Table of Contents
What is Paper Chromatography?
- Paper chromatography is a sort of planar chromatography in which chromatographic techniques are carried out on specialized paper. Because of its capacity to separate, identify, and quantitatively quantify both organic and inorganic substances, it is largely considered as the simplest and most often used chromatographic method.
- Christian Friedrich Schonbein, a German physicist, pioneered the concept of paper chromatography in 1865. Originally, it was used to separate colored compounds or substances as an analytical procedure. It has, however, mostly evolved into a teaching tool, with alternative chromatography techniques, such as thin-layer chromatography (TLC), taking its place in laboratory applications.
- Three fundamental components comprise the paper chromatography setup. Through capillary action, the mobile phase, which is a solution, travels up to the stationary phase. The mobile phase is often a combination of non-polar organic solvents, whereas the stationary phase usually a polar inorganic solvent such as water. The paper serves as a support for the stationary phase (water) and has a cellulose network that binds polar water molecules in its void spaces. It is crucial to highlight that in paper chromatography, the stationary phase is less absorbent paper, as opposed to TLC, where the stationary phase is often a layer of adsorbent material such as silica gel or aluminum oxide.
- Two-dimensional chromatography is a form of paper chromatography that requires utilizing two solvents and rotating the paper by 90° in between. This approach is very effective for isolating complicated combinations of comparable polarity chemicals, such as amino acids.
- Paper chromatography uses paper sheets or strips as the stationary phase through which a solution is forced to flow. The solution’s dissolved chemical components segregate depending on their differing migration speeds over the paper sheets. This low-cost approach yields a powerful analytical tool while requiring only a little amount of ingredients.
- In general, paper chromatography is a useful technique for isolating and studying chemical compounds. While it has originally emerged as an instructional tool, its simplicity and adaptability make it a helpful approach in a variety of scientific domains.
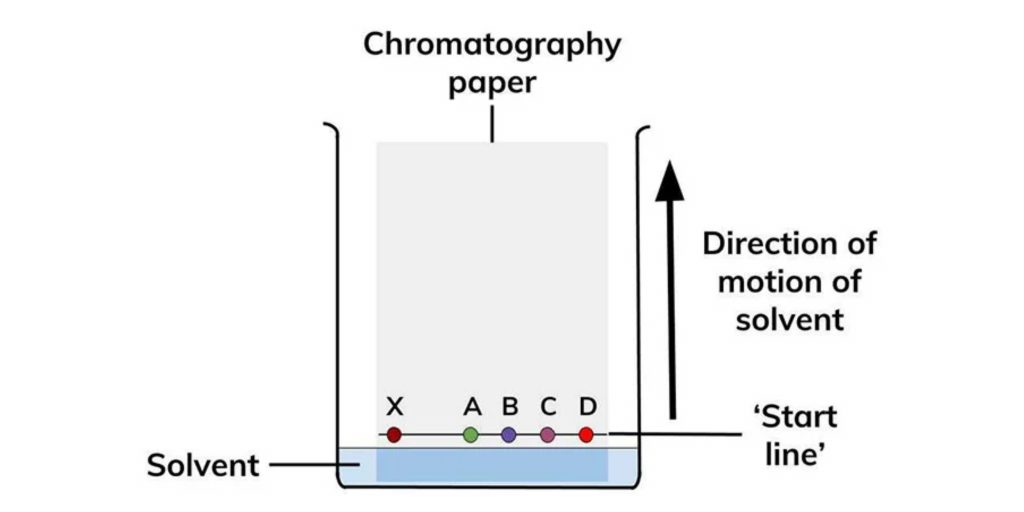
Types of Paper chromatography
There are two main types of paper chromatography: paper adsorption chromatography and paper partition chromatography.
- Paper Adsorption Chromatography: In paper adsorption chromatography, the stationary phase is created by impregnating the paper with silica or alumina. The adsorbent material, such as silica gel or alumina, is evenly distributed throughout the paper. The mobile phase, on the other hand, is a solvent that moves through the paper, carrying the analytes with it. As the mobile phase travels up the paper, the compounds in the sample are adsorbed onto the adsorbent material based on their affinity for it. The separation occurs as different compounds are adsorbed to different degrees, causing them to migrate at different rates up the paper. This type of paper chromatography is commonly used for separating mixtures of compounds based on their adsorption characteristics.
- Paper Partition Chromatography: In paper partition chromatography, the stationary phase is created by the moisture or water present in the pores of the cellulose fibers in the filter paper. The mobile phase is another solvent that is used to carry the analytes through the paper. In this technique, the separation is based on the partitioning of the analytes between the stationary phase (moisture in the paper) and the mobile phase (solvent). The different compounds in the sample distribute themselves between the two phases, with some being more soluble in the mobile phase and others having a greater affinity for the stationary phase. As the mobile phase moves up the paper, the analytes separate into distinct bands or spots based on their partitioning behavior. Paper partition chromatography is often referred to simply as paper chromatography, as it is the more commonly used type of paper chromatography.
Both paper adsorption chromatography and paper partition chromatography are valuable techniques for separating and analyzing mixtures of compounds. They rely on the principles of adsorption or partitioning to achieve separation, and the choice between the two depends on the specific requirements of the analysis and the properties of the compounds being studied.
Principle of Paper chromatography
The principle of paper chromatography is based on the distribution or partitioning of substances between a stationary phase and a mobile phase. In the case of paper chromatography, the cellulose fibers in the filter paper act as the stationary phase, while organic solvents or buffers are used as the mobile phase.
The separation occurs as the mobile phase travels up the stationary phase, carrying the sample with it. The components of the sample separate based on their affinity for the stationary phase and their solubility in the mobile phase. Substances that strongly adsorb onto the stationary phase will migrate more slowly, while those that readily dissolve in the mobile phase will move faster.
The principle involved in paper chromatography can be either partition chromatography or adsorption chromatography. In partition chromatography, the substances in the mixture are partitioned or distributed between liquid phases. The water held in the pores of the filter paper acts as one phase, while the mobile phase serves as the other. As the mobile phase moves, the separation of the mixture takes place. The compounds separate based on their different affinities for the stationary and mobile phase solvents under the capillary action within the pores of the paper.
On the other hand, adsorption chromatography involves the interaction between a solid surface (the paper) and a liquid phase (the mobile phase). In this case, the solid surface of the paper acts as the stationary phase, while the liquid phase serves as the mobile phase. The compounds in the mixture adsorb to different extents onto the solid surface, leading to their separation as the mobile phase moves.
In summary, the principle of paper chromatography relies on the distribution or partitioning of substances between a stationary phase (the cellulose fibers in the filter paper) and a mobile phase (organic solvents or buffers). The separation occurs based on the differences in affinity for the stationary and mobile phases, either through partition chromatography or adsorption chromatography.
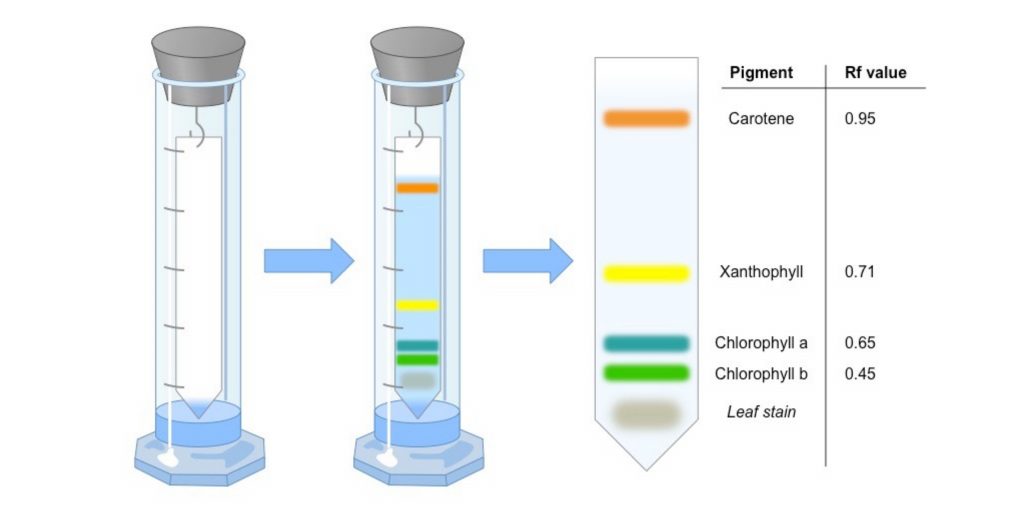
Instrumentation of Paper chromatography
- Stationary phase & papers used
- Mobile phase
- Developing Chamber
- Detecting or Visualizing agents
1. Stationary phase & papers
The type of paper used in paper chromatography is critical since it dictates the properties of the stationary phase. The following papers are widely used in paper chromatography:
- Whatman filter papers: Whatman filter papers are available in a variety of grades, including No. 1, No. 2, No. 3, No. 4, No. 20, No. 40, No. 42, and so on. These sheets contain varying pore diameters and thicknesses, providing for greater adaptability in separating various chemicals.
- Modified papers: In addition to normal filter papers, there are modified papers made particularly for chromatographic purposes. These papers are subjected to various treatments or alterations in order to improve their qualities. Acid or base washed filter papers, for example, have been treated to eliminate contaminants that may interfere with the analysis. Glass fiber sheets are also utilized because they have better chemical resistance and stability.
- Hydrophilic papers: Hydrophilic papers are papers that have been treated with chemicals such as methanol, formamide, glycol, glycerol, or other hydrophilic compounds. These treatments increase the paper’s affinity for the mobile phase, allowing for improved analyte separation and migration.
- Hydrophobic papers: These papers have had the hydroxyl (OH) groups on the cellulose fibers acetylated. This process makes the paper hydrophobic, making it acceptable for reverse phase chromatography. The stationary phase in reverse phase chromatography is non-polar, whereas the mobile phase is polar, allowing for the separation of non-polar substances.
- Impregnated papers: Papers can also be impregnated with various chemicals to change their characteristics. Impregnating the paper matrix with silica, alumina, or ion exchange resins, for example. Depending on the intended application, these impregnations can give various selectivity and separation capabilities.
The stationary phase in paper chromatography is a complicated matrix of water and paper rather than just the paper itself. The cellulose fibers in the paper attach to water molecules in the surrounding air as well as moisture present during the production process. The combination of water and paper produces the stationary phase, which is important in compound separation because the mobile phase transports the sample through the paper.
The selection of stationary phase, which is represented by various types of papers and modifications, is critical in defining the separation properties and overall performance of paper chromatography.
2. Mobile phase
In paper chromatography, the mobile phase is the solvent or mixture of solvents used to transport the mixture being studied through the stationary phase, which is the paper.
Depending on the nature of the chemicals to be separated, many types of mobile phases can be employed in paper chromatography. Mobile phases include the following:
- Hydrophilic mobile phases: Hydrophilic mobile phases are those that have a strong affinity for water. Isopropanol:ammonia:water (9:1:2), methanol:water (4:1), and n-butanol:glacial acetic acid:water (4:1:5) are examples of hydrophilic mobile phases. These solvent mixes can be used to separate polar substances.
- Hydrophobic mobile phases: These are nonpolar mobile phases that have a decreased affinity for water. Dimethyl ether:cyclohexane and kerosene:70% isopropanol are examples of hydrophobic mobile phases. Nonpolar chemicals are separated using these solvent mixes.
The mobile phase used is determined by the type of the compounds being examined. Polar solvents are often used, however the specific solvents or solvent mixes used are dictated by the polarity and properties of the chemicals to be separated.
If pure solvents do not offer acceptable separation, a polarity-appropriate combination of solvents can be utilized to maximize the separation. The solvent combination in the mobile phase can be changed to improve the resolution and separation of the components in the mixture.
The mobile phase, generally a nonpolar solvent, moves up the paper by capillary action in paper chromatography. As the mobile phase travels, it transports the various components of the mixture with it. Because the components of the mixture have different affinities for the mobile phase and the stationary phase (paper), they will separate and produce unique spots or bands on the paper.
Overall, the mobile phase selection is critical in paper chromatography for successful separation and analysis. It is chosen depending on the polarity and properties of the compounds under investigation, and the mobile phase aids in the movement and separation of the components on the paper.
3. Chromatographic Chamber
- A chromatographic chamber is an important component in paper chromatography because it provides the conditions for the separation process to take place. Chromatographic chambers are commonly made of glass, plastic, or stainless steel, with glass tanks being the preferred material due to their clarity and chemical inertness.
- The dimensions and size of the chromatographic chamber might vary based on the length of the paper used and the development procedure used. Chambers are offered in a variety of sizes to accommodate different paper lengths and development procedures.
- The environment within the chromatographic chamber is an important factor. The solvent vapor in the chamber environment must be saturated. This is accomplished by depositing an appropriate amount of the mobile phase or solvent combination at the bottom of the chamber. The solvent evaporates and saturates the chamber atmosphere, resulting in a consistent and regulated condition for separation. Because of the saturated environment, the mobile phase moves equally and consistently across the paper, resulting in precise and reliable separations.
- The saturation of the chamber environment with solvent vapor is critical for effective and repeatable chromatographic separations. It keeps the paper wet throughout the procedure, allowing the analytes to migrate and preserving the connection between the stationary phase (paper) and the mobile phase (solvent). This regulated environment allows the components in the mixture to separate and distinct spots or bands to develop on the paper.
- To summarize, chromatographic chambers are essential in paper chromatography because they provide a controlled environment for the separation process. They are often made out of glass, plastic, or stainless steel. The chambers are available in a variety of sizes to fit varied paper lengths and development procedures. To provide uniform and dependable separations, the chamber environment must be saturated with solvent vapor. The saturated environment keeps the paper wet and allows analytes to migrate, resulting in effective chromatographic separations.
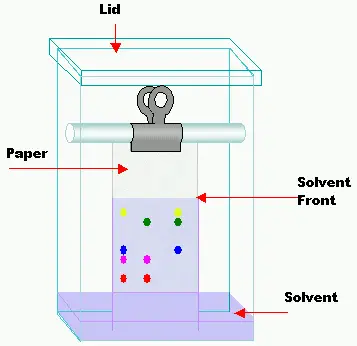
Steps in Paper Chromatography
Paper chromatography involves several steps in order to separate and analyze a sample mixture effectively. The key steps in paper chromatography are as follows:
- Selection of Solid Support: High-quality cellulose paper with defined porosity and high resolution is chosen as the solid support. It should allow for good movement of the solvent while minimizing sample diffusion.
- Selection of Mobile Phase: The mobile phase, which is the solvent or solvent mixture, is selected based on the nature of the analyte being separated. Different combinations of organic and inorganic solvents can be used. For example, a suitable solvent for separating amino acids is butanol:acetic acid:water (12:3:5).
- Saturation of Tank: The inner wall of the chromatography tank is wrapped with filter paper to ensure better resolution. This pre-saturation of the tank helps maintain a uniform solvent atmosphere during the separation.
- Sample Preparation and Loading: If the sample is in solid form, it is dissolved in a suitable solvent. A small volume of the sample (2-20 μL) is applied as a spot on the baseline of the filter paper using a micropipette. The spot is air-dried to prevent diffusion.
- Development of the Chromatogram: The sample-loaded filter paper is carefully placed into the chromatography tank, ensuring that the solvent level does not exceed a height of 1 cm. The chromatogram is developed by allowing the solvent to move up the paper through capillary action. Different development techniques can be used, such as ascending, descending, ascending-descending, or circular/radial development, depending on the desired separation outcome.
- Ascending Development: The solvent flows against gravity in this procedure. The sample is put to the bottom of the paper, which is then placed in a chamber with the mobile phase solvent at the bottom. The components of the sample separate and migrate higher as the solvent travels up the paper via capillary action. This is the most popular and traditional paper chromatography method.
- Descending Development: This process is performed in a specific chamber with the solvent container at the top. The sample is put to the top of the paper, and the solvent is allowed to trickle down the paper. The solvent migrates from the top to the bottom, causing the components to separate and migrate downward. This is referred to as descending chromatography.
- Ascending-Descending Development: A combination of ascending and descending development. By executing both ascending and descending chromatography sequentially, the separation duration is enhanced. Initially, the solvent flows from the bottom to the top (ascending), then reverses direction and flows from the top to the bottom (descending). This approach enables longer separation distances and higher resolution.
- Circular/Radial Development: A circular paper is utilized in this procedure, and the sample spot is applied in the middle. The solvent is injected through a wick in the middle and spreads equally in all directions, resulting in a radial flow pattern. This approach is appropriate for assessing materials that require a wider separation range or when numerous components must be resolved at the same time.
- Drying of Chromatogram: After the development process, the solvent front is marked, and the chromatogram is left to dry in a dry cabinet or oven. Drying ensures that the separated components are immobilized on the paper.
- Detection: Once the chromatogram is dry, the separated components need to be visualized or detected. Colorless analytes can be detected by staining the chromatogram with reagents such as iodine vapor or ninhydrin. Radiolabeled or fluorescently labeled analytes can be detected by measuring radioactivity or fluorescence, respectively.
By following these steps, paper chromatography allows for the separation and analysis of different components within a sample mixture, providing valuable information about their composition and relative concentrations.
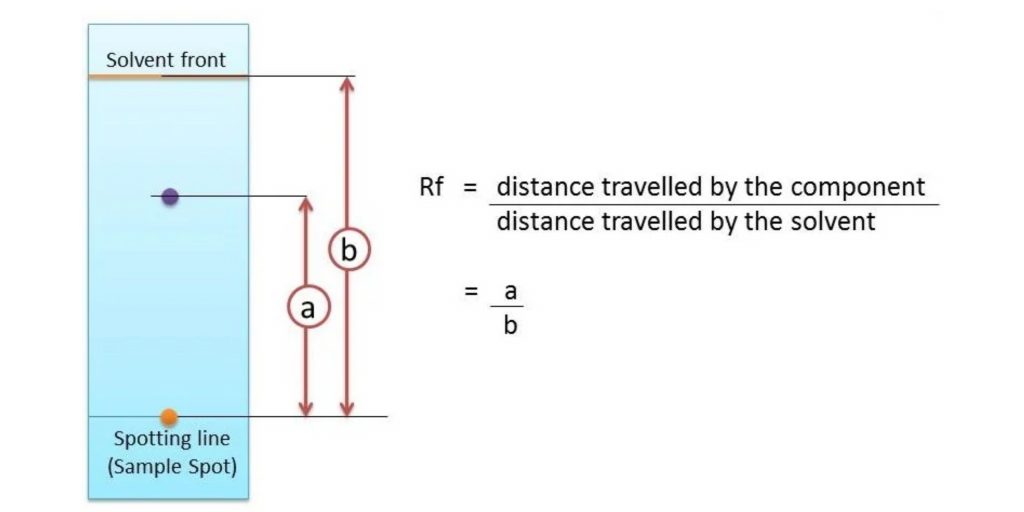
Rf values – Retention factors
In paper chromatography, the Rf (retention factor) value is a significant parameter used to measure the extent of movement of a component in a chromatographic experiment. The Rf value is defined as the ratio of the distance traveled by the component from the application point to the distance traveled by the solvent front.
The Rf value is calculated by dividing the distance traveled by the component by the total distance traveled by the solvent. This value is a constant for a particular compound under specific conditions such as the type of paper and the composition of the solvent.
The Rf value provides valuable information about the relative affinity of a compound for the stationary phase and the mobile phase. It ranges between 0 and 1, with different values indicating different behaviors:
- If the Rf value is 0, it means the solute has no affinity for the mobile phase and remains immobilized in the stationary phase.
- If the Rf value is 1, the solute has no affinity for the stationary phase and travels with the solvent front.
For example, if a compound travels a distance of 9.9 cm and the solvent front travels a distance of 12.7 cm, the Rf value would be calculated as (9.9/12.7) = 0.779 or 0.78.
The Rf value is influenced by factors such as temperature and the choice of solvent. Different solvents can result in different Rf values for the same mixture of compounds. Therefore, by using various solvents, it is possible to obtain different Rf values and achieve better separation and identification of components.
Rf values are particularly useful because they serve as characteristic identifiers for components under specific experimental conditions. By comparing the Rf values obtained from an unknown sample with those in a database or reference standards, it becomes possible to identify the components present in the mixture.
In summary, Rf values play a crucial role in paper chromatography as they provide a quantitative measure of the relative migration of components. They allow for comparisons, identification, and characterization of compounds in a sample, aiding in the analysis and interpretation of chromatographic results.
Applications of Paper Chromatography
Paper chromatography has several uses in a variety of industries. Among the most important uses are:
- Pharmaceutical purity control: Paper chromatography is used to assure the purity of pharmaceutical medications and the absence of impurities or contaminants.
- Adulterant detection: It is used to identify the presence of adulterants in a variety of commodities, including food, beverages, and cosmetics.
- Contaminant analysis in foods and drinks: Paper chromatography aids in the identification and quantification of pollutants in food and beverage items, assuring their safety and quality.
- Ripening and fermentation research: This method is used to investigate the ripening and fermentation processes in foods and drinks, offering insights on chemical changes and quality features.
- Detection of drugs and doping compounds: In sports and forensic sciences, paper chromatography is used to identify drugs and doping substances in biological samples such as urine or blood.
- Cosmetic analysis: It is used to determine the composition and purity of cosmetic items to ensure they fulfill regulatory criteria and are safe to use.
- Analysis of reaction mixtures in biochemical labs: Paper chromatography assists in the analysis of reaction mixtures in biochemical laboratories, allowing researchers to monitor the course of reactions and detect the presence of certain substances.
Furthermore, paper chromatography is used to analyze a wide range of chemicals such as amino acids, organic acids, alkaloids, polysaccharides, proteins and peptides, natural and synthetic colors, inorganic cations, and plant extracts.
In addition to these specific applications, paper chromatography has larger applications such as:
- Reaction monitoring: It allows for the monitoring of reaction progress by creating chromatograms at different time intervals and identifying the reactants.
- Isolation and purification: Paper chromatography aids in the isolation and purification of components from mixtures, allowing for further characterisation and analysis.
- Pharmaceutical R&D: It gives information on the creation of novel medication compounds, the completion of reactions, and the advancement of production procedures. It is a low-cost approach for detecting color fluctuations and monitoring active components in medication compositions.
- Forensics: In forensic investigation, paper chromatography is critical for the identification and comparison of drugs and their metabolites. It is very beneficial when working with tiny sample sizes.
- Food analysis: It aids with the examination of food colors in synthetic drinks, beverages, ice creams, and desserts, assuring compliance with legislation governing the use of approved edible colors.
Overall, paper chromatography is a flexible and effective analytical method that provides qualitative and quantitative information on substances and mixtures in a variety of businesses and research domains.
Advantages of Paper Chromatography
Paper chromatography has a number of features that make it a popular analytical method. These benefits include:
- Simplicity: Paper chromatography is a straightforward and easy procedure. It does not require any specialist equipment or training, making it accessible to a wide spectrum of consumers.
- Speed: Because this approach produces speedy findings, it allows for quick analysis and decision-making. In compared to other chromatographic procedures, the separation and identification of components on the paper occur rather fast.
- Minimal sample quantity required: Paper chromatography takes just a tiny amount of the sample for analysis. This approach can successfully examine even tiny levels of chemicals, saving significant resources.
- Cost-effective: Paper chromatography is a low-cost technology when compared to other chromatographic methods. Paper chromatography requires few supplies, such as filter paper, solvents, and developing chambers, making it an appealing alternative for research and regular analysis.
- Versatility: Paper chromatography is versatile in that it can detect both undiscovered inorganic and organic substances. It has a wide range of applications spanning multiple classes of substances and may be employed in a variety of industries such as pharmaceuticals, forensics, food analysis, and others.
- Space-efficient: Paper chromatography is space-efficient. Paper chromatography apparatus and setup are small, making it suited for laboratories with limited space.
- Excellent resolving power: Paper chromatography has very excellent resolving power and may separate components within a mixture. It can discriminate between closely related molecules and provide extensive information about the sample’s makeup.
Paper chromatography is an appealing alternative for qualitative and semi-quantitative analysis, screening tests, and early separations because of these benefits. Its ease of use, speed, low cost, and adaptability make it a vital tool in a variety of scientific fields and enterprises.
Limitations of Paper Chromatography
While paper chromatography has many advantages, it also has certain drawbacks that should be addressed. These constraints are as follows:
- Sample size limitation: Paper chromatography is not ideal for large-scale analysis since it requires a tiny amount of material. This might be a disadvantage when working with restricted or rare samples since it may not offer enough material for analysis or allow for repeated testing.
- Limited quantitative analysis: Paper chromatography is typically employed as a qualitative or semi-quantitative method with limited quantitative analysis. It is unsuitable for exact quantitative analysis, which requires accurate measurements and precise quantification of components. For quantitative analysis, other chromatographic methods such as high-performance liquid chromatography (HPLC) or high-performance thin-layer chromatography (HPTLC) are recommended.
- Inability to separate complicated mixes: Paper chromatography may fail to separate complex mixtures including several components with comparable characteristics. Paper chromatography has a lower resolving power than more modern chromatographic technologies. More advanced methods, such as column chromatography or gas chromatography, may be required for improved separation and identification of complex combinations.
- Lower accuracy: Paper chromatography is often thought to be less precise than methods such as HPLC or HPTLC. Visual identification and measurement of Rf values in paper chromatography might impose subjectivity and accuracy limits. For high-accuracy analysis, analytical techniques that produce more precise and accurate measurements, such as instrumental detection methods, are chosen.
When choosing a chromatographic technique for a given analysis, it is critical to keep these constraints in mind. While paper chromatography is useful for qualitative analysis and early separations, it may not be appropriate for all analytical demands, especially when better precision, accuracy, and quantitative analysis are required.
FAQ
What is paper chromatography?
Paper chromatography is a technique used to separate and analyze the components of a mixture based on their differential migration through a paper or cellulose matrix. It relies on the principles of partition and adsorption chromatography.
Can paper chromatography be used for quantitative analysis?
Paper chromatography is not typically used for quantitative analysis due to limitations in accuracy and reproducibility. It is more commonly employed as a qualitative technique for separation, identification, and comparison of components in a mixture.
What are the different development techniques in paper chromatography?
Different development techniques in paper chromatography include ascending development (solvent flows against gravity), descending development (solvent flows from top to bottom), ascending-descending development (a hybrid of ascending and descending techniques), and circular/radial development (solvent spreads uniformly in all directions from a central spot on a circular paper).
How can the components on a paper chromatogram be detected?
Components on a paper chromatogram can be detected using various methods. Colorless analytes can be visualized by treating the paper with reagents such as iodine vapor or ninhydrin, which produce color reactions. Radiolabeled or fluorescently labeled analytes can be detected by measuring radioactivity or fluorescence, respectively.
How can I ensure the best results in paper chromatography?
To achieve the best results in paper chromatography, it is important to carefully select the appropriate paper and mobile phase, ensure proper saturation of the chromatographic chamber with solvent vapor, apply the sample accurately and in small quantities, and optimize the development technique and conditions for the specific analyte or mixture being analyzed.
What are the limitations of paper chromatography?
Some limitations of paper chromatography include the inability to handle large sample quantities, lack of effectiveness in quantitative analysis, difficulty in separating complex mixtures, and lower accuracy compared to more advanced chromatographic techniques like high-performance liquid chromatography (HPLC) or high-performance thin-layer chromatography (HPTLC).
How is an Rf value calculated in paper chromatography?
The Rf (retention factor) value is calculated by dividing the distance traveled by a component from the application point to the distance traveled by the solvent front. It is a measure of the relative mobility of a component in the chromatogram and is constant under specific experimental conditions.
How does paper chromatography work?
In paper chromatography, a sample mixture is applied to a piece of filter paper, and the edge of the paper is immersed in a solvent or mobile phase. The solvent moves up the paper through capillary action, carrying the components of the mixture with it. As the solvent moves, the components separate based on their affinity for the stationary phase (paper) and the mobile phase (solvent), resulting in distinct bands or spots on the paper known as chromatograms.
What are the applications of paper chromatography?
Paper chromatography has a wide range of applications, including analyzing the purity of pharmaceuticals, detecting adulterants in food and drinks, studying ripening and fermentation processes, identifying drugs and doping substances in humans and animals, analyzing cosmetics, and examining reaction mixtures in biochemical labs. It can also be used to analyze specific classes of compounds such as amino acids, alkaloids, polysaccharides, proteins, pigments, and plant extracts.
What are the advantages of paper chromatography?
Paper chromatography offers several advantages, including its simplicity, rapidness, requirement of small sample quantities, low cost compared to other chromatography methods, ability to identify both organic and inorganic compounds, compact setup, and excellent resolving power.
References
- Skoog, D.A., West, D.M., Holler, F.J., Crouch, S.R. (2013). Fundamentals of Analytical Chemistry. Cengage Learning. Chapter 27: Paper Chromatography.
- Bhattacharya, S., Banerjee, R. (2018). Thin-Layer and Paper Chromatography: A Laboratory Handbook. CRC Press.
- Sherma, J., Fried, B. (2003). Handbook of Thin-Layer Chromatography. Marcel Dekker.
- Stahl, E. (1969). Thin-Layer Chromatography: A Laboratory Handbook. Springer.
- Smith, R.M. (1995). Introduction to Paper Chromatography. CRC Press.
