain intact.
B. Secondary Immunodeficiencies
- Secondary immunodeficiencies are caused by a variety of diseases or situations, as well as by treatments that suppress the immune system.
- The majority of immunodeficient people have secondary forms of immunodeficiency due to pathological diseases that damage the immune system or the administration of immunosuppressive substances.
- Acquired immunodeficiency syndrome (or AIDS), which is caused by infection with the human immunodeficiency virus, is by far the most prevalent secondary immunodeficiency (HIV).
- AIDS, chemotherapy by immunosuppressive drugs (e.g., corticosteroids and nonsteroidal anti-inflammatory drugs), psychological depression, burns, radiation, Alzheimer’s disease, celiac disease, sarcoidosis, lymphoproliferative disease, Waldenstrom’s macroglobulinemia, multiple myeloma, aplastic anaemia, sickle cell disease, malnutrition, ageing, neoplasia, and diabetes mell
- Secondary immunodeficiencies may be characterised as (a) B-cell deficiencies, (b) T-cell deficiencies, (c) complement deficiencies, and (d) phagocytic deficiencies as follows:
1. B-Cell Deficiencies
Common variable hypogammaglobulinemia
- This disorder is caused by faulty T-cell signalling, which prevents the body from producing IgG. This occurs in persons between the ages of 13 and 35 years.
- The number of B cells is normal, but the ability to make IgG and other immunoglobulins is severely diminished.
- The cell-mediated immunity is normal. These patients are extremely susceptible to infections caused by S. pneumoniae, H. influenzae, and other pyogenic bacteria.
- Gamma globulin administered intravenously lowers illnesses caused by these bacteria.
Malnutrition
- IgG production is diminished in malnutrition due to a deficiency of amino acids.
- Therefore, malnourished individuals are susceptible to infection by pyogenic bacteria.
2. T-Cell Deficiencies
Acquired immunodeficiency syndrome (AIDS)
- Many opportunistic pathogens, such as bacteria, viruses, fungi, and parasites, pose a significant risk of infection to patients with HIV-associated AIDS.
- This is due to the inactivity of T-cell helper cells. The virus infects and destroys only cells with CD4 surface receptors.
- The immune system is severely compromised, and the absence of immunological monitoring results in a high incidence of malignancies.
Measles
- In measles patients, T-cell function is impaired, although immunoglobulins are normal.
- Patients have a transient reduction in delayed hypersensitivity.
3. Complement Deficiencies
Liver failure
- In chronic hepatitis B or C, as well as in liver failure due to alcoholic cirrhosis, complement protein production is drastically diminished.
- These patients are therefore extremely susceptible to infection by pyogenic bacteria.
Malnutrition
- In extreme malnutrition, the liver synthesises fewer complement proteins due to a lack of amino acids.
- Consequently, malnourished individuals are prone to infection by pyogenic bacteria.
4. Phagocyte Deficiencies
Neutropenia
- The syndrome is defined by a low neutrophil count (less than 500/L), which is typically caused by cytotoxic medications, such as those used in cancer treatment.
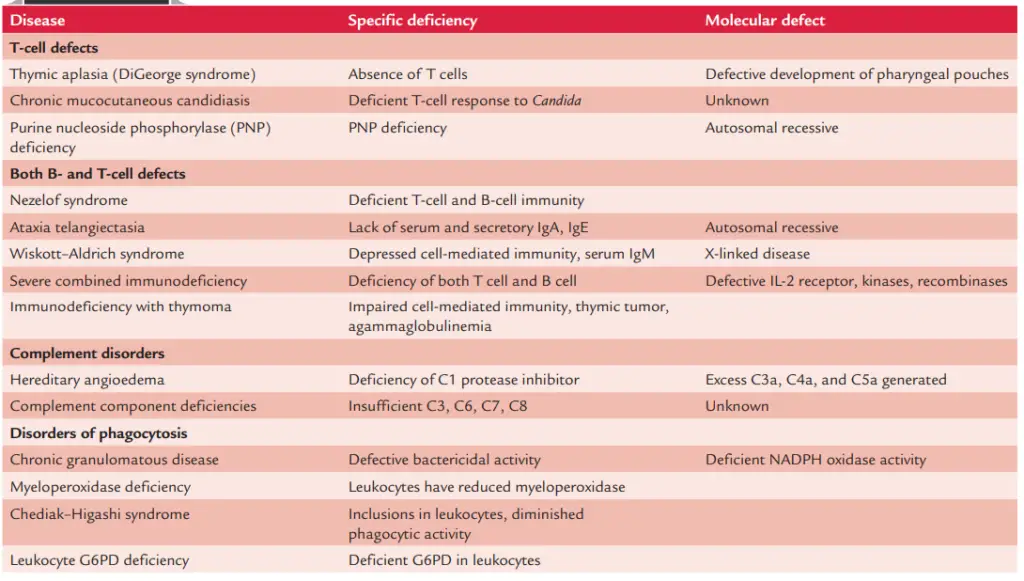
- Patients are prone to severe bacterial infections brought on by pyogenic bacteria, such as S. aureus and S. pneumoniae. In Table 18-1, immunodeficiency illnesses are summarised.
Experimental Models of Immunodeficiency Include Genetically Altered Animals
Immunologists utilise two extensively researched animal models of primary immunodeficiency for a range of experimental purposes. One is the athymic, or hairless, mouse, while the other is the SCID, or severe combined immunodeficiency, mouse.
Nude (Athymic) Mice
- In some mice, a recessive gene on chromosome 11 controls a hereditary characteristic named nu.
- Homozygous (nu/nu) mice for this feature are hairless and have a vestigial thymus. The heterozygote siblings nu/+ have hair and a normal thymus. It is unknown whether the same gene causes both baldness and thymus dysfunction.
- It is plausible that two closely linked genes are responsible for these apparently unrelated abnormalities that occur simultaneously in this mutant animal.
- A gene that regulates development may be involved, as the mechanism that leads to the differential development of the thymus and skin epithelial cells is connected.
- In normal conditions, the nu/nu mouse has a 100% mortality rate within 25 weeks and a 50% mortality rate within the first two weeks after birth.
- Therefore, when these animals are to be utilised for experimental reasons, they must be housed in a manner that prevents infection.
- Included in the precautions are the use of sterile food, drink, cages, and bedding. Individual cages are shielded from dust by placing them in a laminar flow rack or by installing air filters on top of each cage.
- They lack cell-mediated immune responses and are incapable of producing antibodies against the majority of antigens.
- A thymic transplant is capable of reversing the immunodeficiency of a naked mouse. Due of their ability to permanently survive both allografts and xenografts, they have a variety of experimental applications.
- For instance, hybridomas or solid tumours of any origin can be produced as ascites or as implanted tumours in a mouse lacking fur.
- It is recognised that the nude mouse does not lack T cells entirely, but rather has a small population that grows with age.
- The origin of these T cells is unknown; an intriguing theory is that mature T cells have an extrathymic origin.
- Nonetheless, it is more probable that T cells originate from the vestigial thymus. In contrast to the majority of cells in the circulation of a normal mouse, the majority of cells in the circulation of a nude mouse carry T-cell receptors of the type.
The Scid Mouse
- In 1983, Melvin and Gayle Bosma, together with their colleagues, identified an autosomal recessive mutation in mice that resulted in a significant lack of mature cells.
- The trait was dubbed SCID due of its similarities to severe combined immunodeficiency in humans.
- The SCID animal was shown to have early B- and T-lineage cells, but there was an almost complete absence of lymphoid cells in the thymus, spleen, lymph nodes, and gut tissue, which are the typical destinations of functioning T and B cells.
- The precursor T and B cells in the SCID mice did not appear capable of maturing into mature, functioning B and T lymphocytes.
- Detailed research has been conducted on inbred mouse strains that possess the SCID defect. SCID mice are incapable of producing antibodies or engaging in delayed-type hypersensitivity (DTH) or graft-rejection reactions.
- If animals are not kept in a sterile environment, they succumb to disease at a young age.
- Red blood cells, monocytes, and granulocytes develop normally in the SCID animal; lymphocytes do not develop normally.
- Through transplantation of stem cells from normal mice, immunocompetent SCID animals can be created.
- The SCID-causing mutation in a DNA protein kinase is a so-called “leaky” mutation because a subset of SCID animals make IgG. About 50% of these SCID mice are also capable of rejecting skin allografts.
- This study implies that the faulty enzyme can partially function in T- and B-cell development, allowing a small percentage of precursor cells to differentiate normally.
- Recombination-activating enzymes (RAG-1 and RAG-2) responsible for the rearrangement of immunoglobulin or T-cell–receptor genes in both B- and T-cell progenitors have been deleted to create SCID-like immunodeficient animals (RAG knockout mice).
- This causes a deficiency in both B and T cells of the animal; neither is able to rearrange the genes for its receptor, and therefore neither develops normally.
- Because cells with aberrant rearrangements are destroyed in vivo, the RAG knockout animal lacks both B and T cells in its lymphoid organs.
- In addition to shedding light on potential causes of combined T- and B-cell immunodeficiency, the SCID mouse has proven to be an invaluable tool for cellular immunology research.
- Due to the ineffectiveness of its rejection systems, the SCID mice can be used to study cells or organs from multiple sources. For instance, human immune precursor cells may be employed to restore the immune system of a SCID mouse.
- These human cells can grow normally, resulting in the presence of human immunoglobulin in the circulation of SCID mice.
- In one major application, HIV-1 is transmitted to SCID mice. Normal mice are not vulnerable to HIV-1 infection; however, the SCID mouse reconstituted with human lymphoid tissue (SCID-Hu mouse) provides an animal model for testing therapeutic or preventative measures against HIV infection of transplanted human lymphoid tissue.
